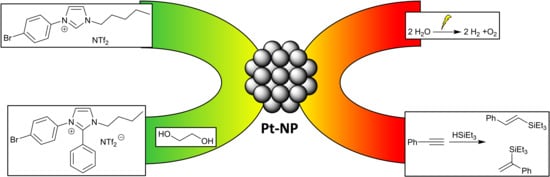
Tunable Aryl Alkyl Ionic Liquid Supported Synthesis of Platinum Nanoparticles and Their Catalytic Activity in the Hydrogen Evolution Reaction and in Hydrosilylation
Abstract
1. Introduction
2. Results and Discussion
2.1. Tunable Aryl Alkyl Ionic Liquids (TAAILs)
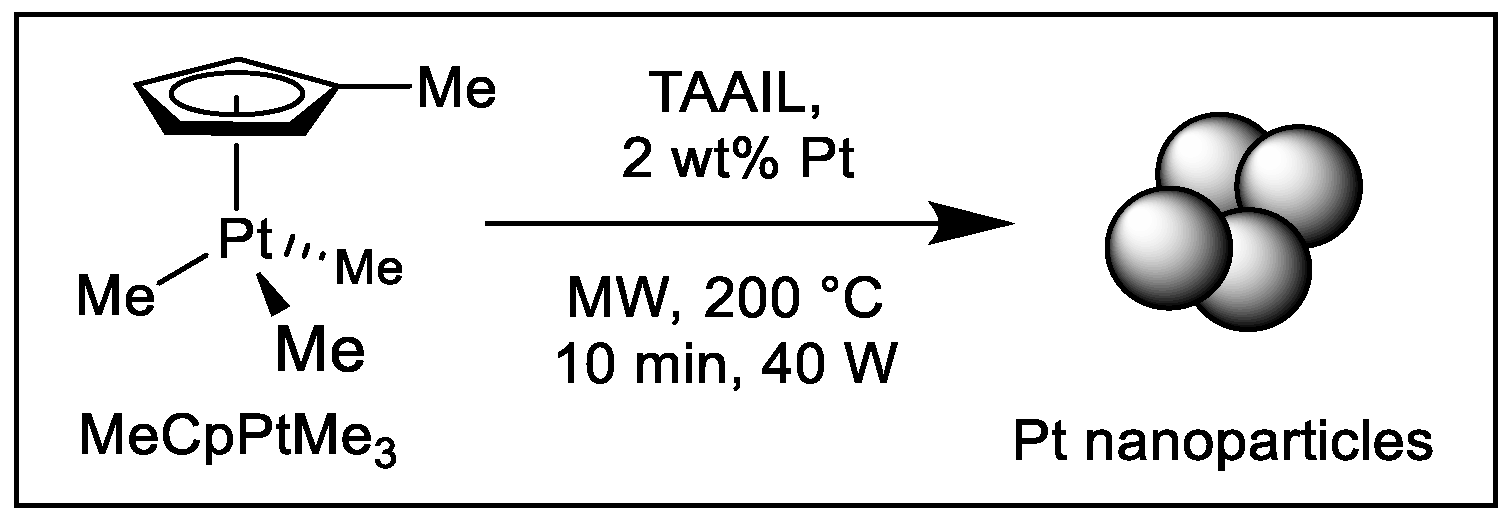
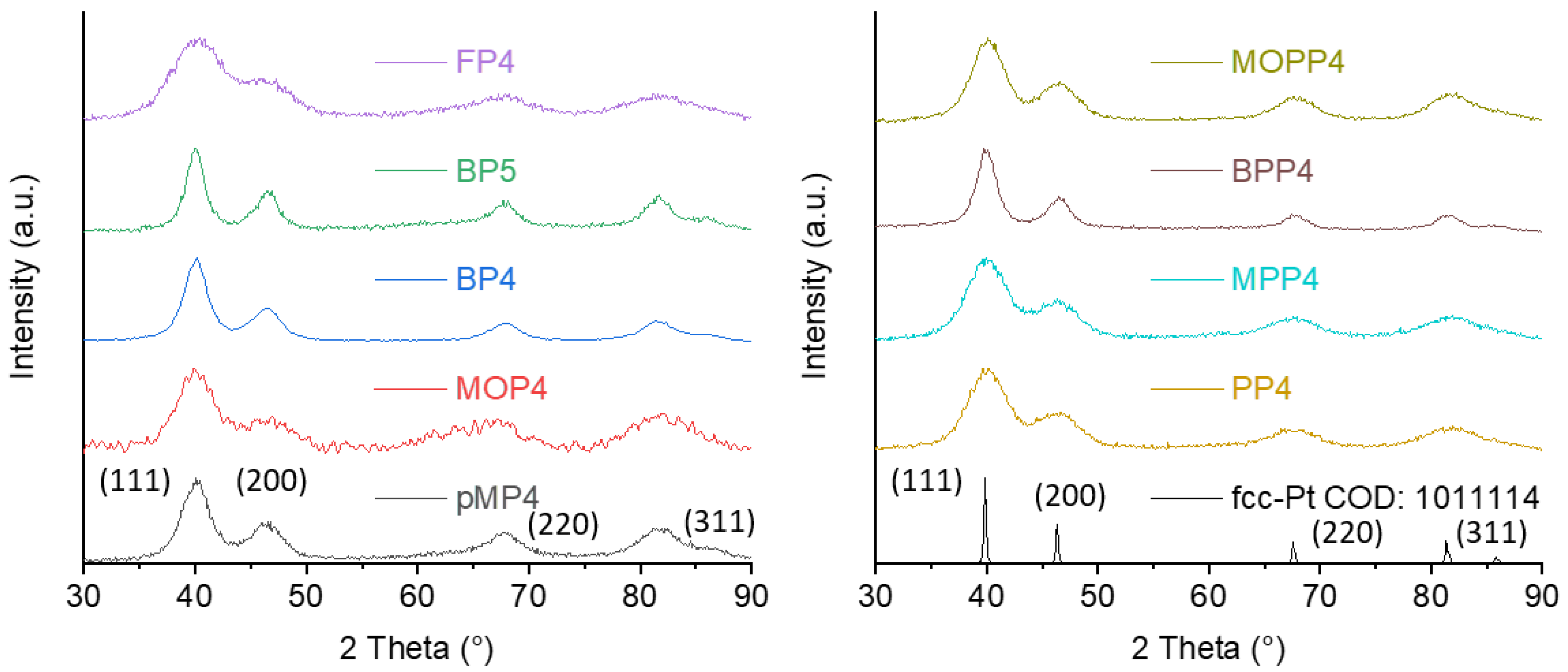
2.2. Synthesis and Characterization of Pt-NPs in TAAILs
2.3. Synthesis and Characterization of Pt-NPs in EG/TAAIL Mixtures
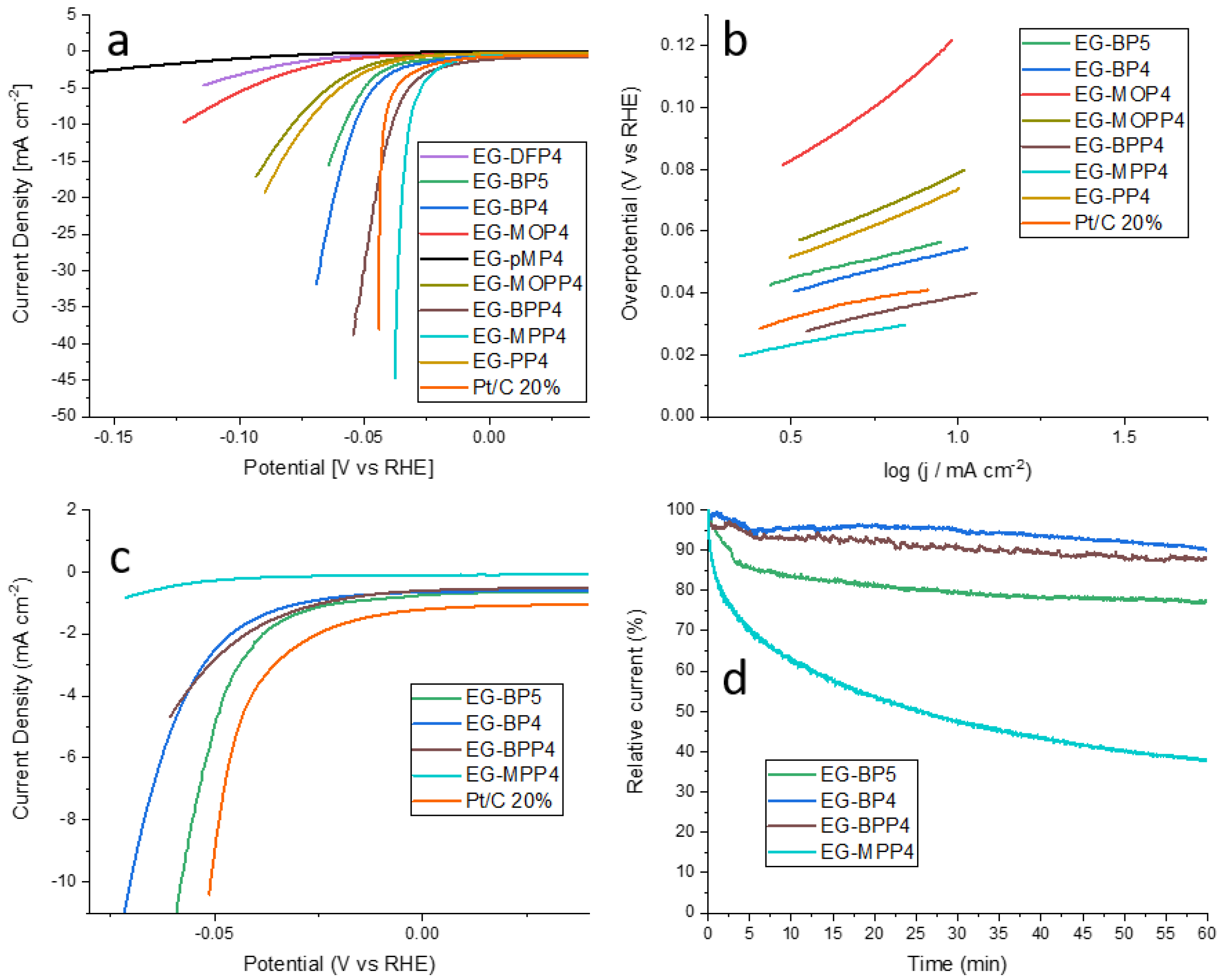
2.4. Hydrogen Evolution Reaction (HER)
2.5. Hydrosilylation Reaction
3. Materials and Methods
3.1. Chemicals and Equipment
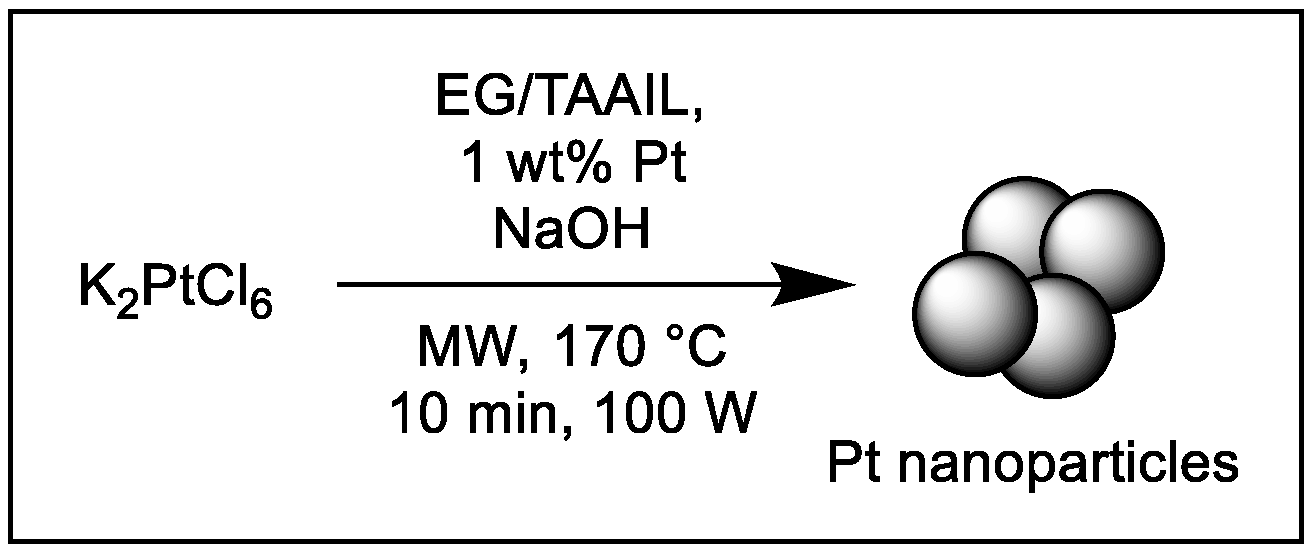
3.2. Synthesis of Pt-NPs in IL and EG/IL mixtures
3.3. Electrochemical Measurements
3.4. Hydrosilylation Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, C.; Niu, Z.; Kim, D.; Li, M.; Yang, P. Surface and Interface Control in Nanoparticle Catalysis. Chem. Rev. 2020, 120, 1184–1249. [Google Scholar] [CrossRef] [PubMed]
- Hartley, F.R. Chemistry of the Platinum Group Metals. Recent Developments; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 9780080933955. [Google Scholar]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- de Almeida, L.D.; Wang, H.; Junge, K.; Cui, X.; Beller, M. Recent Advances in Catalytic Hydrosilylations: Developments beyond Traditional Platinum Catalysts. Angew. Chem. Int. Ed. 2021, 60, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Mahata, A.; Nair, A.S.; Pathak, B. Recent advancements in Pt-nanostructure-based electrocatalysts for the oxygen reduction reaction. Catal. Sci. Technol. 2019, 9, 4835–4863. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Cheng, T.; Fortunelli, A.; Chen, C.-Y.; Yu, R.; Zhang, Q.; Gu, L.; Merinov, B.V.; Lin, Z.; et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 2016, 354, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Chen, H.; Wang, G.; Gao, T.; Chen, Y.; Liao, H.; Guo, X.; Li, H.; Liu, R.; Dou, M.; Nan, S.; et al. Effect of Atomic Ordering Transformation of PtNi Nanoparticles on Alkaline Hydrogen Evolution: Unexpected Superior Activity of the Disordered Phase. J. Phys. Chem. C 2020, 124, 5036–5045. [Google Scholar] [CrossRef]
- Bao, J.; Wang, J.; Zhou, Y.; Hu, Y.; Zhang, Z.; Li, T.; Xue, Y.; Guo, C.; Zhang, Y. Anchoring ultrafine PtNi nanoparticles on N-doped graphene for highly efficient hydrogen evolution reaction. Catal. Sci. Technol. 2019, 9, 4961–4969. [Google Scholar] [CrossRef]
- Vollath, D.; Fischer, F.D.; Holec, D. Surface energy of nanoparticles—Influence of particle size and structure. Beilstein J. Nanotechnol. 2018, 9, 2265–2276. [Google Scholar] [CrossRef]
- Garlyyev, B.; Kratzl, K.; Rück, M.; Michalička, J.; Fichtner, J.; Macak, J.M.; Kratky, T.; Günther, S.; Cokoja, M.; Bandarenka, A.S.; et al. Optimizing the Size of Platinum Nanoparticles for Enhanced Mass Activity in the Electrochemical Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2019, 58, 9596–9600. [Google Scholar] [CrossRef]
- Xu, D.; Lv, H.; Liu, B. Encapsulation of Metal Nanoparticle Catalysts Within Mesoporous Zeolites and Their Enhanced Catalytic Performances: A Review. Front. Chem. 2018, 6, 550. [Google Scholar] [CrossRef]
- Bera, S.; Mondal, D. A role for ultrasound in the fabrication of carbohydrate-supported nanomaterials. J. Ultrasound 2019, 22, 131–156. [Google Scholar] [CrossRef]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Ong, S.Y.; Zhang, C.; Dong, X.; Yao, S.Q. Recent Advances in Polymeric Nanoparticles for Enhanced Fluorescence and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2021, 60, 17797–17809. [Google Scholar] [CrossRef]
- Marquardt, D.; Beckert, F.; Pennetreau, F.; Tölle, F.; Mülhaupt, R.; Riant, O.; Hermans, S.; Barthel, J.; Janiak, C. Hybrid materials of platinum nanoparticles and thiol-functionalized graphene derivatives. Carbon 2014, 66, 285–294. [Google Scholar] [CrossRef]
- Wegner, S.; Janiak, C. Metal Nanoparticles in Ionic Liquids. Top. Curr. Chem. 2017, 375, 65. [Google Scholar] [CrossRef]
- Seidl, V.; Romero, A.H.; Heinemann, F.W.; Scheurer, A.; Vogel, C.S.; Unruh, T.; Wasserscheid, P.; Meyer, K. A New Class of Task-Specific Imidazolium Salts and Ionic Liquids and Their Corresponding Transition-Metal Complexes for Immobilization on Electrochemically Active Surfaces. Chemistry 2022, 28, e202200100. [Google Scholar] [CrossRef]
- Migowski, P.; Machado, G.; Texeira, S.R.; Alves, M.C.M.; Morais, J.; Traverse, A.; Dupont, J. Synthesis and characterization of nickel nanoparticles dispersed in imidazolium ionic liquids. Phys. Chem. Chem. Phys. 2007, 9, 4814–4821. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Lee, J.R.; Kim, S.J.; Jeong, H.; Jung, S.; Lee, J.-H.; Park, J.-C.; Kim, T.-W. Concerns and breakthroughs of combining ionic liquids with microwave irradiation for the synthesis of Ru nanoparticles via decarbonylation. J. Colloid Interface Sci. 2021, 599, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Woitassek, D.; Lerch, S.; Jiang, W.; Shviro, M.; Roitsch, S.; Strassner, T.; Janiak, C. The Facile Deposition of Pt Nanoparticles on Reduced Graphite Oxide in Tunable Aryl Alkyl Ionic Liquids for ORR Catalysts. Molecules 2022, 27, 1018. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.; Barthel, J.; Braun, M.; Ganter, C.; Janiak, C. Weakly-coordinated stable platinum nanocrystals. CrystEngComm 2012, 14, 7607. [Google Scholar] [CrossRef]
- Scheeren, C.W.; Machado, G.; Teixeira, S.R.; Morais, J.; Domingos, J.B.; Dupont, J. Synthesis and characterization of Pt0 nanoparticles in imidazolium ionic liquids. J. Phys. Chem. B 2006, 110, 13011–13020. [Google Scholar] [CrossRef] [PubMed]
- Schmolke, L.; Lerch, S.; Bülow, M.; Siebels, M.; Schmitz, A.; Thomas, J.; Dehm, G.; Held, C.; Strassner, T.; Janiak, C. Aggregation control of Ru and Ir nanoparticles by tunable aryl alkyl imidazolium ionic liquids. Nanoscale 2019, 11, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.G.; Mukherjee, D.; Reddy, B.M. Novel approaches for preparation of nanoparticles. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323461429. [Google Scholar]
- Liu, Z.; Lee, J.Y.; Chen, W.; Han, M.; Gan, L.M. Physical and electrochemical characterizations of microwave-assisted polyol preparation of carbon-supported PtRu nanoparticles. Langmuir 2004, 20, 181–187. [Google Scholar] [CrossRef]
- Fang, B.; Chaudhari, N.K.; Kim, M.-S.; Kim, J.H.; Yu, J.-S. Homogeneous deposition of platinum nanoparticles on carbon black for proton exchange membrane fuel cell. J. Am. Chem. Soc. 2009, 131, 15330–15338. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chen, F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar] [CrossRef] [PubMed]
- Şen, F.; Gökaǧaç, G. Improving Catalytic Efficiency in the Methanol Oxidation Reaction by Inserting Ru in Face-Centered Cubic Pt Nanoparticles Prepared by a New Surfactant, tert-Octanethiol. Energy Fuels 2008, 22, 1858–1864. [Google Scholar] [CrossRef]
- Quinson, J.; Inaba, M.; Neumann, S.; Swane, A.A.; Bucher, J.; Simonsen, S.B.; Theil Kuhn, L.; Kirkensgaard, J.J.K.; Jensen, K.M.O.; Oezaslan, M.; et al. Investigating Particle Size Effects in Catalysis by Applying a Size-Controlled and Surfactant-Free Synthesis of Colloidal Nanoparticles in Alkaline Ethylene Glycol: Case Study of the Oxygen Reduction Reaction on Pt. ACS Catal. 2018, 8, 6627–6635. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.; Deng, K.; Gui, L.; Tang, Y. Preparation of Tractable Platinum, Rhodium, and Ruthenium Nanoclusters with Small Particle Size in Organic Media. Chem. Mater. 2000, 12, 1622–1627. [Google Scholar] [CrossRef]
- Neumann, S.; Grotheer, S.; Tielke, J.; Schrader, I.; Quinson, J.; Zana, A.; Oezaslan, M.; Arenz, M.; Kunz, S. Nanoparticles in a box: A concept to isolate, store and re-use colloidal surfactant-free precious metal nanoparticles. J. Mater. Chem. A 2017, 5, 6140–6145. [Google Scholar] [CrossRef]
- Dewan, M.; Kumar, A.; Saxena, A.; De, A.; Mozumdar, S. Using hydrophilic ionic liquid, bmimBF4-ethylene glycol system as a novel media for the rapid synthesis of copper nanoparticles. PLoS ONE 2012, 7, e29131. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, W.J.; Hong, S.H.; Kim, J.E.; Suh, K.S. Ionic-liquid-assisted formation of silver nanowires. Angew. Chem. Int. Ed. 2009, 48, 3806–3809. [Google Scholar] [CrossRef]
- Freire, M.G.; Louros, C.L.S.; Rebelo, L.P.N.; Coutinho, J.A.P. Aqueous biphasic systems composed of a water-stable ionic liquid + carbohydrates and their applications. Green Chem. 2011, 13, 1536–1545. [Google Scholar] [CrossRef]
- Rodríguez, H.; Rogers, R.D. Liquid mixtures of ionic liquids and polymers as solvent systems. Fluid Phase Equilib. 2010, 294, 7–14. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Y.; Li, X.; Xiao, B.-Y.; Wu, L.; Song, J.-P.; Xiao, Y.-X.; Wu, S.-M.; Hu, J.; Wang, Y.; et al. Hierarchically Dual-Mesoporous TiO2 Microspheres for Enhanced Photocatalytic Properties and Lithium Storage. Chem. Eur. J. 2018, 24, 13246–13252. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, J.E.; Kim, T.; Suh, K.S. Ionic liquid-assisted synthesis of highly branched Ag:AgCl hybrids and their photocatalytic activity. J. Alloys Compd. 2015, 621, 378–382. [Google Scholar] [CrossRef]
- Li, M.; Bo, X.; Zhang, Y.; Han, C.; Guo, L. One-pot ionic liquid-assisted synthesis of highly dispersed PtPd nanoparticles/reduced graphene oxide composites for nonenzymatic glucose detection. Biosens. Bioelectron. 2014, 56, 223–230. [Google Scholar] [CrossRef]
- Okoli, C.U.; Kuttiyiel, K.A.; Cole, J.; McCutchen, J.; Tawfik, H.; Adzic, R.R.; Mahajan, D. Solvent effect in sonochemical synthesis of metal-alloy nanoparticles for use as electrocatalysts. Ultrason. Sonochem. 2018, 41, 427–434. [Google Scholar] [CrossRef]
- He, X.; Sun, Z.; Zou, Q.; Wu, L.; Jiang, J. Electrochemical Behavior of Co(II) Reduction for Preparing Nanocrystalline Co Catalyst for Hydrogen Evolution Reaction from 1-ethyl-3-methylimidazolium Bisulfate and Ethylene Glycol System. J. Electrochem. Soc. 2019, 166, D57–D64. [Google Scholar] [CrossRef]
- He, X.; Sun, Z.; Zou, Q.; Yang, J.; Wu, L. Codeposition of Nanocrystalline Co-Ni Catalyst Based on 1-ethyl-3-methylimidazolium Bisulfate and Ethylene Glycol System for Hydrogen Evolution Reaction. J. Electrochem. Soc. 2019, 166, D908–D915. [Google Scholar] [CrossRef]
- Biller, H.; Strassner, T. Synthesis and Physical Properties of Tunable Aryl Alkyl Ionic Liquids (TAAILs) Comprising Imidazolium Cations Blocked with Methyl-, Propyl- and Phenyl-Groups at the C2 Position. Chem. Eur. J. 2022, 29, e202202795. [Google Scholar] [CrossRef]
- Kukawka, R.; Pawlowska-Zygarowicz, A.; Dutkiewicz, M.; Maciejewski, H.; Smiglak, M. New approach to hydrosilylation reaction in ionic liquids as solvent in microreactor system. RSC Adv. 2016, 6, 61860–61868. [Google Scholar] [CrossRef]
- Zielinski, W.; Kukawka, R.; Maciejewski, H.; Smiglak, M. Ionic Liquids as Solvents for Rhodium and Platinum Catalysts Used in Hydrosilylation Reaction. Molecules 2016, 21, 1115. [Google Scholar] [CrossRef]
- Geldbach, T.J.; Zhao, D.; Castillo, N.C.; Laurenczy, G.; Weyershausen, B.; Dyson, P.J. Biphasic hydrosilylation in ionic liquids: A process set for industrial implementation. J. Am. Chem. Soc. 2006, 128, 9773–9780. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, N.; Bauer, A.; Frey, T.; Auer, M.; Stanjek, V.; Schulz, P.S.; Taccardi, N.; Wasserscheid, P. Liquid-Liquid Biphasic, Platinum-Catalyzed Hydrosilylation of Allyl Chloride with Trichlorosilane using an Ionic Liquid Catalyst Phase in a Continuous Loop Reactor. Adv. Synth. Catal. 2008, 350, 2599–2609. [Google Scholar] [CrossRef]
- Lerch, S.; Strassner, T. Expanding the Electrochemical Window: New Tunable Aryl Alkyl Ionic Liquids (TAAILs) with Dicyanamide Anions. Chem. Eur. J. 2019, 25, 16251–16256. [Google Scholar] [CrossRef] [PubMed]
- Lerch, S.; Strassner, T. Synthesis and Physical Properties of Tunable Aryl Alkyl Ionic Liquids (TAAILs). Chem. Eur. J. 2021, 27, 15554–15557. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef]
- Maton, C.; de Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Lubers, A.M.; Muhich, C.L.; Anderson, K.M.; Weimer, A.W. Mechanistic studies for depositing highly dispersed Pt nanoparticles on carbon by use of trimethyl(methylcyclopentadienyl)platinum(IV) reactions with O2 and H2. J. Nanopart. Res. 2015, 17, 85. [Google Scholar] [CrossRef]
- Hill, J.M.; Marchant, T.R. Modelling microwave heating. Appl. Math. Model. 1996, 20, 3–15. [Google Scholar] [CrossRef]
- Tierney, J.P.; Lidström, P. Microwave Assisted Organic Synthesis; Blackwell Publishing: Oxford, UK, 2005. [Google Scholar] [CrossRef]
- Prechtl, M.H.G.; Scholten, J.D.; Dupont, J. Carbon-carbon cross coupling reactions in ionic liquids catalysed by palladium metal nanoparticles. Molecules 2010, 15, 3441–3461. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, C.; Janiak, C. Naked metal nanoparticles from metal carbonyls in ionic liquids: Easy synthesis and stabilization. Coord. Chem. Rev. 2011, 255, 2039–2057. [Google Scholar] [CrossRef]
- Yang, R.; Qiu, X.; Zhang, H.; Li, J.; Zhu, W.; Wang, Z.; Huang, X.; Chen, L. Monodispersed hard carbon spherules as a catalyst support for the electrooxidation of methanol. Carbon 2005, 43, 11–16. [Google Scholar] [CrossRef]
- Meng, H.; Zhan, Y.; Zeng, D.; Zhang, X.; Zhang, G.; Jaouen, F. Factors Influencing the Growth of Pt Nanowires via Chemical Self-Assembly and their Fuel Cell Performance. Small 2015, 11, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Bizzotto, F.; Quinson, J.; Zana, A.; Kirkensgaard, J.J.K.; Dworzak, A.; Oezaslan, M.; Arenz, M. Ir nanoparticles with ultrahigh dispersion as oxygen evolution reaction (OER) catalysts: Synthesis and activity benchmarking. Catal. Sci. Technol. 2019, 9, 6345–6356. [Google Scholar] [CrossRef]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F. Hydrogen energy. Philos. Trans. Royal Soc. A 2007, 365, 1043–1056. [Google Scholar] [CrossRef]
- Chen, W.-F.; Sasaki, K.; Ma, C.; Frenkel, A.I.; Marinkovic, N.; Muckerman, J.T.; Zhu, Y.; Adzic, R.R. Hydrogen-evolution catalysts based on non-noble metal nickel-molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 2012, 51, 6131–6135. [Google Scholar] [CrossRef]
- Ravula, S.; Zhang, C.; Essner, J.B.; Robertson, J.D.; Lin, J.; Baker, G.A. Ionic Liquid-Assisted Synthesis of Nanoscale (MoS2)x(SnO2)1-x on Reduced Graphene Oxide for the Electrocatalytic Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 8065–8074. [Google Scholar] [CrossRef]
- Rademacher, L.; Beglau, T.H.Y.; Karakas, Ö.; Spieß, A.; Woschko, D.; Heinen, T.; Barthel, J.; Janiak, C. Synthesis of tin nanoparticles on Ketjen Black in ionic liquid and water for the hydrogen evolution reaction. Electrochem. Commun. 2022, 136, 107243. [Google Scholar] [CrossRef]
- Qiao, S.; Zhang, B.; Li, Q.; Li, Z.; Wang, W.; Zhao, J.; Zhang, X.; Hu, Y. Pore Surface Engineering of Covalent Triazine Frameworks@MoS2 Electrocatalyst for the Hydrogen Evolution Reaction. ChemSusChem 2019, 12, 5032–5040. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, L.; Beglau, T.H.Y.; Heinen, T.; Barthel, J.; Janiak, C. Microwave-assisted synthesis of iridium oxide and palladium nanoparticles supported on a nitrogen-rich covalent triazine framework as superior electrocatalysts for the hydrogen evolution and oxygen reduction reaction. Front. Chem. 2022, 10, 945261. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, X.; Chen, S.; Yan, H.; Wang, C.; Wu, C.; Haleem, Y.A.; Duan, S.; Lu, J.; Ge, B.; et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.-K.; Liu, L.-M.; et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun 2016, 7, 13638. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhang, J.; Wan, S.; Guo, S.; Lu, G.; Yao, J.; Huang, X. Precise tuning in platinum-nickel/nickel sulfide interface nanowires for synergistic hydrogen evolution catalysis. Nat. Commun 2017, 8, 14580. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Chalk, A.J.; Harrod, J.F. Homogeneous Catalysis. II. The Mechanism of the Hydrosilation of Olefins Catalyzed by Group VIII Metal Complexes 1. J. Am. Chem. Soc. 1965, 87, 16–21. [Google Scholar] [CrossRef]
- Naganawa, Y.; Inomata, K.; Sato, K.; Nakajima, Y. Hydrosilylation reactions of functionalized alkenes. Tetrahedron Lett. 2020, 61, 151513. [Google Scholar] [CrossRef]
- Troegel, D.; Stohrer, J. Recent advances and actual challenges in late transition metal catalyzed hydrosilylation of olefins from an industrial point of view. Coord. Chem. Rev. 2011, 255, 1440–1459. [Google Scholar] [CrossRef]
- Nakajima, Y.; Shimada, S. Hydrosilylation reaction of olefins: Recent advances and perspectives. RSC Adv. 2015, 5, 20603–20616. [Google Scholar] [CrossRef]
- Komiyama, T.; Minami, Y.; Hiyama, T. Recent Advances in Transition-Metal-Catalyzed Synthetic Transformations of Organosilicon Reagents. ACS Catal. 2017, 7, 631–651. [Google Scholar] [CrossRef]
- Rivero-Crespo, M.; Oliver-Meseguer, J.; Kapłońska, K.; Kuśtrowski, P.; Pardo, E.; Cerón-Carrasco, J.P.; Leyva-Pérez, A. Cyclic metal(oid) clusters control platinum-catalysed hydrosilylation reactions: From soluble to zeolite and MOF catalysts. Chem. Sci. 2020, 11, 8113–8124. [Google Scholar] [CrossRef] [PubMed]
- Dobó, D.G.; Sipos, D.; Sipos, D.; Sápi, A.; London, G.; Juhász, K.; Kukovecz, Á.; Kónya, Z. Tuning the Activity and Selectivity of Phenylacetylene Hydrosilylation with Triethylsilane in the Liquid Phase over Size Controlled Pt Nanoparticles. Catalysts 2018, 8, 22. [Google Scholar] [CrossRef]
- Chauhan, M.; Hauck, B.J.; Keller, L.P.; Boudjouk, P. Hydrosilylation of alkynes catalyzed by platinum on carbon. J. Organomet. Chem. 2002, 645, 1–13. [Google Scholar] [CrossRef]
- Fernández, G.; Pleixats, R. Soluble Pt Nanoparticles Stabilized by a Tris-imidazolium Tetrafluoroborate as Efficient and Recyclable Catalyst for the Stereoselective Hydrosilylation of Alkynes. ChemistrySelect 2018, 3, 11486–11493. [Google Scholar] [CrossRef]
- Chauhan, B.P.S.; Sarkar, A. Functionalized vinylsilanes via highly efficient and recyclable Pt-nanoparticle catalysed hydrosilylation of alkynes. Dalton Trans. 2017, 46, 8709–8715. [Google Scholar] [CrossRef]
- Fang, H.; Chen, J.; Xiao, Y.; Zhang, J. Platinum nanoparticles confined in imidazolium-based ionic polymer for assembling a microfluidic reactor with enhanced catalytic activity. Appl. Catal. A Gen. 2019, 585, 117186. [Google Scholar] [CrossRef]
- Woitassek, D.; Moya-Cancino, J.G.; Sun, Y.; Song, Y.; Woschko, D.; Roitsch, S.; Janiak, C. Sweet, Sugar-Coated Hierarchical Platinum Nanostructures for Easy Support, Heterogenization and Separation. Chemistry 2022, 4, 1147–1160. [Google Scholar] [CrossRef]
- Abramov, A.; Diaz Diaz, D. Katalysatoren immobilisieren. Nachr. Chem. 2022, 70, 75–78. [Google Scholar] [CrossRef]
- Lambert, J.M. The nature of platinum in silicones for biomedical and healthcare use. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 167–180. [Google Scholar] [CrossRef]
- Wixtrom, R.; Glicksman, C.; Kadin, M.; Lawrence, M.; Haws, M.; Ferenz, S.; Sung, J.; McGuire, P. Heavy Metals in Breast Implant Capsules and Breast Tissue: Findings from the Systemic Symptoms in Women-Biospecimen Analysis Study: Part 2. Aesthet. Surg. J. 2022, 42, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Strouse, M.J.; Shuh, D.K.; Knobler, C.B.; Kaesz, H.D.; Hicks, R.F.; Williams, R.S. Characterization of (methylcyclopentadienyl)trimethylplatinum and low-temperature organometallic chemical vapor deposition of platinum metal. J. Am. Chem. Soc. 1989, 111, 8779–8784. [Google Scholar] [CrossRef]
- Beermann, V.; Gocyla, M.; Kühl, S.; Padgett, E.; Schmies, H.; Goerlin, M.; Erini, N.; Shviro, M.; Heggen, M.; Dunin-Borkowski, R.E.; et al. Tuning the Electrocatalytic Oxygen Reduction Reaction Activity and Stability of Shape-Controlled Pt-Ni Nanoparticles by Thermal Annealing—Elucidating the Surface Atomic Structural and Compositional Changes. J. Am. Chem. Soc. 2017, 139, 16536–16547. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.; Kirleis, K.; Butenschön, H. Stereodivergent Formation of Alkenylsilanes:syn oranti Hydrosilylation of Alkynes Catalyzed by a Cyclopentadienylcobalt(I) Chelate Bearing a Pendant Phosphane Tether. Adv. Synth. Catal. 2006, 348, 833–836. [Google Scholar] [CrossRef]
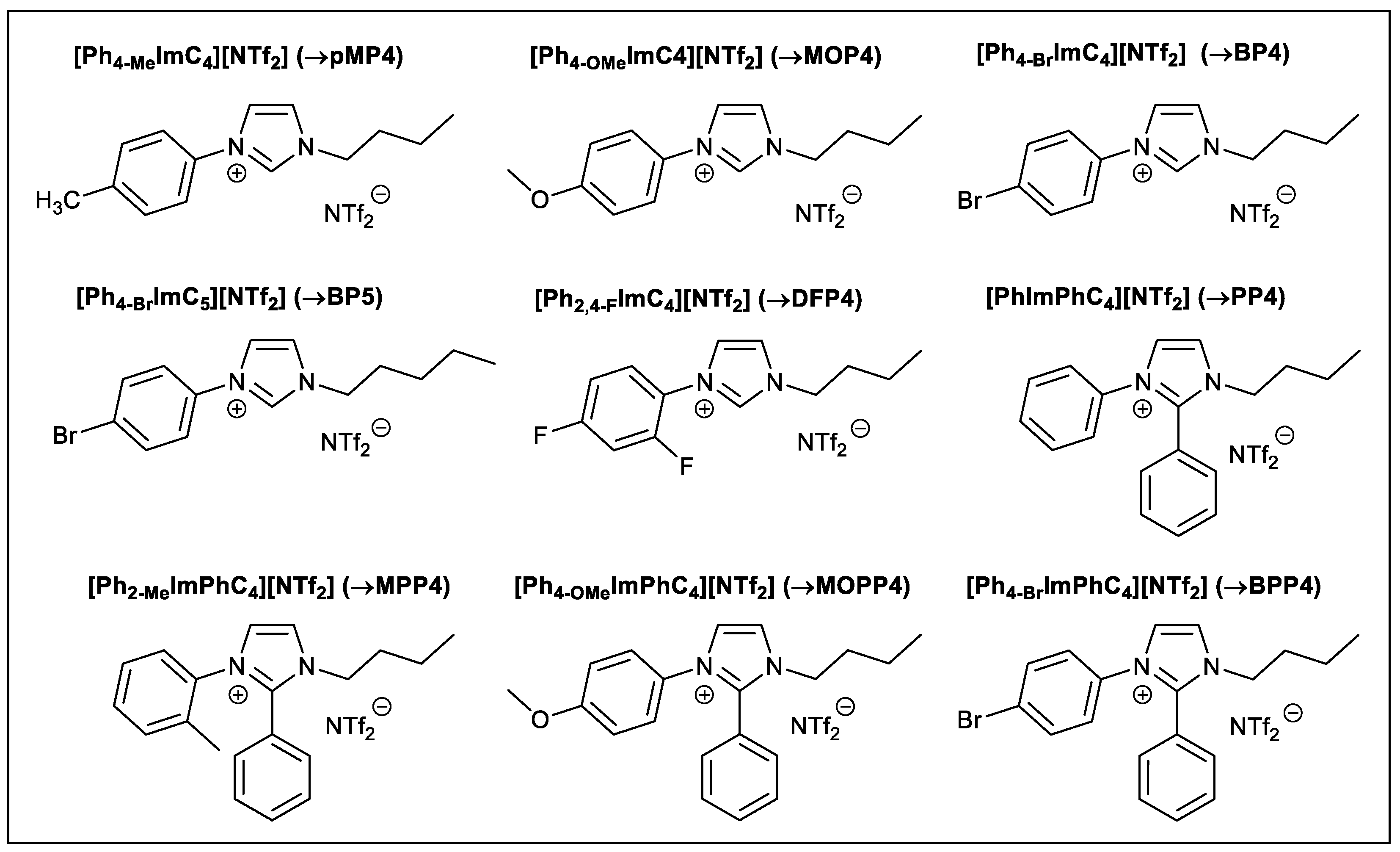
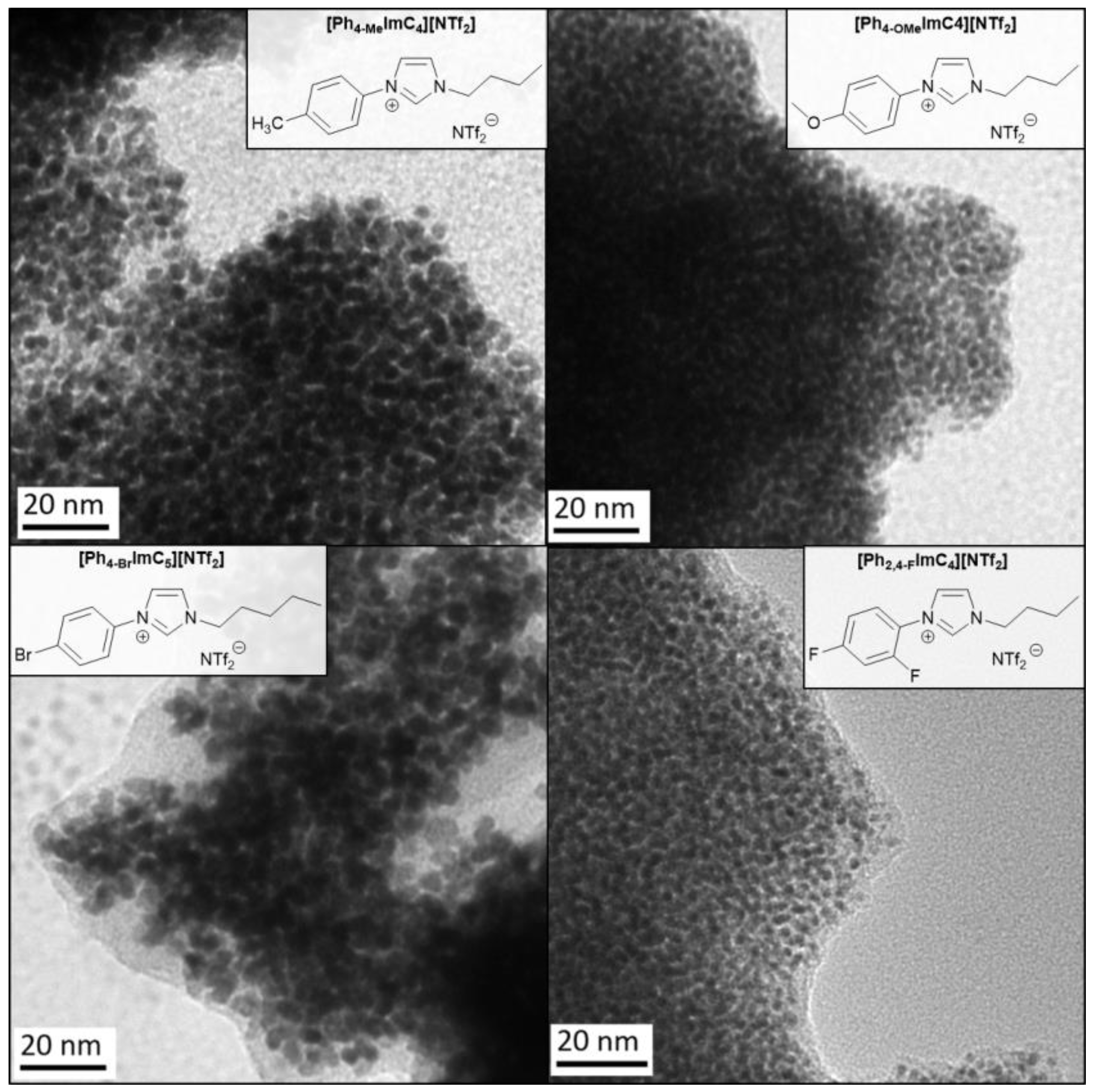
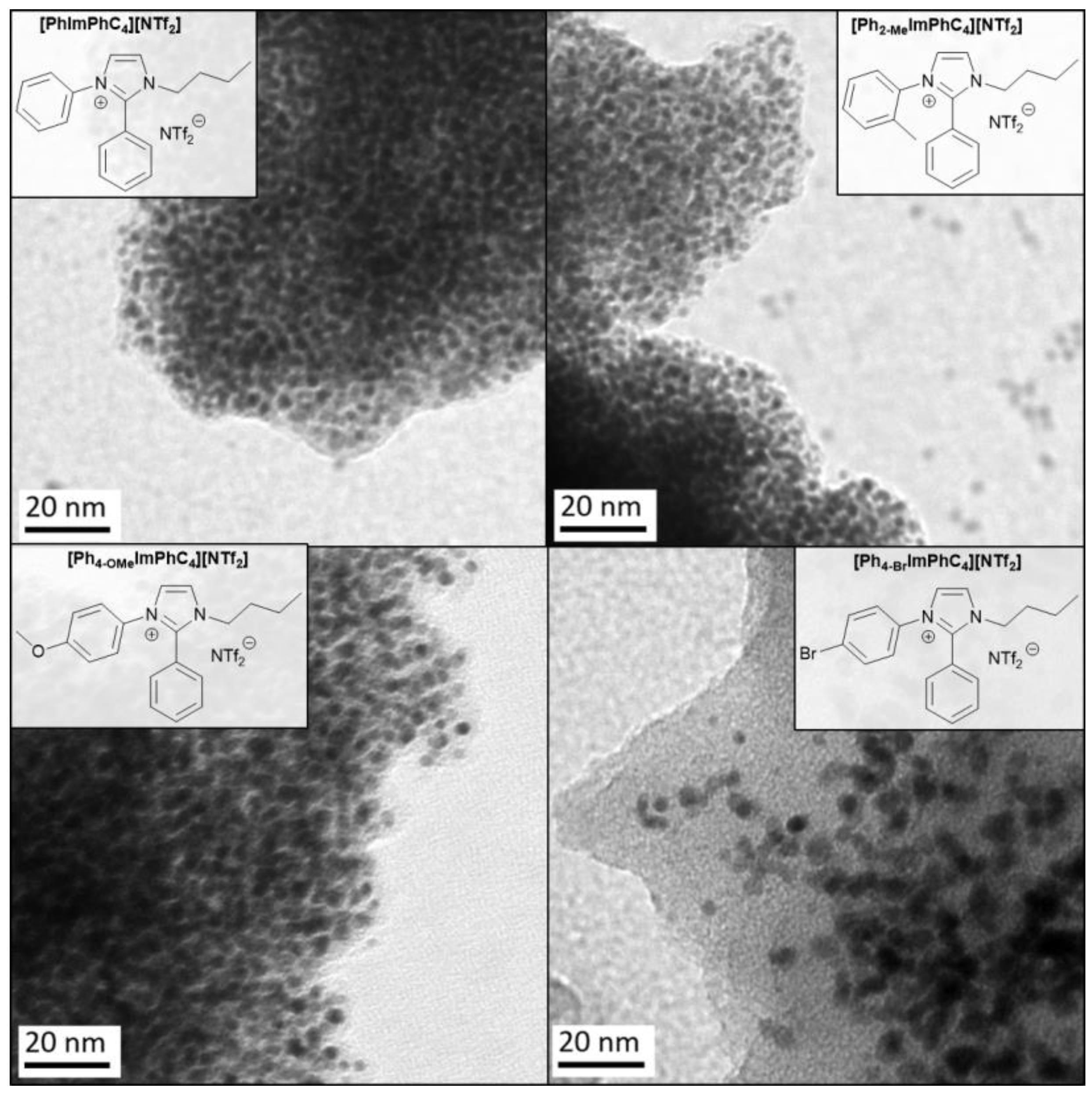



| TAAIL | Color | Anion Purity 1 (wt%) | IL purity 2 (wt%) | TDec 3 (°C) |
|---|---|---|---|---|
| [Ph4-MeImC4][NTf2] | orange | 98.4 | 92 | 403 |
| [Ph4-OMeImC4][NTf2] | brown | >99 | 95 | 417 |
| [Ph4-BrImC4][NTf2] | orange | 97.6 | 98 | 410 |
| [Ph4-BrImC5][NTf2] | brown | 98.4 | 102 | 399 |
| [Ph2,4-FImC4][NTf2] | brown | >99 | 97 | 394 |
| [PhImPhC4][NTf2] | orange | >99 | 94 | 414 |
| [Ph2-MeImPhC4][NTf2] | yellow | 98.3 | 93 | 414 |
| [Ph4-OMeImPhC4][NTf2] | brown | >99 | 102 | 410 |
| [Ph4-BrImPhC4][NTf2] | black | 97.6 | 94 | 413 |
| Sample | TAAIL Used in Synthesis | Crystallite Size 1 (nm) | Particle Size 2 (nm) |
|---|---|---|---|
| pMP4 | [Ph4-MeImC4][NTf2] | 4 | 3.1 ± 0.6 |
| MOP4 | [Ph4-OMeImC4][NTf2] | 3 | 1.8 ± 0.3 |
| BP4 | [Ph4-BrImC4][NTf2] | 4 | 3.2 ± 0.5 |
| BP5 | [Ph4-BrImC5][NTf2] | 5 | 3.3 ± 0.6 |
| DFP4 | [Ph2,4-FImC4][NTf2] | 3 | 2.2 ± 0.6 |
| PP4 | [PhImPhC4][NTf2] | 3 | 2.3 ± 0.4 |
| MPP4 | [Ph2-MeImPhC4][NTf2] | 3 | 2.4 ± 0.4 |
| MOPP4 | [Ph4-OMeImPhC4][NTf2] | 3 ± 1 | 2.9 ± 0.4 |
| BPP4 | [Ph4-BrImPhC4][NTf2] | 4 | 5.0 ± 1.0 |
| Sample Name | TAAIL Used in Synthesis | Crystallite Size 1 (nm) | Particle Size 2 (nm) |
|---|---|---|---|
| EG-pMP4 | [Ph4-MeImC4][NTf2] | 2 | / |
| EG-MOP4 | [Ph4-OMeImC4][NTf2] | 4 | 1.8 ± 0.3 |
| EG-BP4 | [Ph4-BrImC4][NTf2] | 2 | / |
| EG-BP5 | [Ph4-BrImC5][NTf2] | 3 | 3.3 ± 0.6 |
| EG-DFP4 | [Ph2,4-FImC4][NTf2] | 3 | 2.2 ± 0.6 |
| EG-PP4 | [PhImPhC4][NTf2] | 5 | 2.3 ± 0.4 |
| EG-MPP4 | [Ph2-MeImPhC4][NTf2] | 3 | 2.4 ± 0.4 |
| EG-MOPP4 | [Ph4-OMeImPhC4][NTf2] | 2 | 2.9 ± 0.4 |
| EG-BPP4 | [Ph4-BrImPhC4][NTf2] | 2 | 5.0 ± 1.0 |
| Sample Name | η10 mA cm−2 after Activation (mV) | Tafel Slope (mV dec−1) 3 | η10 mA cm−2 after Stability Test (mV) |
|---|---|---|---|
| (TAAIL)Pt-NPs | |||
| pMP4 | 64 | 24 | / |
| BP4 | n.a. 1 | / | / |
| DFP4 | n.a. 1 | / | / |
| PP4 | n.a. 1 | / | / |
| MPP4 | n.a. 1 | / | / |
| MOPP4 | 83 | 54 | / |
| BPP4 | 108 | 81 | / |
| (EG/TAAIL)Pt-NPs | |||
| EG-pMP4 | n.a. 1 | / | / |
| EG-MOP4 | 123 | 78 | / |
| EG-BP4 | 54 | 27 | 70 |
| EG-BP5 | 58 | 26 | / |
| EG-DFP4 | n.a. 1 | / | / |
| EG-PP4 | 74 | 44 | / |
| EG-MPP4 | 32 | 20 | n.a. 1 |
| EG-MOPP4 | 79 | 46 | / |
| EG-BPP4 | 39 | 24 | n.a. 1 |
| Reference materials | |||
| Pt/C 20 wt% | 42 | 25 | 51 |
| Pt1/OLC [66] | 38 | 35 | 38 4 |
| Pt3Ni2 NWs-S/C [67] | 27 | / | / |
| ALD50Pt/NGNs [68] | 50 2 | 29 | 50 |
| Sample Name | Time (h) | Temp. (°C) | Molar Substrate/Pt Ratio 2 | Conversion (%) 3 | d/p Ratio 4 |
|---|---|---|---|---|---|
| Method 1, (EG/TAAIL)K2PtCl6 | |||||
| (EG/[Ph4-BrImC4][NTf2])K2PtCl6 | 0.25 | 110 | 9620 | 96 | 3.1 |
| (EG/[Ph4-BrImC5][NTf2])K2PtCl6 | 0.25 | 110 | 9620 | 96 | 2.5 |
| (EG/[PhImPhC4][NTf2])K2PtCl6 | 0.25 | 110 | 9940 | 99 | 3.0 |
| (EG/[Ph2-MeImPhC4][NTf2])K2PtCl6 | 0.25 | 110 | 10,100 | >99 | 3.1 |
| (EG/[Ph4-BrImPhC4][NTf2])K2PtCl6 | 0.25 | 110 | 8790 | >99 | 2.2 |
| Method 2, (EG/TAAIL)Pt-NPs | |||||
| EG-BP4 | 0.25 | 110 | 1050 | 38 | 3.6 |
| EG-PP4 | 0.25 | 110 | 980 | 57 | 3.1 |
| EG-BPP4 | 0.25 | 110 | 980 | 75 | 3.4 |
| Method 3 | |||||
| (EG/TAAIL)Pt-NPs | |||||
| EG-BP4 | 0.083 | 200 | 880 | >99 | 1.5 |
| EG-BP5 | 0.083 | 200 | 980 | 67 | 2.2 |
| EG-MPP4 | 0.083 | 200 | 950 | >99 | 2.0 |
| EG-BPP4 | 0.083 | 200 | 990 | 99 | 1.8 |
| (TAAIL)Pt-NPs | |||||
| BP4 | 0.083 | 200 | 1030 | 82 | 2.6 |
| BP5 | 0.083 | 200 | 1140 | 95 | 2.2 |
| PP4 | 0.083 | 200 | 880 | 99 | 1.5 |
| MPP4 | 0.083 | 200 | 990 | >99 | 2.0 |
| Sample Name | Time (h) | Temp. (°C) | Molar Substrate/Pt Ratio 2 | Conversion (%) 3 | d/p Ratio 4 |
|---|---|---|---|---|---|
| [P44414][NTf2]/Karstedt [44] 1 | 1 | 110 | 10,000 | >99 | - |
| [S222][NTf2]/K2PtCl6 [45] 1 | 1 | 110 | 10,000 | ~88 | - |
| Pt1/NaY [75] | 24 | 110 | 2440 | 82 | 0.3 |
| 7.0 nm Pt/SBA-15 [76] | 6 | 70 | 390 | 6.8 | 1.8 |
| Pt/C [77] | 4.5 | 70 | 4880 | 91 | 3.3 |
| Pt-NP [78] | 1.3 | 60 | 200 | 82 | 4.9 |
| Pt-NP [78] | 24 | 60 | 200 | 94 | 9.0 |
| Pt-NP [79] | 10 | rt | 1000 | 98 | 6.7 |
| C-Pt/ImIP-2BrB [80] | 4 | 80 | 2000 | 79 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woitassek, D.; Strothmann, T.; Biller, H.; Lerch, S.; Schmitz, H.; Song, Y.; Roitsch, S.; Strassner, T.; Janiak, C. Tunable Aryl Alkyl Ionic Liquid Supported Synthesis of Platinum Nanoparticles and Their Catalytic Activity in the Hydrogen Evolution Reaction and in Hydrosilylation. Molecules 2023, 28, 405. https://doi.org/10.3390/molecules28010405
Woitassek D, Strothmann T, Biller H, Lerch S, Schmitz H, Song Y, Roitsch S, Strassner T, Janiak C. Tunable Aryl Alkyl Ionic Liquid Supported Synthesis of Platinum Nanoparticles and Their Catalytic Activity in the Hydrogen Evolution Reaction and in Hydrosilylation. Molecules. 2023; 28(1):405. https://doi.org/10.3390/molecules28010405
Chicago/Turabian StyleWoitassek, Dennis, Till Strothmann, Harry Biller, Swantje Lerch, Henning Schmitz, Yefan Song, Stefan Roitsch, Thomas Strassner, and Christoph Janiak. 2023. "Tunable Aryl Alkyl Ionic Liquid Supported Synthesis of Platinum Nanoparticles and Their Catalytic Activity in the Hydrogen Evolution Reaction and in Hydrosilylation" Molecules 28, no. 1: 405. https://doi.org/10.3390/molecules28010405
APA StyleWoitassek, D., Strothmann, T., Biller, H., Lerch, S., Schmitz, H., Song, Y., Roitsch, S., Strassner, T., & Janiak, C. (2023). Tunable Aryl Alkyl Ionic Liquid Supported Synthesis of Platinum Nanoparticles and Their Catalytic Activity in the Hydrogen Evolution Reaction and in Hydrosilylation. Molecules, 28(1), 405. https://doi.org/10.3390/molecules28010405