Inhibition of Leishmania infantum Trypanothione Reductase by New Aminopropanone Derivatives Interacting with the NADPH Binding Site
Abstract
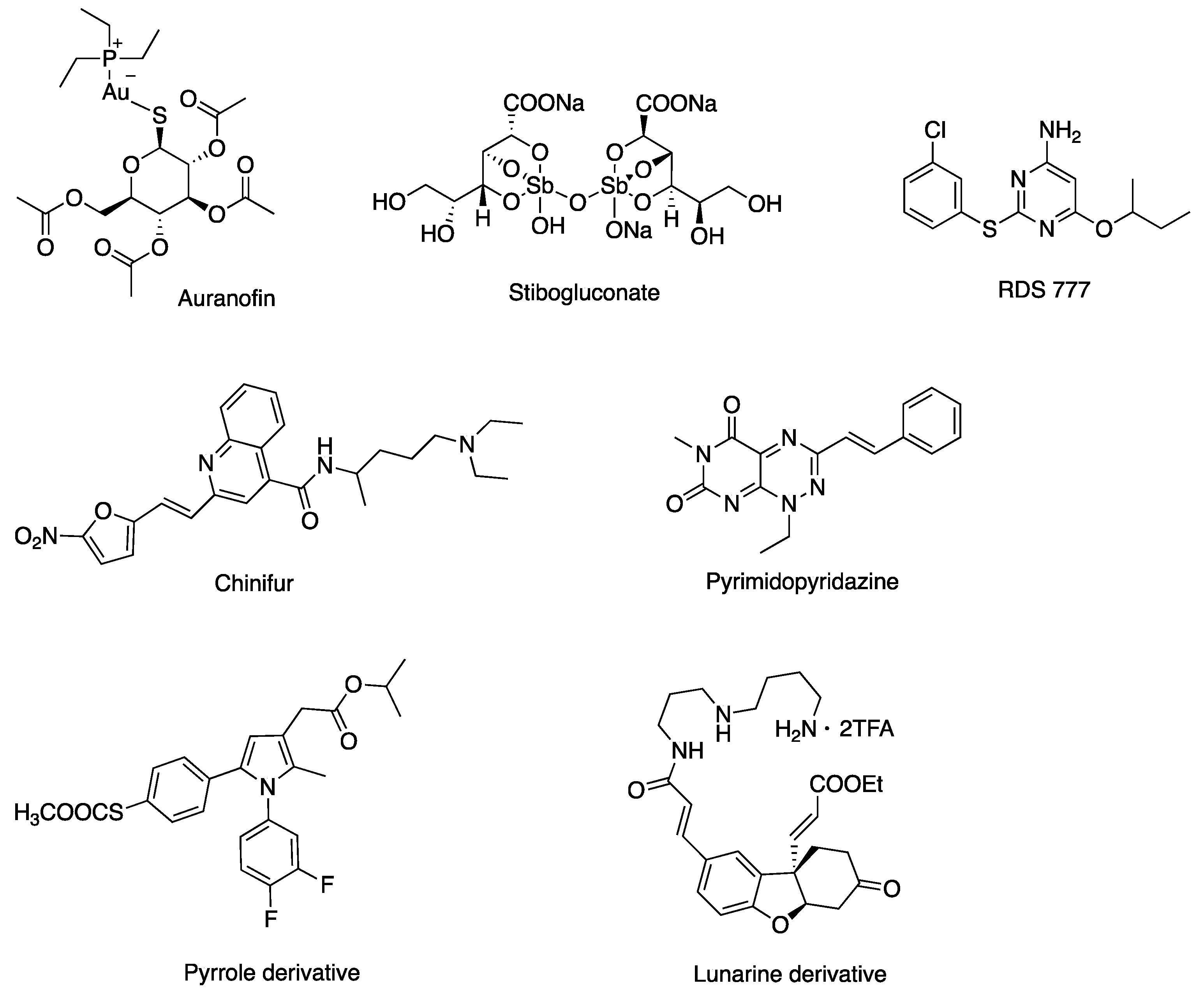
1. Introduction
2. Results and Discussion
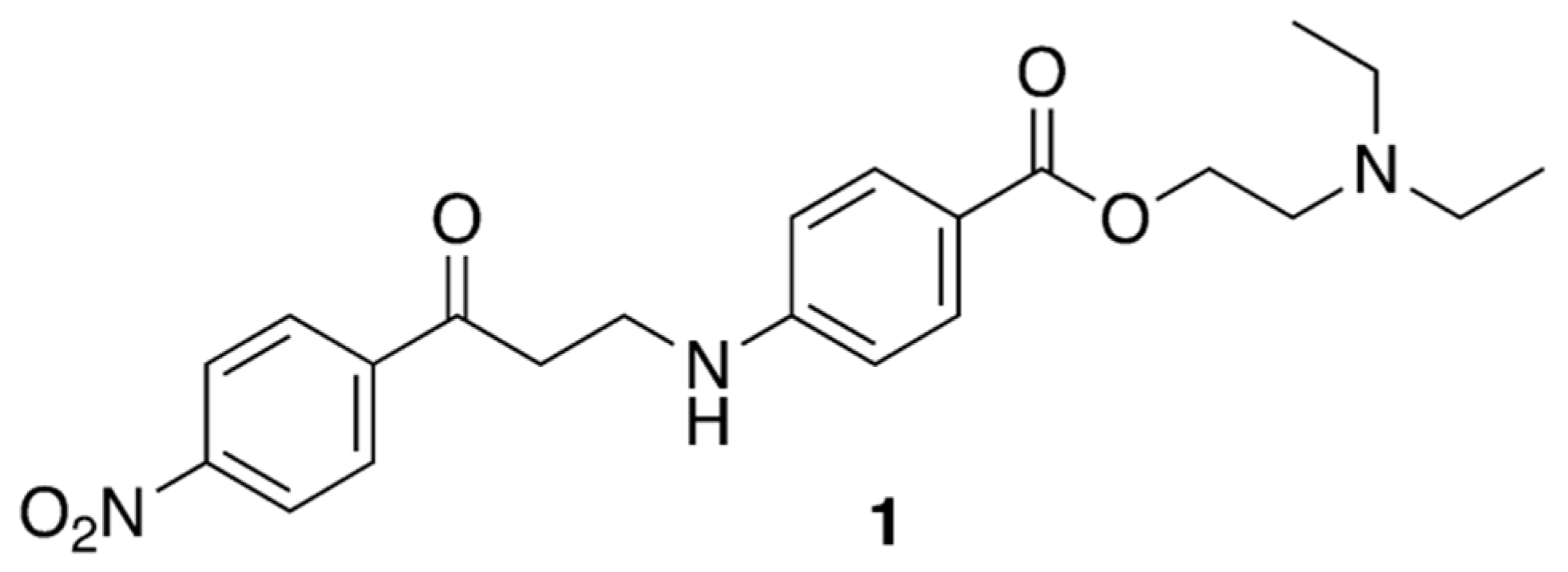
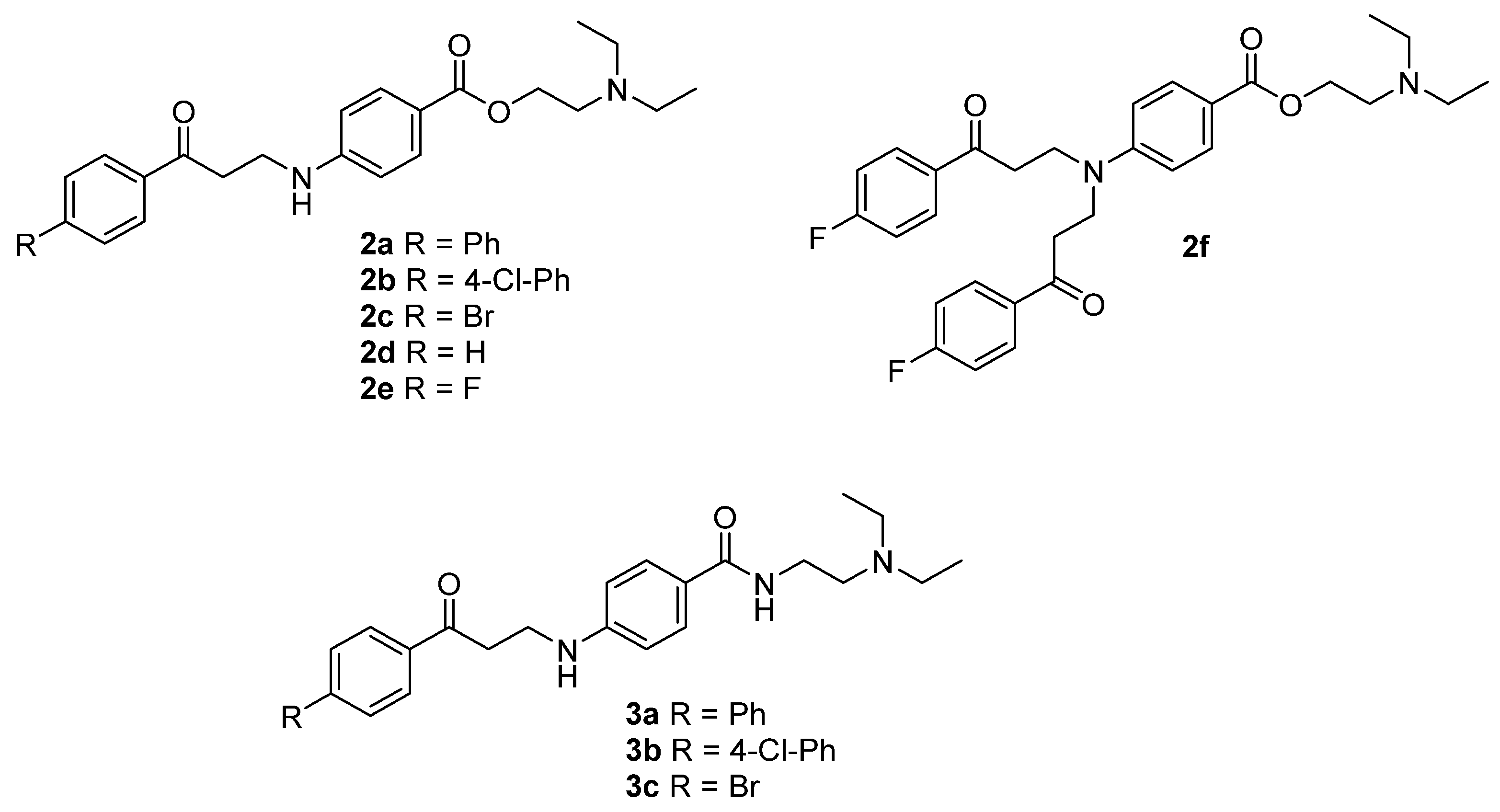
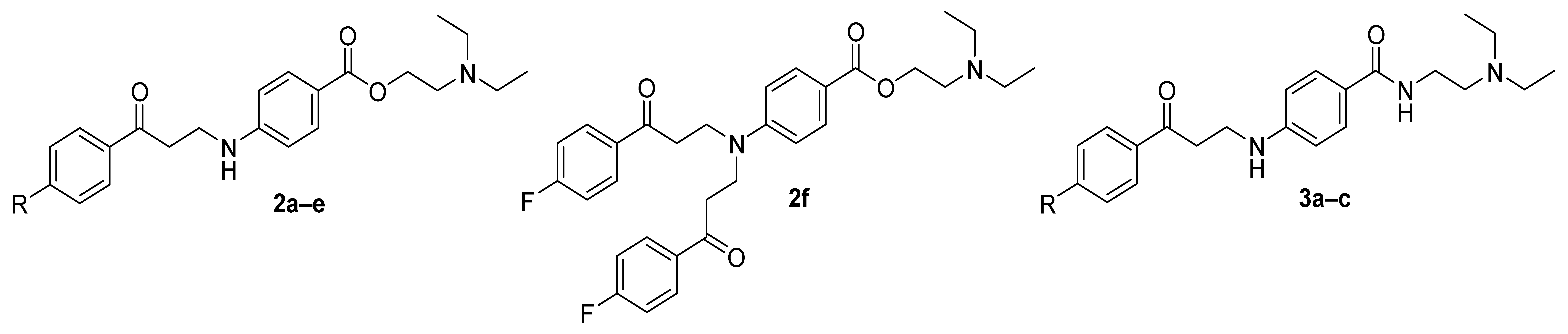
2.1. Chemistry
2.2. In Vitro Enzymatic Assay
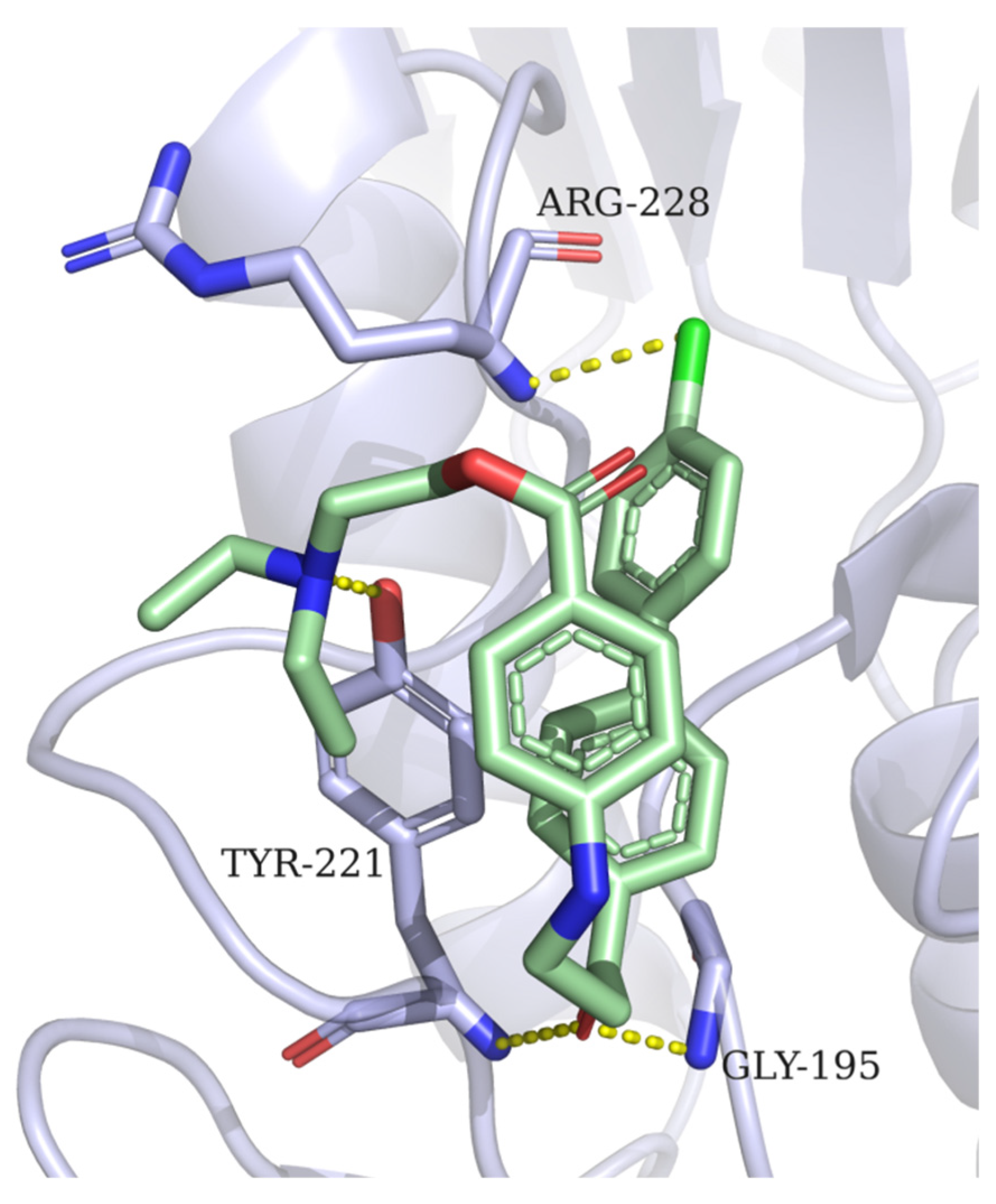
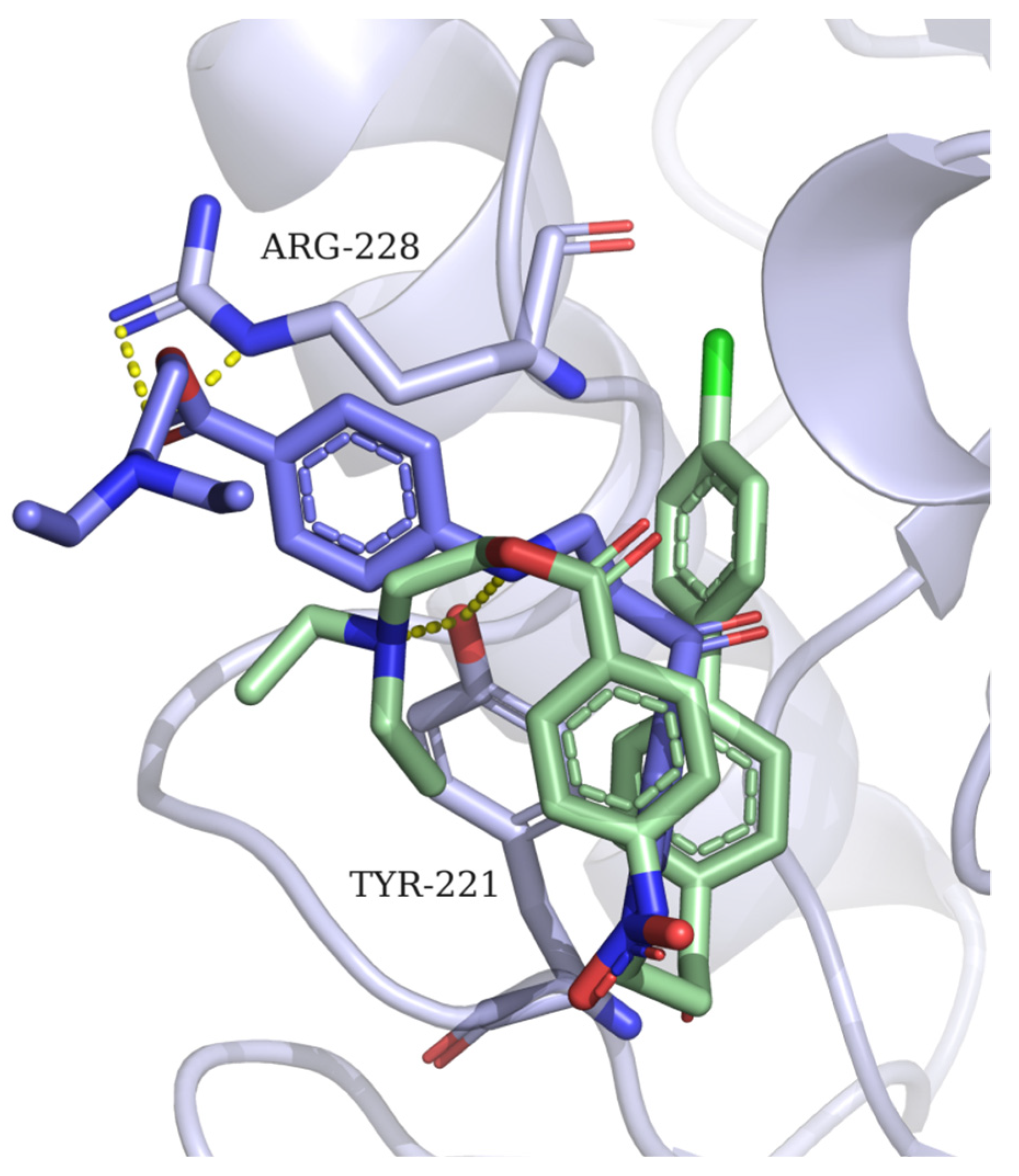
2.3. Docking Experiments
3. Materials and Methods
3.1. Chemistry
3.1.1. General Instrumentation
3.1.2. General Experimental Procedures
3.1.3. Characterization
3.2. Biological Assays
3.2.1. Protein Expression and Purification
3.2.2. Enzymatic Assay
3.2.3. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Esch, K.J.; Petersen, C.A. Transmission and Epidemiology of Zoonotic Protozoal Diseases of Companion Animals. Clin. Microbiol. Rev. 2013, 26, 58–85. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Wamai, R.G.; Kahn, J.; McGloin, J.; Ziaggi, G. Visceral leishmaniasis: A global overview. J. Glob. Health Sci. 2020, 2, e3. [Google Scholar] [CrossRef]
- CDC. Epidemiology and Risk Factors. Available online: https://www.cdc.gov/parasites/leishmaniasis/epi.html (accessed on 15 October 2022).
- Lockwood, D.; Moore, E. Treatment of visceral leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 151–158. [Google Scholar] [CrossRef]
- Saccoliti, F.; Madia, V.N.; Tudino, V.; De Leo, A.; Pescatori, L.; Messore, A.; De Vita, D.; Scipione, L.; Brun, R.; Kaiser, M.; et al. Biological evaluation and structure-activity relationships of imidazole-based compounds as antiprotozoal agents. Eur. J. Med. Chem. 2018, 156, 53–60. [Google Scholar] [CrossRef]
- Saccoliti, F.; Angiulli, G.; Pupo, G.; Pescatori, L.; Madia, V.N.; Messore, A.; Colotti, G.; Fiorillo, A.; Scipione, L.; Gramiccia, M.; et al. Inhibition of Leishmania infantum trypanothione reductase by diaryl sulfide derivatives. J. Enzym. Inhib. Med. Chem. 2017, 32, 304–310. [Google Scholar] [CrossRef]
- Colotti, G.; Saccoliti, F.; Gramiccia, M.; Di Muccio, T.; Prakash, J.; Yadav, S.; Dubey, V.K.; Vistoli, G.; Battista, T.; Mocci, S.; et al. Structure-guided approach to identify a novel class of anti-leishmaniasis diaryl sulfide compounds targeting the trypanothione metabolism. Amino Acids 2020, 52, 247–259. [Google Scholar] [CrossRef]
- Turcano, L.; Torrente, E.; Missineo, A.; Andreini, M.; Gramiccia, M.; Di Muccio, T.; Genovese, I.; Fiorillo, A.; Harper, S.; Bresciani, A.; et al. Identification and binding mode of a novel Leishmania Trypanothione reductase inhibitor from high throughput screening. PLoS Negl. Trop. Dis. 2018, 12, e0006969. [Google Scholar] [CrossRef]
- Krieger, S.; Schwarz, W.; Ariyanayagam, M.R.; Fairlamb, A.; Krauth-Siegel, R.L.; Clayton, C. Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol. Microbiol. 2000, 35, 542–552. [Google Scholar] [CrossRef]
- Fairlamb, A.H.; Patterson, S. Current and Future Prospects of Nitro-compounds as Drugs for Trypanosomiasis and Leishmaniasis. Curr. Med. Chem. 2019, 26, 4454–4475. [Google Scholar] [CrossRef]
- Battista, T.; Colotti, G.; Ilari, A.; Fiorillo, A. Targeting Trypanothione Reductase, a Key Enzyme in the Redox Trypanosomatid Metabolism, to Develop New Drugs against Leishmaniasis and Trypanosomiases. Molecules 2020, 25, 1924. [Google Scholar] [CrossRef]
- Nepali, K.; Lee, H.-Y.; Liou, J.-P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. [Google Scholar] [CrossRef] [PubMed]
- Bosquesi, P.L.; Santos, J.L. A Prodrug Approach to Improve the Physico-Chemical Properties and Decrease the Genotoxicity of Nitro Compounds. Curr. Pharm. Des. 2011, 17, 3515–3526. [Google Scholar] [CrossRef]
- Chung, K.T.; Murdock, C.A.; Zhou, Y.; Stevens, S.E., Jr.; Li, Y.S.; Wei, C.I.; Fernando, S.Y.; Chou, M.W. Effects of the nitro-group on the mutagenicity and toxicity of some benzamines. Environ. Mol. Mutagen. 1996, 27, 67–74. [Google Scholar] [CrossRef]
- Enanga, B.; Ariyanayagam, M.R.; Stewart, M.L.; Barrett, M.P. Activity of Megazol, a Trypanocidal Nitroimidazole, Is Associated with DNA Damage. Antimicrob. Agents Chemother. 2003, 47, 3368–3370. [Google Scholar] [CrossRef] [PubMed]
- Buschini, A.; Ferrarini, L.; Franzoni, S.; Galati, S.; Lazzaretti, M.; Mussi, F.; de Albuquerque, C.N.; Zucchi, T.M.A.D.; Poli, P. Genotoxicity Revaluation of Three Commercial Nitroheterocyclic Drugs: Nifurtimox, Benznidazole, and Metronidazole. J. Parasitol. Res. 2009, 2009, 463575. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Liu, D.; Zhang, W. Asymmetric Hydrogenation of β-Secondary Amino Ketones Catalyzed by a Ruthenocenyl Phosphino-oxazoline-ruthenium Complex (RuPHOX-Ru): The Synthesis of γ-Secondary Amino Alcohols. Adv. Synth. Catal. 2015, 357, 3262–3272. [Google Scholar] [CrossRef]
- Thompson, N.J.; Goodby, J.W.; Toyne, K.J. Liquid Crystalline Polymesomorphism in Copper(II) Complexes of Diketones: The Effect of the Position of a Polar Substituent. Mol. Cryst. Liq. Cryst. 1992, 214, 81–95. [Google Scholar] [CrossRef]
- De Vita, D.; Angeli, A.; Pandolfi, F.; Bortolami, M.; Costi, R.; Di Santo, R.; Suffredini, E.; Ceruso, M.; Del Prete, S.; Capasso, C.; et al. Inhibition of the α-carbonic anhydrase from Vibrio cholerae with amides and sulfonamides incorporating imidazole moieties. J. Enzym. Inhib. Med. Chem. 2017, 32, 798–804. [Google Scholar] [CrossRef]
- Cirilli, R.; Costi, R.; Di Santo, R.; La Torre, F.; Pierini, M.; Siani, G. Perturbing Effects of Chiral Stationary Phase on Enantiomerization Second-Order Rate Constants Determined by Enantioselective Dynamic High-Performance Liquid Chromatography: A Practical Tool to Quantify the Accessible Acid and Basic Catalytic Sites Bonded on Chromatographic Supports. Anal. Chem. 2009, 81, 3560–3570. [Google Scholar] [CrossRef] [PubMed]
- Baiocco, P.; Franceschini, S.; Ilari, A.; Colotti, G. Trypanothione Reductase from Leishmania infantum: Cloning, Expression, Purification, Crystallization and Preliminary X-Ray Data Analysis. Protein Pept. Lett. 2009, 16, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]



 | ||
|---|---|---|
| Cpd | R | LiTR Activity (% at 100 µM) 1 |
| 2a | Ph | 55.4 ± 13.3 |
| 2b | 4-Cl-Ph | 47.0 ± 9.7 |
| 2c | Br | 73.8 ± 4.6 |
| 2d | H | 73.8 ± 4.4 |
| 2e | F | 60.5 ± 4.1 |
| 2f | - | 75.0 ± 2.4 |
| 3a | Ph | 68.4 ± 6.7 |
| 3b | 4-Cl-Ph | 59.4 ± 2.4 |
| 3c | Br | 87.3 ± 1.9 |
| 1 | - | <10.0 [10] |
| Name | Lowest Binding Energy | Number of Conformations in Cluster |
|---|---|---|
| 2a | −3.65 kcal/mol | 17 |
| 2b | −3.62 kcal/mol | 27 |
| 2c | −3.61 kcal/mol | 16 |
| 2d | −3.50 kcal/mol | 14 |
| 2e | −2.91 kcal/mol | 13 |
| 2f | −3.49 kcal/mol | 5 |
| 3a | −3.45 kcal/mol | 23 |
| 3b | −3.59 kcal/mol | 24 |
| 3c | −3.93 kcal/mol | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madia, V.N.; Ialongo, D.; Patacchini, E.; Exertier, C.; Antonelli, L.; Colotti, G.; Messore, A.; Tudino, V.; Saccoliti, F.; Scipione, L.; et al. Inhibition of Leishmania infantum Trypanothione Reductase by New Aminopropanone Derivatives Interacting with the NADPH Binding Site. Molecules 2023, 28, 338. https://doi.org/10.3390/molecules28010338
Madia VN, Ialongo D, Patacchini E, Exertier C, Antonelli L, Colotti G, Messore A, Tudino V, Saccoliti F, Scipione L, et al. Inhibition of Leishmania infantum Trypanothione Reductase by New Aminopropanone Derivatives Interacting with the NADPH Binding Site. Molecules. 2023; 28(1):338. https://doi.org/10.3390/molecules28010338
Chicago/Turabian StyleMadia, Valentina Noemi, Davide Ialongo, Elisa Patacchini, Cécile Exertier, Lorenzo Antonelli, Gianni Colotti, Antonella Messore, Valeria Tudino, Francesco Saccoliti, Luigi Scipione, and et al. 2023. "Inhibition of Leishmania infantum Trypanothione Reductase by New Aminopropanone Derivatives Interacting with the NADPH Binding Site" Molecules 28, no. 1: 338. https://doi.org/10.3390/molecules28010338
APA StyleMadia, V. N., Ialongo, D., Patacchini, E., Exertier, C., Antonelli, L., Colotti, G., Messore, A., Tudino, V., Saccoliti, F., Scipione, L., Ilari, A., Costi, R., & Di Santo, R. (2023). Inhibition of Leishmania infantum Trypanothione Reductase by New Aminopropanone Derivatives Interacting with the NADPH Binding Site. Molecules, 28(1), 338. https://doi.org/10.3390/molecules28010338











