Bismuth-Based Halide Perovskites for Photocatalytic H2 Evolution Application
Abstract
1. Introduction
1.1. Metal Halide Perovskite Photocatalysts
1.2. Lead-Free Metal Halide Photocatalysts
1.3. Design and Mechanism of H2 Production by Metal Halide Photocatalysts
2. Hybrid Bi-Based Halide Perovskites
2D and 2D Bi-Based Halide Perovskites
3. Inorganic Bi-Based Halide Perovskite
2D and 3D Double Bi-Based Halide Perovskite
4. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.-Y.; Jin, S. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- Roknuzzaman, M.; Zhang, C.; Ostrikov, K.; Du, A.; Wang, H.; Wang, L.; Tesfamichael, T. Electronic and optical properties of lead-free hybrid double perovskites for photovoltaic and optoelectronic applications. Sci. Rep. 2019, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Malavasi, L. Solar-Driven Hydrogen Generation by Metal Halide Perovskites: Materials, Approaches, and Mechanistic View. ACS Omega 2020, 5, 25511–25519. [Google Scholar] [CrossRef]
- Armenise, V.; Colella, S.; Fracassi, F.; Listorti, A. Lead-free metal halide perovskites for hydrogen evolution from aqueous solutions. Nanomaterials 2021, 11, 433. [Google Scholar] [CrossRef]
- Li, J.; Duan, J.; Yang, X.; Duan, Y.; Yang, P.; Tang, Q. Review on recent progress of lead-free halide perovskites in optoelectronic applications. Nano Energy 2021, 80, 105526. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, W.; Yang, H.; Fan, Q.; Xiong, F.; Liu, S.; Li, D.-S.; Liu, B. Halide perovskite composites for photocatalysis: A mini review. EcoMat 2021, 3, e12079. [Google Scholar] [CrossRef]
- Stanley, J.C.; Mayr, F.; Gagliardi, A. Machine Learning Stability and Bandgaps of Lead-Free Perovskites for Photovoltaics. Adv. Theory Simul. 2020, 3, 1900178. [Google Scholar] [CrossRef]
- Corti, M.; Bonomi, S.; Chiara, R.; Romani, L.; Quadrelli, P.; Malavasi, L. Application of metal halide perovskites as photocatalysts in organic reactions. Inorganics 2021, 9, 56. [Google Scholar] [CrossRef]
- Stroyuk, O. Lead-free hybrid perovskites for photovoltaics. Beilstein J. Nanotechnol. 2018, 9, 2209–2235. [Google Scholar] [CrossRef]
- Gao, P.; Grätzel, M.; Nazeeruddin, M.K. Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 2448–2463. [Google Scholar] [CrossRef]
- Noel, N.K.; Stranks, S.D.; Abate, A.; Wehrenfennig, C.; Guarnera, S.; Haghighirad, A.-A.; Sadhanala, A.; Eperon, G.E.; Pathak, S.K.; Johnston, M.B.; et al. Lead-free organic-inorganic tin halide perovskites for photovoltaic applications. Energy Environ. Sci. 2014, 7, 3061–3068. [Google Scholar] [CrossRef]
- Mosconi, E.; Amat, A.; Nazeeruddin, M.K.; Grätzel, M.; de Angelis, F. First-principles modeling of mixed halide organometal perovskites for photovoltaic applications. J. Phys. Chem. C 2013, 117, 13902–13913. [Google Scholar] [CrossRef]
- Hoefler, S.F.; Trimmel, G.; Rath, T. Progress on lead-free metal halide perovskites for photovoltaic applications: A review. Monatsh. Chem. 2017, 148, 795–826. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.G.; Dai, J.; Ma, L.; Zeng, X.C. Lead-Free Mixed Tin and Germanium Perovskites for Photovoltaic Application. J. Am. Chem. Soc. 2017, 139, 8038–8043. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, J.; Chen, Y.; Li, Q.; Pan, Y.; Zhang, H.; Xia, Y.; Huang, W. Lead-Free Organic–Inorganic Hybrid Perovskites for Photovoltaic Applications: Recent Advances and Perspectives. Adv. Mater. 2017, 29, 1605005. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Li, X.; Hoffman, J.M.; Kanatzidis, M.G. The 2D halide perovskite rulebook: How the spacer influences everything from the structure to optoelectronic device efficiency. Chem. Rev. 2021, 121, 2230–2291. [Google Scholar] [CrossRef]
- Tang, Y.; Mak, C.H.; Kai, K.-C.C.-J.; Meng, F.; Niu, W.; Li, F.-F.; Shen, H.-H.; Zhu, X.; Chen, H.M.; Hsu, H.-Y. Lead-free hybrid perovskite photocatalysts: Surface engineering, charge-carrier behaviors, and solar-driven applications. J. Mater. Chem. A 2022, 10, 12296–12316. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, X.; Huang, Y.; Lai, J.; Yang, J.; Tian, C.; He, P.; Huang, Q.; Tang, X. Synthesis and CO2Photoreduction of Lead-Free Cesium Bismuth Halide Perovskite Nanocrystals. J. Phys. Chem. C 2021, 125, 18328–18333. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Kim, D.; Lee, D.K.; Kim, S.M.; Park, W.; Sim, U. Photoelectrochemical water splitting reaction system based on metal-organic halide perovskites. Materials 2020, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Kosco, J.; Bidwell, M.; Cha, H.; Martin, T.; Howells, C.; Sachs, M.; Anjum, D.; Lopez, S.G.; Zou, L.; Wadsworth, A.; et al. Enhanced photocatalytic hydrogen evolution from organic semiconductor heterojunction nanoparticles. Nat. Mater. 2020, 19, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. Development of novel photocatalyst and cocatalyst materials for water splitting under visible light. Bull. Chem. Soc. Jpn. 2016, 89, 627–648. [Google Scholar] [CrossRef]
- Tao, R.; Sun, Z.; Li, F.; Fang, W.; Xu, L. Achieving Organic Metal Halide Perovskite into a Conventional Photoelectrode: Outstanding Stability in Aqueous Solution and High-Efficient Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2019, 2, 1969–1976. [Google Scholar] [CrossRef]
- Bosso, P.; Milella, A.; Barucca, G.; Mengucci, P.; Armenise, V.; Fanelli, F.; Giannuzzi, R.; Maiorano, V.; Fracassi, F. Plasma-assisted deposition of iron oxide thin films for photoelectrochemical water splitting. Plasma Process. Polym. 2021, 18, 2000121. [Google Scholar] [CrossRef]
- Guerrero, A.; Bisquert, J. Perovskite semiconductors for photoelectrochemical water splitting applications. Curr. Opin. Electrochem. 2017, 2, 144–147. [Google Scholar] [CrossRef]
- Zhai, P.; Haussener, S.; Ager, J.; Sathre, R.; Walczak, K.; Greenblatta, J.; McKone, T. Net primary energy balance of a solar-driven photoelectrochemical water-splitting device. Energy Environ. Sci. 2013, 6, 2380–2389. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on Ti02 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, Y.; Zhang, B.; Xu, T.; Wang, C. PtIx/[(CH3)2NH2]3[BiI6] as a well-dispersed photocatalyst for hydrogen production in hydroiodic acid. Nano Energy 2018, 50, 665–674. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, G.; Li, Z.; Lou, Y.; Chen, J.; Zhao, Y. Stable Lead-Free (CH3NH3)3Bi2I9 Perovskite for Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2019, 7, 15080–15085. [Google Scholar] [CrossRef]
- Tang, Y.; Mak, C.H.; Liu, R.; Wang, Z.; Ji, L.; Song, H.; Tan, C.; Barrière, F.; Hsu, H.-Y. In Situ Formation of Bismuth-Based Perovskite Heterostructures for High-Performance Cocatalyst-Free Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2020, 30, 2006919. [Google Scholar] [CrossRef]
- Liu, G.N.; Zhao, R.-Y.; Xu, B.; Sun, Y.; Jiang, X.-M.; Hu, X.; Li, C. Design, Synthesis, and Photocatalytic Application of Moisture-Stable Hybrid Lead-Free Perovskite. ACS Appl. Mater. Interfaces 2020, 12, 54694–54702. [Google Scholar] [CrossRef]
- Zhao, H.; Chordiya, K.; Leukkunen, P.; Popov, A.; Kahaly, M.U.; Kordas, K.; Ojala, S. Dimethylammonium iodide stabilized bismuth halide perovskite photocatalyst for hydrogen evolution. Nano Res. 2021, 14, 1116–1125. [Google Scholar] [CrossRef]
- Chen, G.; Wang, P.; Wu, Y.; Zhang, Q.; Wu, Q.; Wang, Z.; Zheng, Z.; Liu, Y.; Dai, Y.; Huang, B. Lead-Free Halide Perovskite Cs3Bi2xSb2–2xI9 (x ≈ 0.3) Possessing the Photocatalytic Activity for Hydrogen Evolution Comparable to that of (CH3NH3)PbI3. Adv. Mater. 2020, 32, 2001344. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Speltini, A.; Dibenedetto, C.N.; Listorti, A.; Ambrosio, F.; Mosconi, E.; Simbula, A.; Saba, M.; Profumo, A.; Quadrelli, P.; et al. Experimental Strategy and Mechanistic View to Boost the Photocatalytic Activity of Cs3Bi2Br9 Lead-Free Perovskite Derivative by g-C3N4 Composite Engineering. Adv. Funct. Mater. 2021, 31, 2104428. [Google Scholar] [CrossRef]
- Igbari, F.; Wang, Z.K.; Liao, L.S. Progress of Lead-Free Halide Double Perovskites. Adv. Energy Mater. 2019, 9, 1803150. [Google Scholar] [CrossRef]
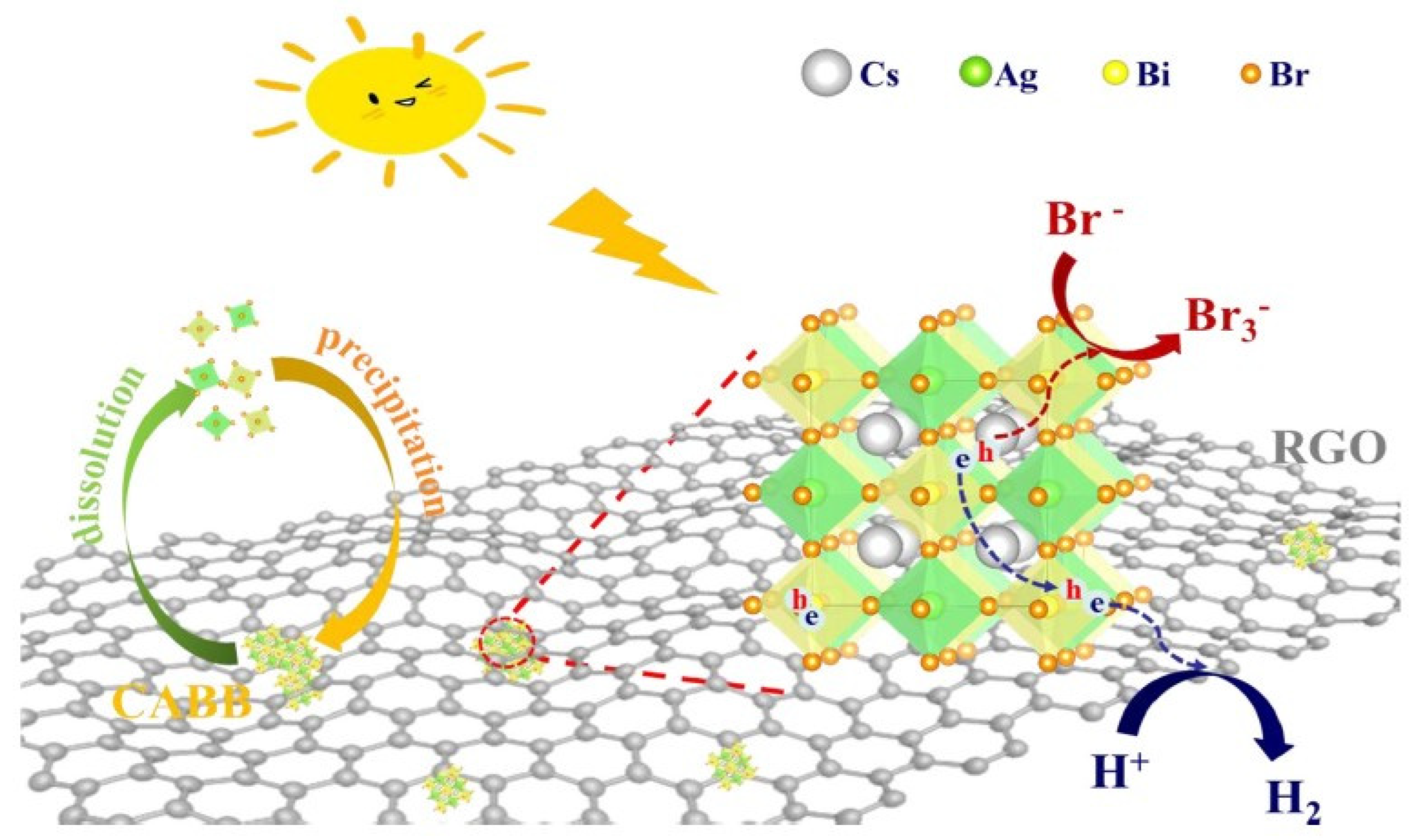
- Wang, T.; Yue, D.; Li, X.; Zhao, Y. Lead-free double perovskite Cs2AgBiBr6/RGO composite for efficient visible light photocatalytic H2 evolution. Appl. Catal. B 2020, 268, 118399. [Google Scholar] [CrossRef]
- He, Z.; Tang, Q.; Liu, X.; Yan, X.; Li, K.; Yue, D. Lead-Free Cs2AgBiBr6Perovskite with Enriched Surface Defects for Efficient Photocatalytic Hydrogen Evolution. Energy Fuels 2021, 35, 15005–15009. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, K.; Wu, X.; Zhu, M.; Zhang, H.; Zhang, K.; Wang, Y.; Loh, K.P.; Shi, Y.; Xu, Q.-H. In Situ Synthesis of Lead-Free Halide Perovskite Cs2AgBiBr6Supported on Nitrogen-Doped Carbon for Efficient Hydrogen Evolution in Aqueous HBr Solution. ACS Appl. Mater. Interfaces 2021, 13, 10037–10046. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, Y.; Chen, J.; Lou, Y.; Zhao, Y. NiCoP modified lead-free double perovskite Cs2AgBiBr6 for efficient photocatalytic hydrogen generation. New J. Chem. 2022, 46, 7395–7402. [Google Scholar] [CrossRef]
- Dai, Y.; Tüysüz, H. Lead-Free Cs3Bi2Br9 Perovskite as Photocatalyst for Ring-Opening Reactions of Epoxides. ChemSusChem 2019, 12, 2587–2592. [Google Scholar] [CrossRef] [PubMed]
- Bresolin, B.M.; Sgarbossa, P.; Bahnemann, D.W.; Sillanpää, M. Cs3Bi2I9/g-C3N4 as a new binary photocatalyst for efficient visible-light photocatalytic processes. Sep. Purif. Technol. 2020, 251, 117320. [Google Scholar] [CrossRef]
- Estrada-Pomares, J.; Ramos-Terrón, S.; Lasarte-Aragonés, G.; Lucena, R.; Cárdenas, S.; Rodríguez-Padrón, D.; Luque, R.; de Miguel, G. Mechanochemically designed bismuth-based halide perovskites for efficient photocatalytic oxidation of vanillyl alcohol. J. Mater. Chem. A Mater. 2022, 10, 11298–11305. [Google Scholar] [CrossRef]
- Shi, M.; Zhou, H.; Tian, W.; Yang, B.; Yang, S.; Han, K.; Li, R.; Li, C. Lead-free B-site bimetallic perovskite photocatalyst for efficient benzylic C–H bond activation. Cell Rep. Phys. Sci. 2021, 2, 100656. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, B.; Zhao, H.-B.; Liao, J.-F.; Zhou, Z.-C.; Liu, T.; He, B.; Wei, Q.; Chen, S.; Chen, H.-Y.; et al. Self-assembled lead-free double perovskite-MXene heterostructure with efficient charge separation for photocatalytic CO2 reduction. Appl. Catal. B 2022, 312, 121358. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Wang, J.; Yuan, Y.; Hills-Kimball, K.; Cai, T.; Wang, P.; Tang, A.; Chen, O. Synthesis of lead-free Cs2AgBix6 (X = Cl, Br, I) double perovskite nanoplatelets and their application in CO2 photocatalytic reduction. Nano Lett. 2021, 21, 1620–1627. [Google Scholar] [CrossRef]
- Wu, D.; Zhao, X.; Huang, Y.; Lai, J.; Li, H.; Yang, J.; Tian, C.; He, P.; Huang, Q.; Tang, X. Lead-free perovskite Cs2AgBiX6nanocrystals with a band gap funnel structure for photocatalytic CO2reduction under visible light. Chem. Mater. 2021, 33, 4971–4976. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Y.F.; Chen, B.X.; Kuang, D.B.; Su, C.Y. Synthesis and Photocatalytic Application of Stable Lead-Free Cs2AgBiBr6 Perovskite Nanocrystals. Small 2018, 14, 1703762. [Google Scholar] [CrossRef]
- Sun, Q.; Ye, W.; Wei, J.; Li, L.; Wang, J.; He, J.-H.; Lu, J.-M. Lead-free perovskite Cs3Bi2Br9 heterojunctions for highly efficient and selective photocatalysis under mild conditions. J. Alloys. Compd. 2022, 893, 162326. [Google Scholar] [CrossRef]
- Xiao, Z.; Song, Z.; Yan, Y. From Lead Halide Perovskites to Lead-Free Metal Halide Perovskites and Perovskite Derivatives. Adv. Mater. 2019, 31, e1803792. [Google Scholar] [CrossRef] [PubMed]






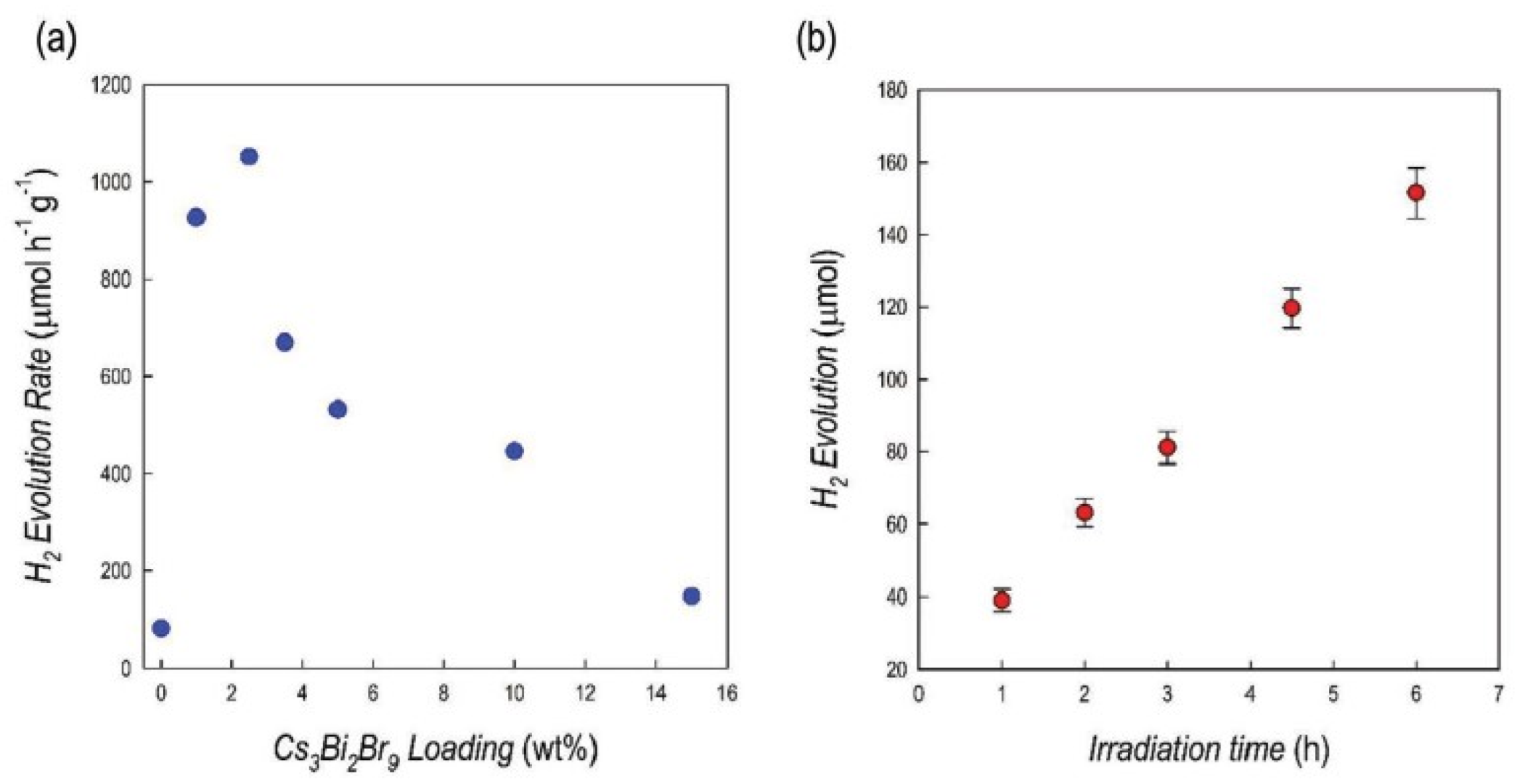



| System | Source Power | Reaction Environment and Sacrificial Electron Donor | H2 (µmol g−1 h−1) | Ref. |
|---|---|---|---|---|
| [DMA]3[BiI6]/PtIx Pt 1 wt% | 300 W Xe lamp (λ = 465 nm) for 100 h | H3PO2, HI sacrificial electron donor (1:4) | 186.5 | [30] |
| MA3Bi2I9/Pt | 300 W Xe lamp (λ ≥ 420 nm) for 10 h | H3PO2, HI sacrificial electron donor, MABI saturated solution | 169.2 | [31] |
| DMA3BiI6/MA3Bi2I9 | 300 W Xe lamp (λ ≥ 420 nm) for 10 h | HI saturated solution, sacrificial electron donor | 198 | [32] |
| (EtbtBi2I10)/Pt TiO2- EtbtBi2I10/Pt TiO2- EtbtBi2I10-rGO/Pt | 300 W Xe lamp (λ ≥ 420 nm) for 10 h | HI sacrificial electron donor, H3PO2 saturated solution | 9.2 59.9 83.8 | [33] |
| Cs3Bi0.6Sb1.4I9/Pt | 300 W Xe lamp (λ ≥ 420 nm) for 10 h | HI sacrificial electron donor, CBI saturated solution and Cs2CO3 | 926 | [35] |
| DMA3BiI6/Pt | Commercial LED for 6 h 425 nm, light intensity 8 mW | HI sacrificial electron donor, DAI solution | 5.7 | [34] |
| g-C3N4/Cs3Bi2Br9 2.5% wt%/Pt 3 wt% | Simulated solar light 500 Wm 2 for 2 for6 h UV filter | Aqueous solution with 10% v/v TEOA sacrificial electron donor | 1050 | [36] |
| Cs2AgBiBr6/2.5%RGO | 300 W Xe lamp (λ ≥ 420 nm) for 3 h | HBr sacrificial electron donor, H3PO2 saturated solution | 489 | [38] |
| Defect-rich Cs2AgBiBr6/Mo3S132− Cs2AgBiBr6Defect-rich Cs2AgBiBr6 Defect-rich Cs2AgBiBr6/Pt | 300 W Xe lamp (λ ≥ 420 nm) for 3 h | CABB saturated solution | 24.7 0.8 4.1 7.3 | [39] |
| Cs2AgBiBr6/N-C-140 | 300 W Xe lamp (λ ≥ 420 nm) for 3 h | HBr sacrificial electron donor, H3PO2 saturated solution | 380 | [40] |
| Cs2AgBiBr6/NiCoP | 300 W Xe lamp (λ ≥ 420 nm) for 3 h | HBr sacrificial electron donor, H3PO2 saturated solution | 373.2 | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedesco, C.; Malavasi, L. Bismuth-Based Halide Perovskites for Photocatalytic H2 Evolution Application. Molecules 2023, 28, 339. https://doi.org/10.3390/molecules28010339
Tedesco C, Malavasi L. Bismuth-Based Halide Perovskites for Photocatalytic H2 Evolution Application. Molecules. 2023; 28(1):339. https://doi.org/10.3390/molecules28010339
Chicago/Turabian StyleTedesco, Costanza, and Lorenzo Malavasi. 2023. "Bismuth-Based Halide Perovskites for Photocatalytic H2 Evolution Application" Molecules 28, no. 1: 339. https://doi.org/10.3390/molecules28010339
APA StyleTedesco, C., & Malavasi, L. (2023). Bismuth-Based Halide Perovskites for Photocatalytic H2 Evolution Application. Molecules, 28(1), 339. https://doi.org/10.3390/molecules28010339





