Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review
Abstract
1. Introduction
2. Tyrosinase Inhibitors against Melanogenesis
2.1. Simple Phenolic Derivatives
2.2. Flavonoids
2.2.1. Flavones
2.2.2. Flavonoles
2.2.3. Isoflavones
2.2.4. Flavanones
3. Anthocyanidins and Curcuminoids
3.1. Coumarins
3.2. Chalcones and Dihydrochalcones
3.3. Stilbenes
4. Quinone and Phenyl Derivatives
5. Pyridine, Piperidine, Pyridinones, Hydroxypyridinone, Azole and Thiazolidine Derivatives
6. Kojic Acid Analogs and Carboxylic Acids Derivatives
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiu, P.; Zhang, M.; Wu, Y.; Liu, Y.; Wang, Y.; Zhang, J.; Song, J.; Feng, S.; Sun, Y.; Tan, L. Cloning and characterization of microphthalmia-associated transcription factor-like gene provide insights into Cyclina sinensis clam shell melanin deposition. Aquac. Res. 2022, 53, 1413–1423. [Google Scholar] [CrossRef]
- De Oliveira, C.; Franco-Belussi, L. Melanic pigmentation in ectothermic vertebrates: Occurrence and function. In Melanin: Biosynthesis, Functions and Health Effects; Ma, X.-P., Sun, X.-X., Eds.; Nova Biomedical: Waltham, MA, USA, 2012; pp. 213–225. [Google Scholar]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin pigment in plants: Current knowledge and future perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, H.; Hani, A.F.M.; Jolivot, R.; Marzani, F. Melanin type and concentration determination using inverse model. In Proceedings of the 2011 National Postgraduate Conference, Perak, Malaysia, 19–20 September 2011; pp. 1–7. [Google Scholar]
- Kishida, R.; Meñez Aspera, S.; Kasai, H. Melanin Chemistry. In Melanin Chemistry Explored by Quantum Mechanics; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–31. [Google Scholar]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, melanin, and melanogenesis: The Yin and Yang relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chai, W.-M.; Yang, Q.; Wei, M.-K.; Peng, Y. 2-(4-Fluorophenyl)-quinazolin-4 (3H)-one as a novel tyrosinase inhibitor: Synthesis, inhibitory activity, and mechanism. Bioorganic Med. Chem. 2016, 24, 4620–4625. [Google Scholar] [CrossRef] [PubMed]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin transfer in the epidermis: The pursuit of skin pigmentation control mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Chemistry of melanins. In The Pigmentary System: Physiology and Pathophysiology, 2nd ed.; Nordlund, J.J., Boissy, R.E., Hearing, V.J., King, R.A., Oetting, W.S., Ortonne, J.-P., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2006; pp. 282–310. [Google Scholar]
- Maymone, M.B.; Neamah, H.H.; Wirya, S.A.; Patzelt, N.M.; Secemsky, E.A.; Zancanaro, P.Q.; Vashi, N.A. The impact of skin hyperpigmentation and hyperchromia on quality of life: A cross-sectional study. J. Am. Acad. Dermatol. 2017, 77, 775–778. [Google Scholar] [CrossRef]
- Raper, H. The aerobic oxidases. Physiol. Rev. 1928, 8, 245–282. [Google Scholar] [CrossRef]
- Chen, Q.-X.; Kubo, I. Kinetics of mushroom tyrosinase inhibition by quercetin. J. Agric. Food Chem. 2002, 50, 4108–4112. [Google Scholar] [CrossRef]
- Roulier, B.; Pérès, B.; Haudecoeur, R. Advances in the design of genuine human tyrosinase inhibitors for targeting melanogenesis and related pigmentations. J. Med. Chem. 2020, 63, 13428–13443. [Google Scholar] [CrossRef]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- TH Khan, M. Novel tyrosinase inhibitors from natural resources–their computational studies. Curr. Med. Chem. 2012, 19, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Bao, K.; Dai, Y.; Zhu, Z.-B.; Tu, F.-J.; Zhang, W.-G.; Yao, X.-S. Design and synthesis of biphenyl derivatives as mushroom tyrosinase inhibitors. Bioorganic Med. Chem. 2010, 18, 6708–6714. [Google Scholar] [CrossRef] [PubMed]
- Zachary, C.M.; Wang, J.V.; Saedi, N. Kojic Acid for Melasma: Popular Ingredient in Skincare Products. Skinmed 2020, 18, 271–273. [Google Scholar] [PubMed]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef]
- O’Donoghue, J. Hydroquinone and its analogues in dermatology—A risk-benefit viewpoint. J. Cosmet. Dermatol. 2006, 5, 196–203. [Google Scholar] [CrossRef]
- Maeda, K.; Fukuda, M. Arbutin: Mechanism of its depigmenting action in human melanocyte culture. J. Pharmacol. Exp. Ther. 1996, 276, 765–769. [Google Scholar]
- Gaskell, M.; McLuckie, K.I.; Farmer, P.B. Genotoxicity of the benzene metabolites para-benzoquinone and hydroquinone. Chem. -Biol. Interact. 2005, 153, 267–270. [Google Scholar] [CrossRef]
- Ogiwara, Y.; Sugiura, M.; Watanabe, K.; Tawara, J.; Endo, E.; Maruyama, H.; Tsuji, S.; Matsue, K.; Yamada, H.; Wako, Y. Evaluation of the repeated-dose liver, bone marrow and peripheral blood micronucleus and comet assays using kojic acid. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2015, 780, 111–116. [Google Scholar] [CrossRef]
- Chang, T.-S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012, 5, 1661–1685. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef]
- Slominski, A.; Paus, R. Towards defining receptors for L-tyrosine and L-dopa. Mol. Cell. Endocrinol. 1994, 99, C7–C11. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Paus, R. Are L-tyrosine and L-dopa hormone-like bioregulators? J. Theor. Biol. 1990, 143, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Moellmann, G.; Kuklinska, E.; Bomirski, A.; Pawelek, J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. J. Cell. Sci. 1988, 89, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Abbas, Q.; Ashraf, Z.; Moustafa, A.A.; Seo, S.-Y. Pharmacoinformatics exploration of polyphenol oxidases leading to novel inhibitors by virtual screening and molecular dynamic simulation study. Comput. Biol. Chem. 2017, 68, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Ozawa, K.; Bonnet-Duquennoy, M.; Bonté, F. Hormone influence on melanogenesis and spots formation. Int. J. Cosmet. Sci. 2005, 27, 56–59. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Handel, A.C.; Miot, L.D.B.; Miot, H.A. Melasma: A clinical and epidemiological review. An. Bras. Dermatol. 2014, 89, 771–782. [Google Scholar] [CrossRef]
- Chen, N.; Hu, Y.; Li, W.H.; Eisinger, M.; Seiberg, M.; Lin, C.B. The role of keratinocyte growth factor in melanogenesis: A possible mechanism for the initiation of solar lentigines. Exp. Dermatol. 2010, 19, 865–872. [Google Scholar] [CrossRef]
- Davis, E.C.; Callender, V.D. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J. Clin. Aesthet. Dermatol. 2010, 3, 20. [Google Scholar] [PubMed]
- Nieman, L.K.; Turner, M.L.C. Addison’s disease. Clin. Dermatol. 2006, 24, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, A.; Felsten, L.M.; Daly, M.; Petronic-Rosic, V. Vitiligo: A comprehensive overview: Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J. Am. Acad. Dermatol. 2011, 65, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hearing, V.J. Melanocytes and their diseases. Cold Spring Harb. Perspect. Med. 2014, 4, a017046. [Google Scholar] [CrossRef] [PubMed]
- Kleszczynski, K.; Fischer, T.W. Melatonin and human skin aging. Dermato-Endocrinology 2012, 4, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A cutaneous perspective on its production, metabolism, and functions. J. Invest. Dermatol. 2018, 138, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. Plant Second. Metab. Occur. Struct. Role Hum. Diet 2006, 1, 1–25. [Google Scholar]
- Sakuma, K.; Ogawa, M.; Sugibayashi, K.; Yamada, K.-i.; Yamamoto, K. Relationship between tyrosinase inhibitory action and oxidation-reduction potential of cosmetic whitening ingredients and phenol derivatives. Arch. Pharmacal Res. 1999, 22, 335–339. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Chiou, R.Y.-Y.; Lin, T.-Y.; Huang, C.-P.; Tang, W.-C.; Chen, S.-T.; Lin, S.-B. Identification of an Alkylhydroquinone from Rhus succedanea as an Inhibitor of Tyrosinase and Melanogenesis. J. Agric. Food Chem. 2009, 57, 2200–2205. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamano, Y.; Sugimoto, S.; Otsuka, H.; Matsunami, K.; Shinzato, T. Phenolic compounds from the leaves of Breynia officinalis and their tyrosinase and melanogenesis inhibitory activities. J. Nat. Med. 2018, 72, 381–389. [Google Scholar] [CrossRef]
- Chawla, S.; DeLong, M.; Visscher, M.; Wickett, R.; Manga, P.; Boissy, R. Mechanism of tyrosinase inhibition by deoxyarbutin and its second-generation derivatives. Br. J. Dermatol. 2008, 159, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Kvalnes, K.; Wickett, R.; Manga, P.; Boissy, R. DeoxyArbutin and its derivatives inhibit tyrosinase activity and melanin synthesis without inducing reactive oxygen species or apoptosis. J. Drugs Dermatol. JDD 2012, 11, e28–e34. [Google Scholar] [PubMed]
- Matsumoto, T.; Nakajima, T.; Iwadate, T.; Nihei, K.-I. Chemical synthesis and tyrosinase-inhibitory activity of isotachioside and its related glycosides. Carbohydr. Res. 2018, 465, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Choi, H.-J.; Park, T.; Lee, M.-J.; Lim, H.-S.; Yang, W.-S.; Hwang, C.-W.; Park, D.; Kim, C.-H. Inhibitory effect of avenanthramides (Avn) on tyrosinase activity and melanogenesis in α-MSH-activated SK-MEL-2 cells: In vitro and in silico analysis. Int. J. Mol. Sci. 2021, 22, 7814. [Google Scholar] [CrossRef] [PubMed]
- Ishioka, W.; Oonuki, S.; Iwadate, T.; Nihei, K.-I. Resorcinol alkyl glucosides as potent tyrosinase inhibitors. Bioorganic Med. Chem. Lett. 2019, 29, 313–316. [Google Scholar] [CrossRef]
- Zhou, W.; Tang, J.; Zhou, X.; Liu, J. Tyrosinase Inhibition by Novel Benzimidazole-thione Schiff Base Derivatives. Lett. Drug Des. Discov. 2022, 19, 782–790. [Google Scholar]
- Laksmiani, N.P.L.; Widiantara, I.W.A.; Pawarrangan, A.B.S. Potency of moringa (Moringa oleifera L.) leaves extract containing quercetin as a depigmentation agent inhibiting the tyrosinase enzyme using in-silico and in-vitro assay. Pharmacia 2022, 69, 85–92. [Google Scholar] [CrossRef]
- Deri, B.; Kanteev, M.; Goldfeder, M.; Lecina, D.; Guallar, V.; Adir, N.; Fishman, A. The unravelling of the complex pattern of tyrosinase inhibition. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Alqahtani, Y.S.; Alyami, B.A.; Alqarni, A.O.; Mahnashi, M.H.; Ali, A.; Javed, Q.; Hassan, M.; Ehsan, M. Cephalosporin as Potent Urease and Tyrosinase Inhibitor: Exploration through Enzyme Inhibition, Kinetic Mechanism, and Molecular Docking Studies. BioMed Res. Int. 2022, 2022, 1092761. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Erdogan Orhan, I.; Tareq Hassan Khan, M. Flavonoid derivatives as potent tyrosinase inhibitors–a survey of recent findings between 2008–2013. Curr. Top. Med. Chem. 2014, 14, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M. Structure and inhibition mechanism of some synthetic compounds and phenolic derivatives as tyrosinase inhibitors: Review and new insight. J. Biomol. Struct. Dyn. 2022, 1–13, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jakimiuk, K.; Sari, S.; Milewski, R.; Supuran, C.T.; Şöhretoğlu, D.; Tomczyk, M. Flavonoids as tyrosinase inhibitors in in silico and in vitro models: Basic framework of SAR using a statistical modelling approach. J. Enzym. Inhib. Med. Chem. 2022, 37, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Promden, W.; Viriyabancha, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Correlation between the potency of flavonoids on mushroom tyrosinase inhibitory activity and melanin synthesis in melanocytes. Molecules 2018, 23, 1403. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. CMLS 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Wairkar, S. Topical nanocrystals of bioflavonoids: A new technology platform for skin ailments. Int. J. Pharm. 2022, 619, 121707. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Nguyen, M.H.K.; Nguyen, H.X.; Nguyen, M.T.T.; Nguyen, N.T. Phenolic constituents from the heartwood of Artocapus altilis and their tyrosinase inhibitory activity. Nat. Prod. Commun. 2012, 7, 1934578X1200700214. [Google Scholar] [CrossRef]
- Shang, C.; Zhang, Y.; You, X.; Guo, N.; Wang, Y.; Fan, Y.; Liu, W. The effect of 7, 8, 4-trihydroxyflavone on tyrosinase activity and conformation: Spectroscopy and docking studies. Luminescence 2018, 33, 681–691. [Google Scholar] [CrossRef]
- Abbas, Q.; Ashraf, Z.; Hassan, M.; Nadeem, H.; Latif, M.; Afzal, S.; Seo, S.-Y. Development of highly potent melanogenesis inhibitor by in vitro, in vivo and computational studies. Drug Des. Dev. Ther. 2017, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Cai, Y.-Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and synthetic flavonoid derivatives as new potential tyrosinase inhibitors: A systematic review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, G.; Yan, J.; Gong, D. Inhibitory effect of morin on tyrosinase: Insights from spectroscopic and molecular docking studies. Food Chem. 2014, 163, 226–233. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Hori, I. Flavonols from saffron flower: Tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 1999, 47, 4121–4125. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Hintz, K.K.; Ren, J. Phytoestrogenic isoflavones daidzein and genistein reduce glucose-toxicity-induced cardiac contractile dysfunction in ventricular myocytes. Endocr. Res. 2004, 30, 215–223. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, D.H.; Lee, J.K.; Lee, J.Y.; Kim, D.H.; Kim, H.K.; Lee, H.-J.; Kim, H.C. Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. Bioorganic Med. Chem. Lett. 2010, 20, 1162–1164. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 111–117. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Zanatta, L.; Alberton, E.H.; Bonorino Figueiredo, M.S.R.; Folador, P.; Damazio, R.G.; Pizzolatti, M.G.; Barreto Silva, F.R.M. Flavonoids: Prospective drug candidates. Mini Rev. Med. Chem. 2008, 8, 1429–1440. [Google Scholar] [CrossRef]
- Jaime, A.; Remsberg, C.M.; Takemoto, J.K.; Vega-Villa, K.R.; Andrews, P.K.; Sayre, C.L.; Martinez, S.E.; Davies, N.M. Polyphenols and flavonoids: An overview. In Flavonoid Pharmacokinetics: Methods of Analysis, Preclinical and Clinical Pharmacokinetics, Safety, and Toxicology; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Si, Y.-X.; Wang, Z.-J.; Park, D.; Chung, H.Y.; Wang, S.-F.; Yan, L.; Yang, J.-M.; Qian, G.-Y.; Yin, S.-J.; Park, Y.-D. Effect of hesperetin on tyrosinase: Inhibition kinetics integrated computational simulation study. Int. J. Biol. Macromol. 2012, 50, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.R.S.; Abbasi, M.A.; Siddiqui, S.Z.; Raza, H.; Hassan, M.; Shah, S.A.A.; Seo, S.-Y. Synthesis, Kinetics, Binding Conformations and Structure-activity Relationship of Potent Tyrosinase Inhibitors: Aralkylated 2-aminothiazole-ethyltriazole Hybrids. Iran. J. Pharm. Res. IJPR 2021, 20, 206. [Google Scholar]
- Abbas, Q.; Raza, H.; Hassan, M.; Phull, A.R.; Kim, S.J.; Seo, S.Y. Acetazolamide inhibits the level of tyrosinase and melanin: An enzyme kinetic, in vitro, in vivo, and in silico studies. Chem. Biodivers. 2017, 14, e1700117. [Google Scholar] [CrossRef]
- Jiang, Y.; Du, Z.; Xue, G.; Chen, Q.; Lu, Y.; Zheng, X.; Conney, A.H.; Zhang, K. Synthesis and biological evaluation of unsymmetrical curcumin analogues as tyrosinase inhibitors. Molecules 2013, 18, 3948–3961. [Google Scholar] [CrossRef] [PubMed]
- Jhan, J.K.; Chung, Y.C.; Chen, G.H.; Chang, C.H.; Lu, Y.C.; Hsu, C.K. Anthocyanin contents in the seed coat of black soya bean and their anti-human tyrosinase activity and antioxidative activity. Int. J. Cosmet. Sci. 2016, 38, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, L.; Sawant, P.; AshaThomas, R.W.; Bhosale, K. Understanding the Molecular Mechanism of Phytoconstituents as Tyrosinase Inhibitors for Treatment of Hyperpigmentation. Saudi J. Med. Pharm. Sci. 2021, 7, 135–144. [Google Scholar] [CrossRef]
- Athipornchai, A.; Niyomtham, N.; Pabuprapap, W.; Ajavakom, V.; Duca, M.; Azoulay, S.; Suksamrarn, A. Potent tyrosinase inhibitory activity of curcuminoid analogues and inhibition kinetics studies. Cosmetics 2021, 8, 35. [Google Scholar] [CrossRef]
- Hassan, M.; Ashraf, Z.; Abbas, Q.; Raza, H.; Seo, S.-Y. Exploration of novel human tyrosinase inhibitors by molecular modeling, docking and simulation studies. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 68–80. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Seo, S.-Y.; Babar, M.M.; Zaidi, N.-U.-S.S. Design, synthesis and bioevaluation of novel umbelliferone analogues as potential mushroom tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 30, 874–883. [Google Scholar] [CrossRef]
- Tocco, G.; Fais, A.; Meli, G.; Begala, M.; Podda, G.; Fadda, M.B.; Corda, M.; Attanasi, O.A.; Filippone, P.; Berretta, S. PEG-immobilization of cardol and soluble polymer-supported synthesis of some cardol–coumarin derivatives: Preliminary evaluation of their inhibitory activity on mushroom tyrosinase. Bioorganic Med. Chem. Lett. 2009, 19, 36–39. [Google Scholar] [CrossRef]
- Fais, A.; Corda, M.; Era, B.; Fadda, M.B.; Matos, M.J.; Quezada, E.; Santana, L.; Picciau, C.; Podda, G.; Delogu, G. Tyrosinase inhibitor activity of coumarin-resveratrol hybrids. Molecules 2009, 14, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Suthar, S.K.; Bansal, S.; Narkhede, N.; Guleria, M.; Alex, A.T.; Joseph, A. Design, synthesis and biological evaluation of oxindole-based chalcones as small-molecule inhibitors of melanogenic tyrosinase. Chem. Pharm. Bull. 2017, 65, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Park, K.C.; Park, J.H.; Lee, C.G.; Ye, S.-K.; Park, J.Y. Inhibition of tyrosinase activity and melanin production by the chalcone derivative 1-(2-cyclohexylmethoxy-6-hydroxy-phenyl)-3-(4-hydroxymethyl-phenyl)-propenone. Biochem. Biophys. Res. Commun. 2016, 480, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Yin, L.; Nie, L.F.; Dou, J.; Zhao, J.Y.; Li, G.; Aisa, H.A. Synthesis and bioactivity of novel isoxazole chalcone derivatives on tyrosinase and melanin synthesis in murine B16 cells for the treatment of vitiligo. Bioorganic Med. Chem. 2016, 24, 5440–5448. [Google Scholar] [CrossRef] [PubMed]
- Nerya, O.; Musa, R.; Khatib, S.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The effect of hydroxyl positions and numbers. Phytochemistry 2004, 65, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Z.; Chen, K.; Chen, Y.-L.; Zhang, C.; Xie, Y.-Y.; Hider, R.C.; Zhou, T. Design and synthesis of novel stilbene-hydroxypyridinone hybrids as tyrosinase inhibitors and their application in the anti-browning of freshly-cut apples. Food Chem. 2022, 385, 132730. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chen, K.; Zhu, Y.Z.; Zhang, C.J.; Chen, Y.L.; Wang, F.; Xie, Y.Y.; Hider, R.C.; Zhou, T. Antioxidant and anti-tyrosinase activity of a novel stilbene analogue as an anti-browning agent. J. Sci. Food Agric. 2022, 102, 3817–3825. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, B.; Xing, S.; Chen, Y.; Liao, Q.; Mo, J.; Chen, Y.; Li, Q.; Sun, H. Medicinal Prospects of Targeting Tyrosinase: A Feature Review. Curr. Med. Chem. 2022. Online ahead of print. [Google Scholar]
- Gunia-Krzyżak, A.; Popiol, J.; Marona, H. Melanogenesis inhibitors: Strategies for searching for and evaluation of active compounds. Curr. Med. Chem. 2016, 23, 3548–3574. [Google Scholar] [CrossRef]
- Ortiz-Ruiz, C.V.; Ballesta de los Santos, M.; Berna, J.; Fenoll, J.; Garcia-Ruiz, P.A.; Tudela, J.; Garcia-Canovas, F. Kinetic characterization of oxyresveratrol as a tyrosinase substrate. Iubmb Life 2015, 67, 828–836. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Medicinal importance, pharmacological activities, and analytical aspects of aloin: A concise report. J. Acute Dis. 2013, 2, 262–269. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Hu, G.; Zhang, Q.; Yang, Y.-X.; Li, Q.-Q.; Hu, Y.-J.; Chen, H.; Yang, F.-Q. Screening and characterizing tyrosinase inhibitors from Salvia miltiorrhiza and Carthamus tinctorius by spectrum-effect relationship analysis and molecular docking. J. Anal. Methods Chem. 2018, 2018, 2141389. [Google Scholar] [CrossRef] [PubMed]
- Borojerdi, S.S.; Haghbeen, K.; Karkhane, A.A.; Fazli, M.; Saboury, A.A. Successful resonance Raman study of cresolase activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 2004, 314, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.-L.; Wang, X.-L.; Chen, K.; Dong, X.-W.; Kong, L.-M.; Zhao, D.-Y.; Hider, R.C.; Zhou, T. Novel hydroxypyridinone derivatives containing an oxime ether moiety: Synthesis, inhibition on mushroom tyrosinase and application in anti-browning of fresh-cut apples. Food Chem. 2018, 242, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-F.; Hu, P.-P.; Liu, M.-S.; Kong, X.-L.; Zhang, J.-C.; Hider, R.C.; Zhou, T. Design and synthesis of hydroxypyridinone-L-phenylalanine conjugates as potential tyrosinase inhibitors. J. Agric. Food Chem. 2013, 61, 6597–6603. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Adhikari, I.; Nandy, A.; Saha, A.; Goswami, B.B. In silico modelling of azole derivatives with tyrosinase inhibition ability: Application of the models for activity prediction of new compounds. Comput. Biol. Chem. 2018, 74, 105–114. [Google Scholar] [CrossRef]
- Mahajan, P.G.; Dige, N.C.; Vanjare, B.D.; Raza, H.; Hassan, M.; Seo, S.-Y.; Kim, C.-H.; Lee, K.H. Facile synthesis of new quinazolinone benzamides as potent tyrosinase inhibitors: Comparative spectroscopic and molecular docking studies. J. Mol. Struct. 2019, 1198, 126915. [Google Scholar] [CrossRef]
- Chekir, S.; Debbabi, M.; Regazzetti, A.; Dargère, D.; Laprévote, O.; Jannet, H.B.; Gharbi, R. Design, synthesis and biological evaluation of novel 1, 2, 3-triazole linked coumarinopyrazole conjugates as potent anticholinesterase, anti-5-lipoxygenase, anti-tyrosinase and anti-cancer agents. Bioorganic Chem. 2018, 80, 189–194. [Google Scholar] [CrossRef]
- Hassan, M.; Vanjare, B.D.; Sim, K.-Y.; Raza, H.; Lee, K.H.; Shahzadi, S.; Kloczkowski, A. Biological and Cheminformatics Studies of Newly Designed Triazole Based Derivatives as Potent Inhibitors against Mushroom Tyrosinase. Molecules 2022, 27, 1731. [Google Scholar] [CrossRef]
- Qamar, R.; Saeed, A.; Larik, F.A.; Abbas, Q.; Hassan, M.; Raza, H.; Seo, S.Y. Novel 1, 3-oxazine-tetrazole hybrids as mushroom tyrosinase inhibitors and free radical scavengers: Synthesis, kinetic mechanism, and molecular docking studies. Chem. Biol. Drug Des. 2019, 93, 123–131. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS ONE 2017, 12, e0178069. [Google Scholar] [CrossRef] [PubMed]
- Ujan, R.; Saeed, A.; Ashraf, S.; Channar, P.A.; Abbas, Q.; Rind, M.A.; Hassan, M.; Raza, H.; Seo, S.-Y.; El-Seedi, H.R. Synthesis, computational studies and enzyme inhibitory kinetics of benzothiazole-linked thioureas as mushroom tyrosinase inhibitors. J. Biomol. Struct. Dyn. 2021, 39, 7035–7043. [Google Scholar] [CrossRef] [PubMed]
- Raza, H.; Abbasi, M.A.; Rehman, A.-u.; Siddiqui, S.Z.; Hassan, M.; Shah, S.A.A.; Shahid, M.; Hong, H.; Seo, S.-Y. Design, synthesis and computational studies of N-(substituted-phenyl)-4-(4-phenyl-1-piperazinyl) butanamides as potent anti-melanogenic and tyrosinase inhibitors. J. Mol. Struct. 2020, 1210, 127969. [Google Scholar] [CrossRef]
- Vanjare, B.D.; Choi, N.G.; Mahajan, P.G.; Raza, H.; Hassan, M.; Han, Y.; Yu, S.-M.; Kim, S.J.; Seo, S.-Y.; Lee, K.H. Novel 1, 3, 4-oxadiazole compounds inhibit the tyrosinase and melanin level: Synthesis, in-vitro, and in-silico studies. Bioorganic Med. Chem. 2021, 41, 116222. [Google Scholar] [CrossRef]
- Saeed, A.; Ejaz, S.A.; Khalid, A.; Channar, P.A.; Aziz, M.; Abbas, Q.; Wani, T.A.; Alsaif, N.A.; Alanazi, M.M.; Al-Hossaini, A.M. Acetophenone-Based 3, 4-Dihydropyrimidine-2 (1H)-Thione as Potential Inhibitor of Tyrosinase and Ribonucleotide Reductase: Facile Synthesis, Crystal Structure, In-Vitro and In-Silico Investigations. Int. J. Mol. Sci. 2022, 23, 13164. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, H.; He, J.; Zhang, J.; Yu, Q.; Luo, C.; Li, S. Synthesis and biological evaluation of novel hydroxybenzaldehyde-based kojic acid analogues as inhibitors of mushroom tyrosinase. Bioorganic Med. Chem. Lett. 2017, 27, 530–532. [Google Scholar] [CrossRef]
- Gheibi, N.; Saboury, A.; Haghbeen, K.; Rajaei, F.; Pahlevan, A. Dual effects of aliphatic carboxylic acids on cresolase and catecholase reactions of mushroom tyrosinase. J. Enzym. Inhib. Med. Chem. 2009, 24, 1076–1081. [Google Scholar] [CrossRef]
- Yu, L. Inhibitory effects of (S)-and (R)-6-hydroxy-2, 5, 7, 8-tetramethylchroman-2-carboxylic acids on tyrosinase activity. J. Agric. Food Chem. 2003, 51, 2344–2347. [Google Scholar] [CrossRef]
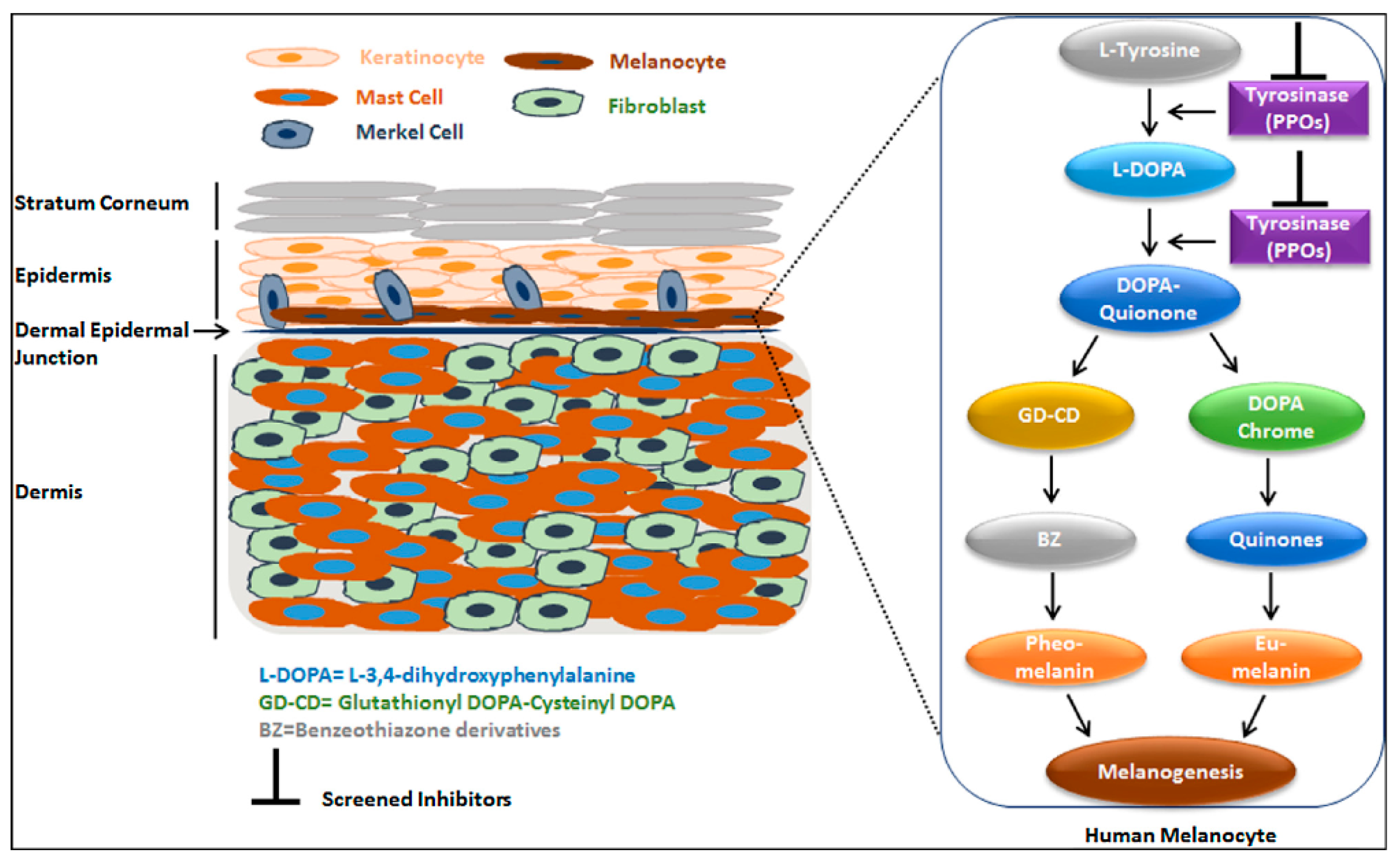

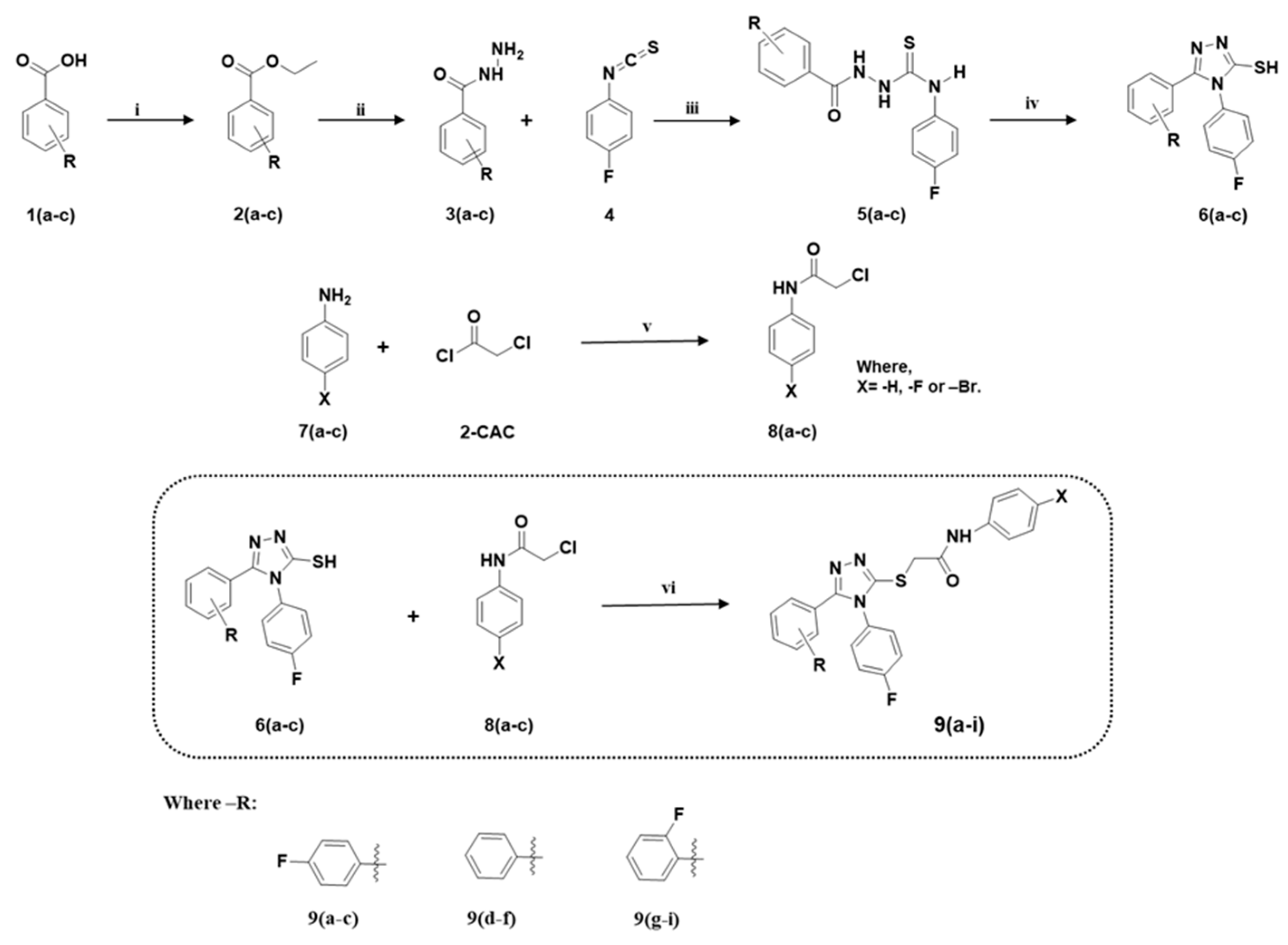
| Compounds | Tyrosinase Activity IC50 ± SEM (µM) |
|---|---|
| 2-(4,5-bis(4-fluorophenyl)-4H-1,2,4-triazol-3-ylthio)-N-phenylacetamide | 0.124 ± 0.077 |
| 2-(4,5-bis(4-fluorophenyl)-4H-1,2,4-triazol-3-ylthio)-N-(4-fluorophenyl) acetamide | no data |
| 2-(4,5-bis(4-fluorophenyl)-4H-1,2,4-triazol-3-ylthio)-N-(4-bromophenyl) acetamide | no data |
| 2-(4-(4-fluorophenyl)-5-phenyl-4H-1,2,4-triazol-3-ylthio)-N-phenylacetamide | 0.219 ± 0.081 |
| N-(4-fluorophenyl)-2-(4-(4-fluorophenyl)-5-phenyl-4H-1,2,4-triazol-3-ylthio) acetamide | 0.379 ± 0.193 |
| N-(4-bromophenyl)-2-(4-(4-fluorophenyl)-5-phenyl-4H-1,2,4-triazol-3-ylthio) acetamide | 0.142 ± 0.068 |
| 2-(5-(2-fluorophenyl)-4-(4-fluorophenyl)-4H-1,2,4-triazol-3-ylthio)-N-phenylacetamide | 0.111 ± 0.021 |
| N-(4-fluorophenyl)-2-(5-(2-fluorophenyl)-4-(4-fluorophenyl)-4H-1,2,4-triazol-3-ylthio) Acetamide | 0.098 ± 0.009 |
| N-(4-bromophenyl)-2-(5-(2-fluorophenyl)-4-(4-fluorophenyl)-4H-1,2,4-triazol-3-ylthio) acetamide | no data |
| Kojic acid | 16.832 ± 1.161 |
| Compounds | Tyrosinase Activity IC50 ± SEM (µM) |
|---|---|
| 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl 3-hydroxybenzoate | 14.9 ± 0.91 |
| 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl 4-hydroxybenzoate | 14.9 ± 2.13 |
| 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl 2,4-dihydroxybenzoate | 6.7 ± 1.20 |
| 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl 3,4-dihydroxybenzoate | 15.9 ± 3.71 |
| 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl 3,5-dihydroxybenzoate | 93.8 ± 9.32 |
| 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl 3,4,5-trihydroxybenzoate | 65.2 ± 3.89 |
| 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl (2E)-3-phenylprop-2-enoate | 7.7 ± 0.70 |
| 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl (2E)-3-(4-hydroxyphenyl)prop-2-enoate | 6.5 ± 0.35 |
| 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl (2E)-3-(2,4-dihydroxyphenyl)prop-2-enoate | 0.0167 ± 0.0011 |
| Kojic acid | 16.69 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules 2023, 28, 378. https://doi.org/10.3390/molecules28010378
Hassan M, Shahzadi S, Kloczkowski A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules. 2023; 28(1):378. https://doi.org/10.3390/molecules28010378
Chicago/Turabian StyleHassan, Mubashir, Saba Shahzadi, and Andrzej Kloczkowski. 2023. "Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review" Molecules 28, no. 1: 378. https://doi.org/10.3390/molecules28010378
APA StyleHassan, M., Shahzadi, S., & Kloczkowski, A. (2023). Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules, 28(1), 378. https://doi.org/10.3390/molecules28010378







