Structural Features of Monoethanolamine Aqueous Solutions with Various Compositions: A Combined Experimental and Theoretical Study Using Vibrational Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
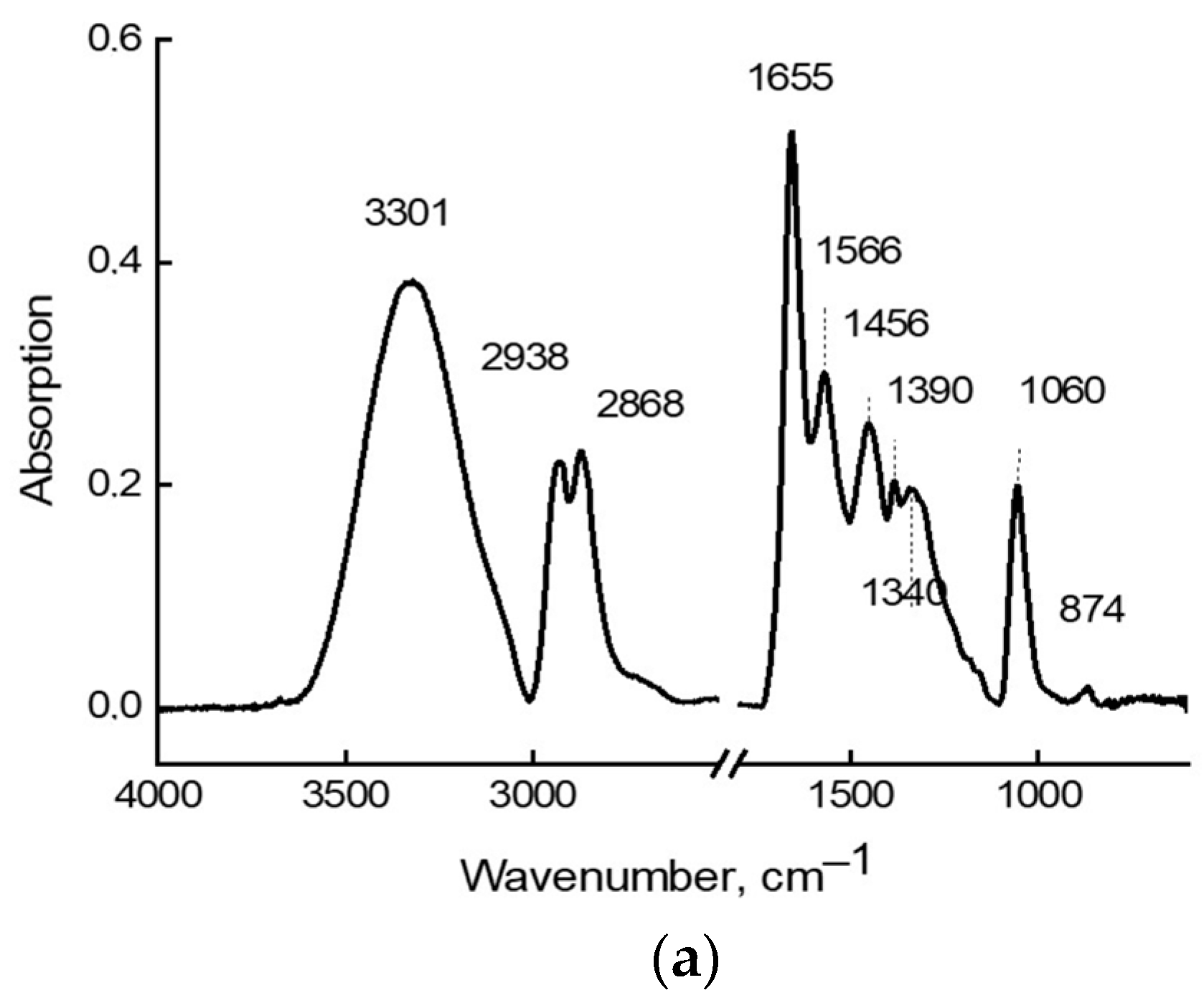
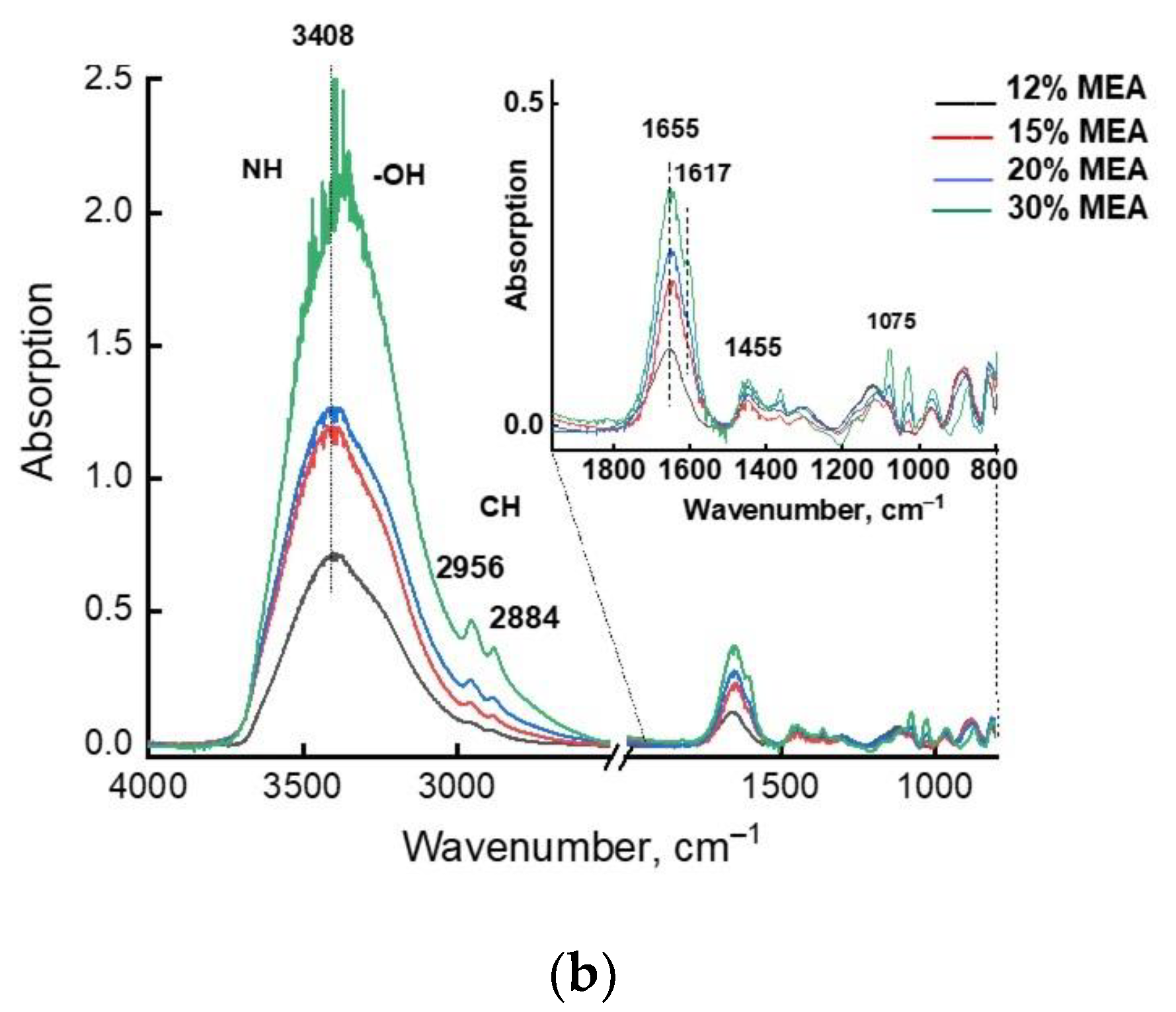
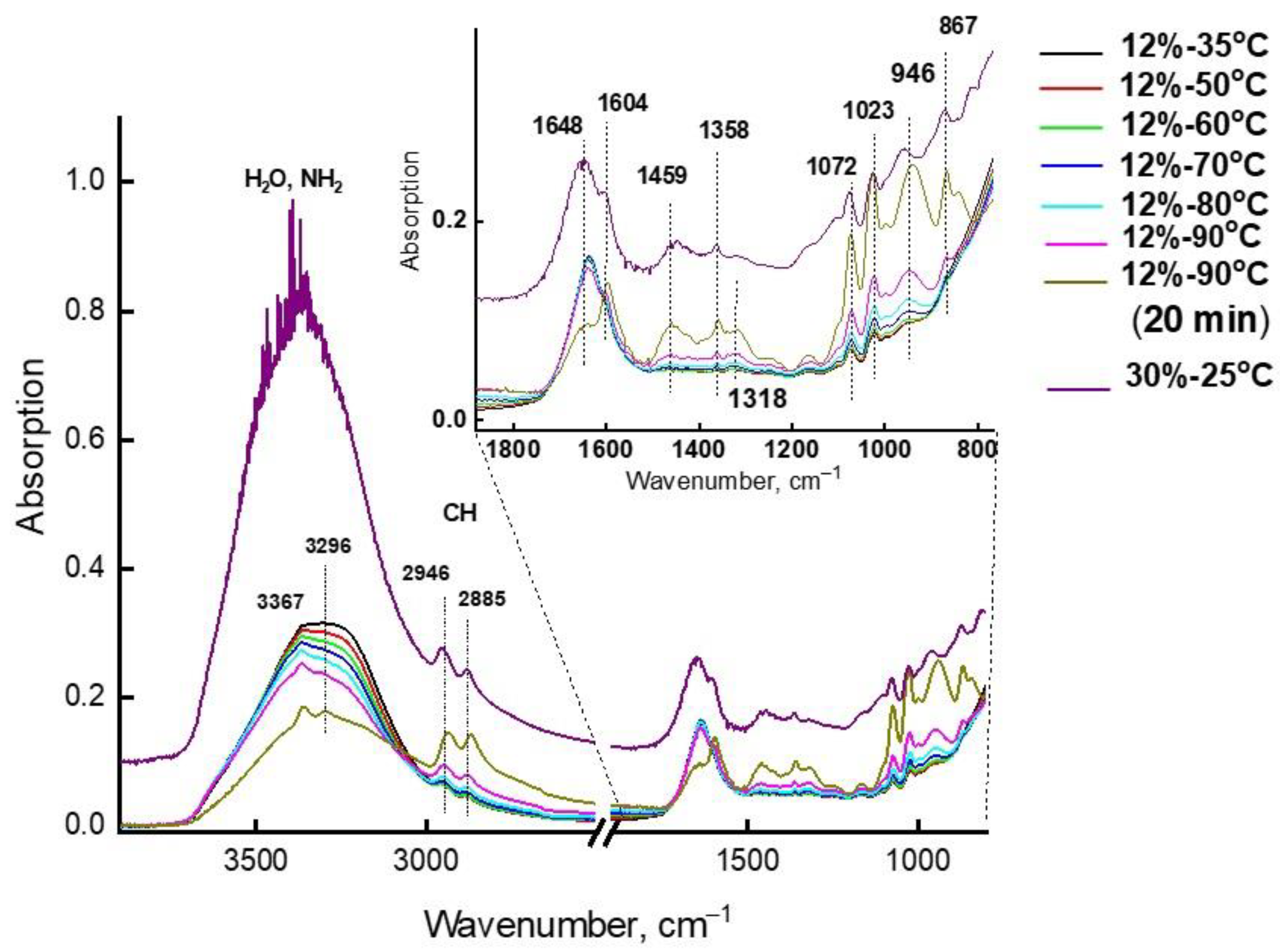
Structure and Vibrational Spectra of Aqueous Solutions of MEA
- MEA molecules with two hydrophilic groups have a high propensity to form associates with each other and with water molecules.
- In aqueous solutions of MEA with a concentration of 7 to 30%, a large number of associate complexes are realized through MEA-H2O hydrogen bonds. The stability and lifetime of these bonds depend on the composition of the associate and on the number and the length of hydrogen bonds.
- In aqueous solutions, where the concentration of which does not exceed 22%, primarily small associates are realized, in which MEA molecules are isolated from each other, due to their environment featuring energy-intensive associates from water molecules. In such solutions, both the absorption of carbon dioxide and its desorption can easily take place.
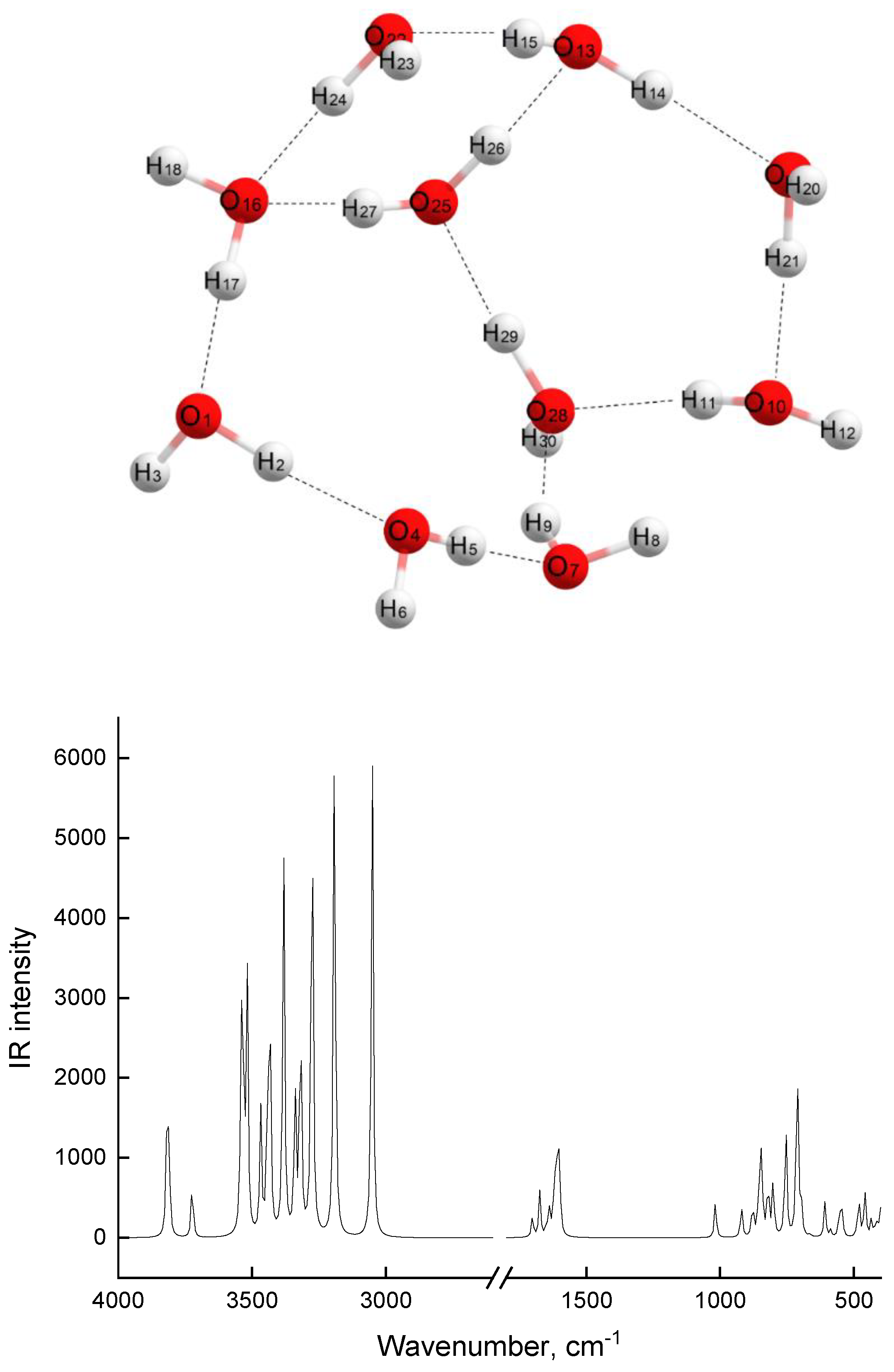
- In aqueous solutions of MEA with a concentration above 22%, the probability of formation of very energy-intensive (67.7 kcal/mol) complexes of MEA-10H2O associates increases, the structure of which makes it possible to retain CO2 more firmly, i.e., it does not interfere with the absorption of acid gases, but hinders their desorption.
- In IR spectra, the bands characterizing the energy-intensive associates of MEA-10H2O were identified (Table 3) and the conditions for the occurrence of such associates in aqueous solutions of MEA were established.
3. Materials and Methods
3.1. Materials and Characterization
3.2. FTIR-Spectroscopy, High-Temperature ATR-FTIR Spectroscopy
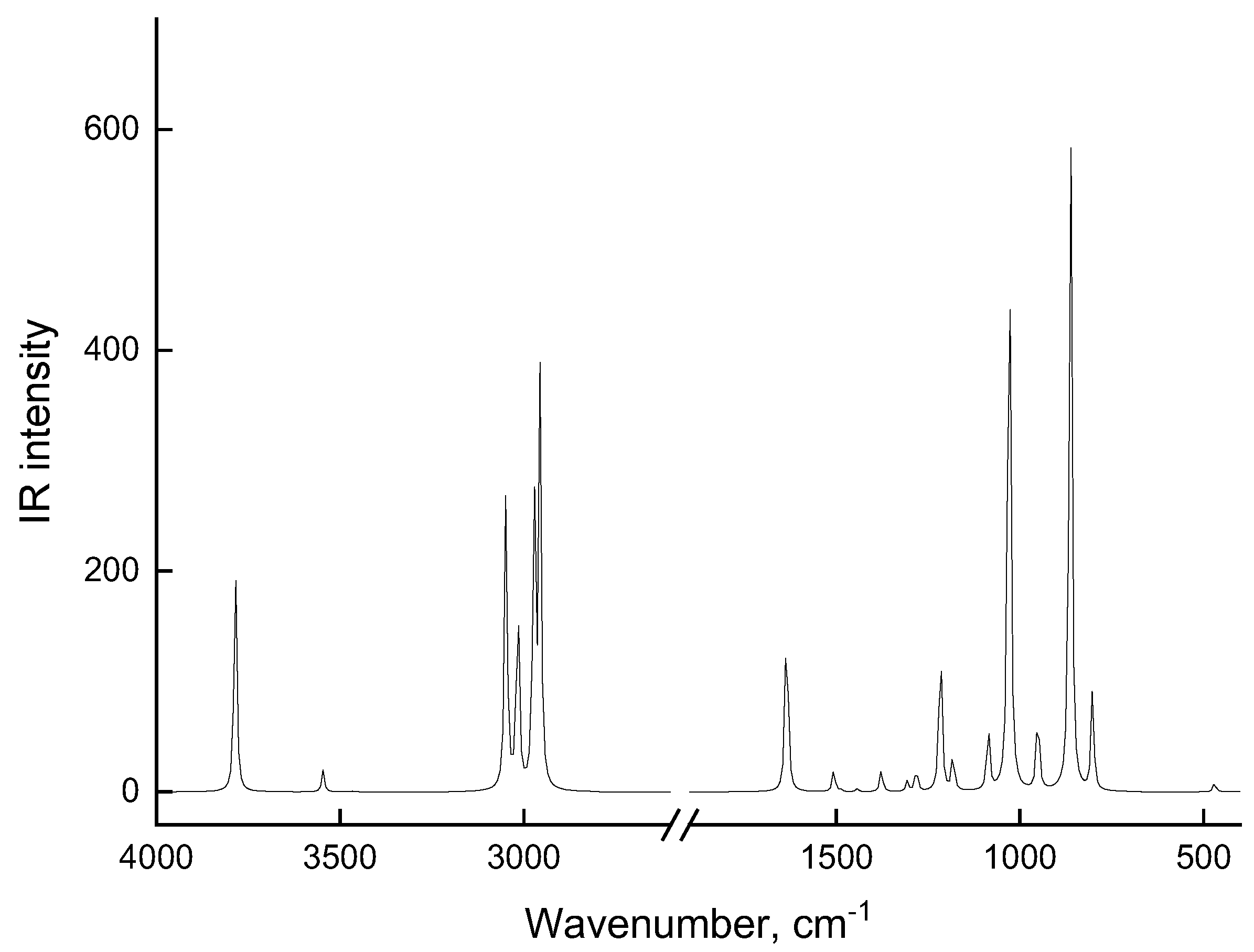
3.3. Quantum-Chemical Calculations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roger, B.R. Process for Separating Acidic Gases. U.S. Patent 1783901, 2 December 1930. [Google Scholar]
- Smith, G.D.; Tooley, N.; Cummings, A.L. Making amine systems sing, GPA Europe. In Proceedings of the Gas Processing Symposium, Barcelona, Spain, 13–15 May 2009. [Google Scholar]
- Talzi, V.P. Investigation of Product Composition Produced by Degradation of Monoethanolamines in Absorption Cleaning of Exhaust Gas. Russ. J. Appl. Chem. 2010, 83, 1139–1146. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Moser, P.; Schmidt, S.; Stahl, K. Investigation of Trace Elements in the Inlet and Outlet Streams of a MEA-Based Post-Combustion Capture Process Results from the Test Programme at the Niederaussem Pilot Plant. Energy Procedia 2011, 4, 473–479. [Google Scholar] [CrossRef]
- Wang, T.; Hovland, J.; Jens, K.J. Amine Reclaiming Technologies in Post-Combustion Carbon Dioxide Capture. J. Environ. Sci. 2015, 27, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Guo, B.; Zhou, Z.; Jing, G. Mechanisms of CO2 Capture into Monoethanolamine Solution with Different CO2 Loading during the Absorption/Desorption Processes. Environ. Sci. Technol. 2015, 49, 10728–10735. [Google Scholar] [CrossRef] [PubMed]
- Talzi, V.P.; Ignashin, S.V. NMR Study of Decomposition of Monoethanolamine under Conditions of Industrial Gas Treatment. Russ. J. Appl. Chem. 2002, 75, 80–85. [Google Scholar] [CrossRef]
- Talzi, V.P. NMR Determination of the Total Composition of Commercial Absorbents Based on Monoethanolamine. Russ. J. Appl. Chem. 2004, 77, 430–434. [Google Scholar] [CrossRef]
- Strazisar, B.R.; Anderson, R.R.; White, C.M. Degradation Pathways for Monoethanolamine in a CO2 Capture Facility. Energy Fuels 2003, 17, 1034–1039. [Google Scholar] [CrossRef]
- Bello, A.; Idem, R.O. Pathways for the Formation of Products of the Oxidative Degradation of CO2-Loaded Concentrated Aqueous Monoethanolamine Solutions during CO2 Absorption from Flue Gases. Ind. Eng. Chem. Res. 2005, 44, 945–969. [Google Scholar] [CrossRef]
- Chi, S.; Rochelle, G.T. Oxidative Degradation of Monoethanolamine. Ind. Eng. Chem. Res. 2002, 41, 4178–4186. [Google Scholar] [CrossRef]
- Jackson, P.; Robinson, K.; Puxty, G.; Attalla, M. In Situ Fourier Transform-Infrared (FT-IR) Analysis of Carbon Dioxide Absorption and Desorption in Amine Solutions. Energy Procedia 2009, 1, 985–994. [Google Scholar] [CrossRef]
- Vasilevskii, V.P.; Novitskii, E.G.; Volkov, V.V. Electro membrane regeneration of amine solutions of gas processing plants. Membrany 2010, 48, 26–28. [Google Scholar]
- Novitskii, E.G.; Vasilevskii, V.P.; Vasil’eva, V.I.; Goleva, E.A.; Grushevenko, E.A.; Volkov, A.V. Effect of Composition and Structure of Aqueous Monoethanolamine Solutions on Carbon Dioxide Sorption and Desorption in Purification of Gas Mixtures. Russ. J. Appl. Chem. 2018, 91, 813–821. [Google Scholar] [CrossRef]



| Associate Model | E∆, kcal/mol | Hydrogen Bond Lengths (Å) | |
|---|---|---|---|
| MEA ⋯ 2H2O | −9.11 | MEA⋯H2O | 1.816 (O1-H13) |
| H2O⋯H2O | 2.539 (O15-H11) | ||
| 3MEA⋯3H2O | −17.87 | MEA⋯H2O | 2007 (N4-H42) |
| 1.987 (O1-H13) | |||
| 1.952 (O29-H14) | |||
| 1.968 (O15-H41) | |||
| 1.878 (O26-H19) | |||
| H2O⋯H2O | 1.904 (O12-H28) | ||
| 3.092 (O26-H14) | |||
| MEA⋯MEA | 2.022 (O15-H33) | ||
| No. | Associate Model | E∆. kcal/mol | Bond Lengths (Å) | Charges on Atoms (e) | Angle H-N-Ho | Dip. Moment (D) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEA | H2O:O-H | HO⋯H | HN⋯H | MEA | H2O | ||||||||||
| O-H | N-H | N | HN | O | Ho | O | H | ||||||||
| 1 | MEA | - | 0.967 | 1.017 | - | - | - | 0.60 | +0.31 | −0.54 | +0.37 | - | - | 106.3 | 2.232 |
| 2 | H2O | - | - | - | 0.967 | - | - | - | - | - | - | - | +0.38 | - | 2.456 |
| 3 | 2 MEA | −3.73 | 0.950 | 1.00 | - | - | 2.090 | −0.78 | +0.31 | −0.68 | +0.36 | - | - | 106.9 | 0.516 |
| 4 | 3 MEA | −3.28 | 0.945 | 1.002 | - | - | 2.281 | −0.78 | +0.31 | −0.68 | +0.36 | - | - | 106.5 | 7.120 |
| 5 | 2H2O | −5.02 | - | - | 0.948 | 2.040 | - | - | - | - | - | −0.76 | +0.42 | - | 3.296 |
| 6 | MEA-H2O | −5.51 | 0.943 | 1.000 | 0.949 | 2.001 | - | −0.63 | +0.29 | −0.63 | +0.37 | −0.78 | +0.44 | 107.6 | 2.884 |
| 7 | MEA-2H2O | −9.11 | 0.968 | 1.020 | 0.983 | 1.815 | 2.359 | −0.61 | +0.33 | −0.57 | +0.40 | −0.84 | +0.45 | 106.9 | 3.589 |
| 8 | 3 MEA-3H2O | −17.87 | 0.955 | 1.002 | 0.957 | 1.878 | 2.007 | −0.77 | +0.30 | −0.72 | +0.38 | −0.75 | +0.41 | 106.2 | 3.650 |
| 9 | 10H2O | −59.70 | - | - | 0.995 | 1.645 | - | - | - | - | - | −1.06 | +0.66 | - | 7.660 |
| 10 | MEA-10H2O | −67.74 | 0.988 | 1.020 | 0.992 | 1.693 | 1.890 | −0.66 | +0.45 | −0.94 | +0.49 | −1.00 | +0.50 | 109.1 | 2.825 |
| No. | Theoretical Frequency, cm−1 | Intensity Con. UNITS | Infrared Band, CM−1 | Attribution |
|---|---|---|---|---|
| 1 * | 27 | 0.8 | - | δOH (H2O) |
| 2 | 32 | 4.9 | - | -“- |
| 3 | 44 | 1.1 | - | -“- |
| 4 | 47 | 3.0 | - | -“- |
| 5 | 52 | 3.9 | - | -“- |
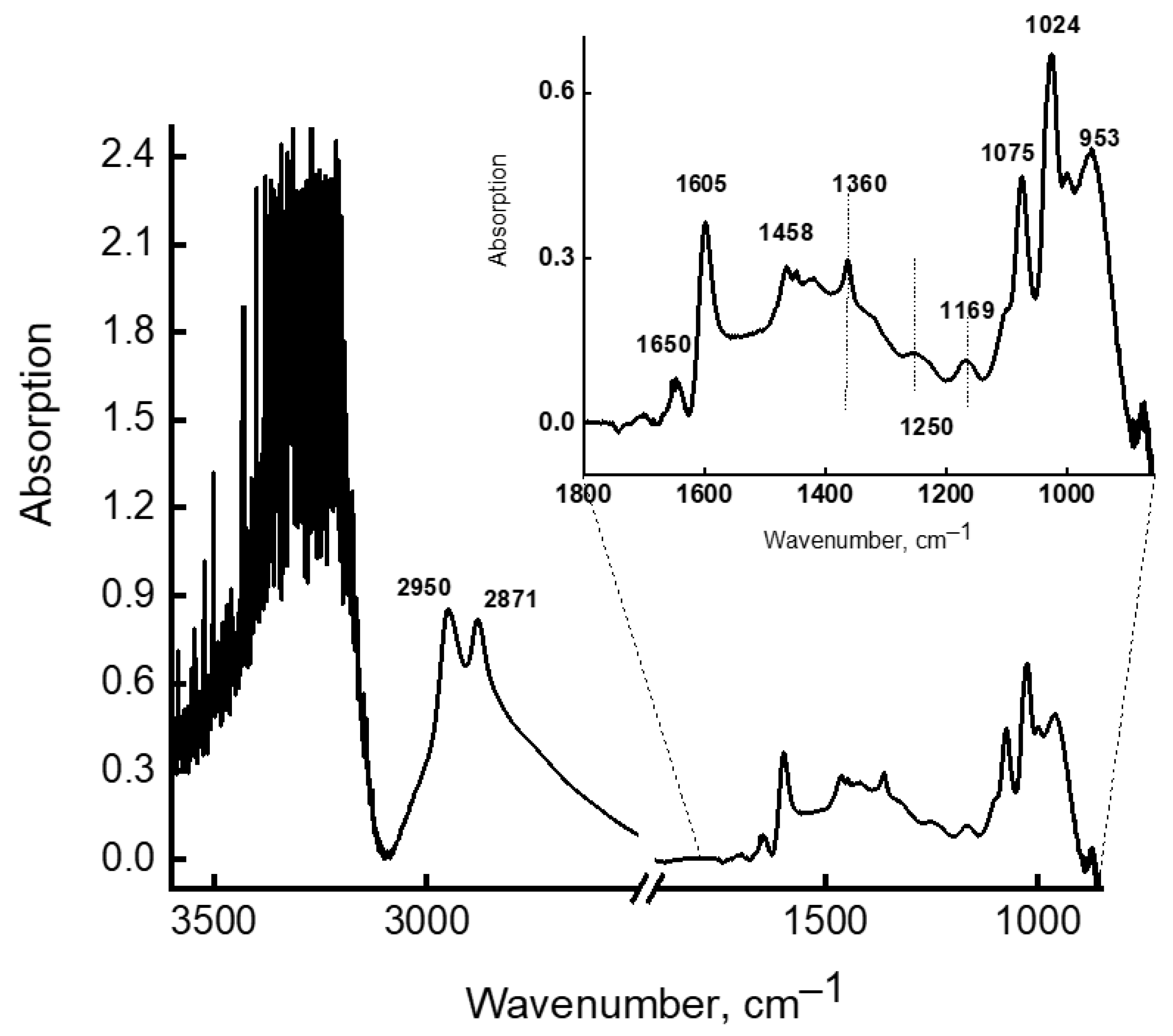
| 1 | 858 + 865 | 293 | 867 m * | H2O⋯H2O⋯H2O |
| 2 | 947 | 166 | 946 m | H2O⋯H2O⋯NH2 |
| 3 | 1027 | 230 | 1024 m | NH2⋯ H2O + OH⋯OH2 |
| 4 | 1080 | 110 | 1075 m | H2O ⋯H2O⋯H2O⋯NH2 |
| 5 | 1147 | 14 | 1169 m | δNH2 + OH⋯OH2 |
| 6 | 1268 | 56 | 1250 m | δNH2-CH2 |
| 7 | 1352 | 12 | 1358 m | δCH2 |
| 8 | 1456 | 112 | 1458 m | δCH2 |
| 9 | 1618 | 252 | 1605 m | H2O⋯H2O⋯H2O |
| 10 | 1653 | 78 | 1650 | NH2⋯H2O |
| Concentration (%) MEA | 7 | 12 | 15 | 20 | 30 |
|---|---|---|---|---|---|
| Number of H2O molecules per 1 MEA molecule | 45.0 | 24.8 | 19.2 | 13.6 | 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legkov, S.A.; Bondarenko, G.N.; Kostina, J.V.; Novitsky, E.G.; Bazhenov, S.D.; Volkov, A.V.; Volkov, V.V. Structural Features of Monoethanolamine Aqueous Solutions with Various Compositions: A Combined Experimental and Theoretical Study Using Vibrational Spectroscopy. Molecules 2023, 28, 403. https://doi.org/10.3390/molecules28010403
Legkov SA, Bondarenko GN, Kostina JV, Novitsky EG, Bazhenov SD, Volkov AV, Volkov VV. Structural Features of Monoethanolamine Aqueous Solutions with Various Compositions: A Combined Experimental and Theoretical Study Using Vibrational Spectroscopy. Molecules. 2023; 28(1):403. https://doi.org/10.3390/molecules28010403
Chicago/Turabian StyleLegkov, Sergey A., Galina N. Bondarenko, Julia V. Kostina, Eduard G. Novitsky, Stepan D. Bazhenov, Alexey V. Volkov, and Vladimir V. Volkov. 2023. "Structural Features of Monoethanolamine Aqueous Solutions with Various Compositions: A Combined Experimental and Theoretical Study Using Vibrational Spectroscopy" Molecules 28, no. 1: 403. https://doi.org/10.3390/molecules28010403
APA StyleLegkov, S. A., Bondarenko, G. N., Kostina, J. V., Novitsky, E. G., Bazhenov, S. D., Volkov, A. V., & Volkov, V. V. (2023). Structural Features of Monoethanolamine Aqueous Solutions with Various Compositions: A Combined Experimental and Theoretical Study Using Vibrational Spectroscopy. Molecules, 28(1), 403. https://doi.org/10.3390/molecules28010403









