Abstract
2,4-Diaminopyrimidines and (dialkylamino)azabicyclo-nonanes possess activity against protozoan parasites. A series of fused hybrids were synthesized and tested in vitro against pathogens of malaria tropica and sleeping sickness. The activities and selectivities of compounds strongly depended on the substitution pattern of both ring systems as well as on the position of the nitrogen atom in the bicycles. The most promising hybrids of 3-azabicyclo-nonane with 2-aminopyrimidine showed activity against P. falciparum NF54 in submicromolar concentration and high selectivity. A hybrid with pyrrolidino substitution of the 2-azabicyclo-nonane as well as of the pyrimidine moiety exhibited promising activity against the multiresistant K1 strain of P. falciparum. A couple of hybrids of 2-azabicyclo-nonanes with 2-(dialkylamino)pyrimidines possessed high activity against Trypanosoma brucei rhodesiense STIB900 and good selectivity.
1. Introduction
Malaria and human African trypanosomiasis (HAT, sleeping sickness) are both tropical diseases transmitted by the bite of infected insects. Malaria is caused by Plasmodium parasites. In 2020 there were about 241 million estimated cases of malaria and about 627,000 reported deaths [1]. There are five types of human malaria parasites, but the majority of infections are caused by Plasmodium falciparum, the deadliest malaria parasite. A lot of strains of Plasmodium falciparum have become resistant to previous generations of medicines [1]. In recent years even resistance to recommended artemisinin-based therapies has become prevalent across an expanding area of Southeast Asia [2,3]. There is growing evidence that resistant strains have reached regions in Africa [1]. Therefore, there is still a necessity to develop new compounds with distinct activity against Plasmodium falciparum.
Human African trypanosomiasis is caused by Trypanosoma brucei parasites. After continued control efforts the number of reported cases dropped below 1000 in 2019. However, as history has shown it may re-emerge and the estimated population at risk is 65 million people. In case of T. b. gambiense infections, the disease is chronic, generally lasting several years without any major signs or symptoms. However, infections with T. b. rhodesiense cause the acute form of the disease, lasting from a few weeks to several months and are lethal if untreated [4]. This East African sleeping sickness is difficult to treat considering the toxicity and complex administration of the drugs currently in use. For the treatment of T. b. rhodesiense infections of the central nervous system, melarsoprol is the only effective drug. Unfortunately, this arsenic-containing medication may cause encephalopathy, killing one in twenty patients [5]. Therefore, the development of new drugs against the East African form of HAT is urgently required.
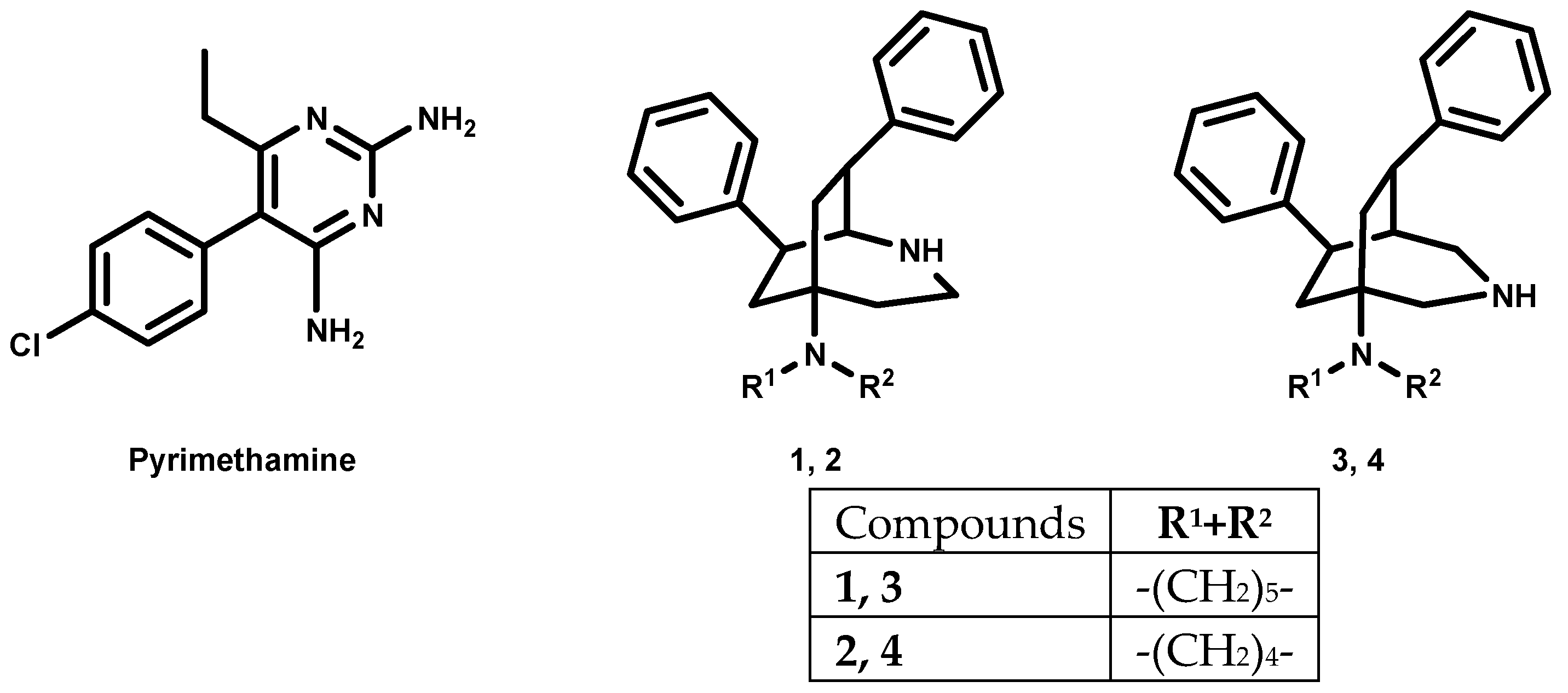
An important drug in the therapy against protozoan parasites (Figure 1) is 2,4-diaminopyrimidine pyrimethamine. It targets the enzymes of the folate metabolism, and so, in the case of malaria it selectively inhibits the plasmodial form of dihydrofolate reductase and reduces the production of folic acid required for nucleic acid synthesis in the Plasmodium parasite [6]. It is effective against the erythrocytic stages of all Plasmodium spp. that are pathogenic for humans, but resistance has significantly limited its usefulness as a single agent [7]. As a consequence, it is used for treatment of acute malaria only in combination with other drugs to prevent resistance. In addition, pyrimethamine is also used for the development of novel antiparasitic treatments [8,9,10]. Trypanosomes are unable to synthesize folates and pterins, which they need for nucleic acid synthesis. They use the extracellular pterin precursors of their hosts. These have to be modified by two reductions to yield active tetrahydro-derivates. Pyrimethamine was found to selectively inhibit the trypanosomal form of DHFR-thymidylate synthase (DHFR-TS) and pteridine reductase 1 (PTR1), and in this way, the production of folic acid required by trypanosomes [11].
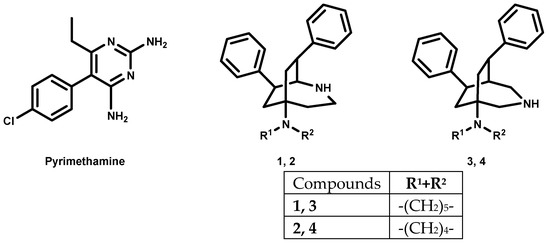
Figure 1.
Pyrimethamine, 2-azabicyclo[3.2.2]nonanes and 3-azabicyclo[3.2.2]nonanes.
The 2-azabicyclo[3.2.2]nonanes 1, 2 and the 3-azabicyclo[3.2.2]nonane 3, 4 show antiplasmodial activity in submicromolar concentration. Their antitrypanosomal activity requires low micromolar concentrations [12,13].
In drug development against plasmodia the combination of different pharmacophores in one hybrid molecule is a potential option to bypass drug resistance, to improve pharmacokinetics or to achieve multistage antimalarial activity [14]. Recently, we reported about the impact of the linkage of tetrazole or sulfonamide cores to antiprotozoal active (dialkylamino)azabicyclo-nonanes [15]. A series of new quinoline-pyrimidine hybrids and their biological activities have been published in the last decade [16,17,18,19,20]. This paper presents the preparation of fused hybrids of (dialkylamino)azabicyclo-nonanes with the 2,4-diaminopyrimidine pharmacophore of pyrimethamine and selected analogues.
2. Results
2.1. Chemistry
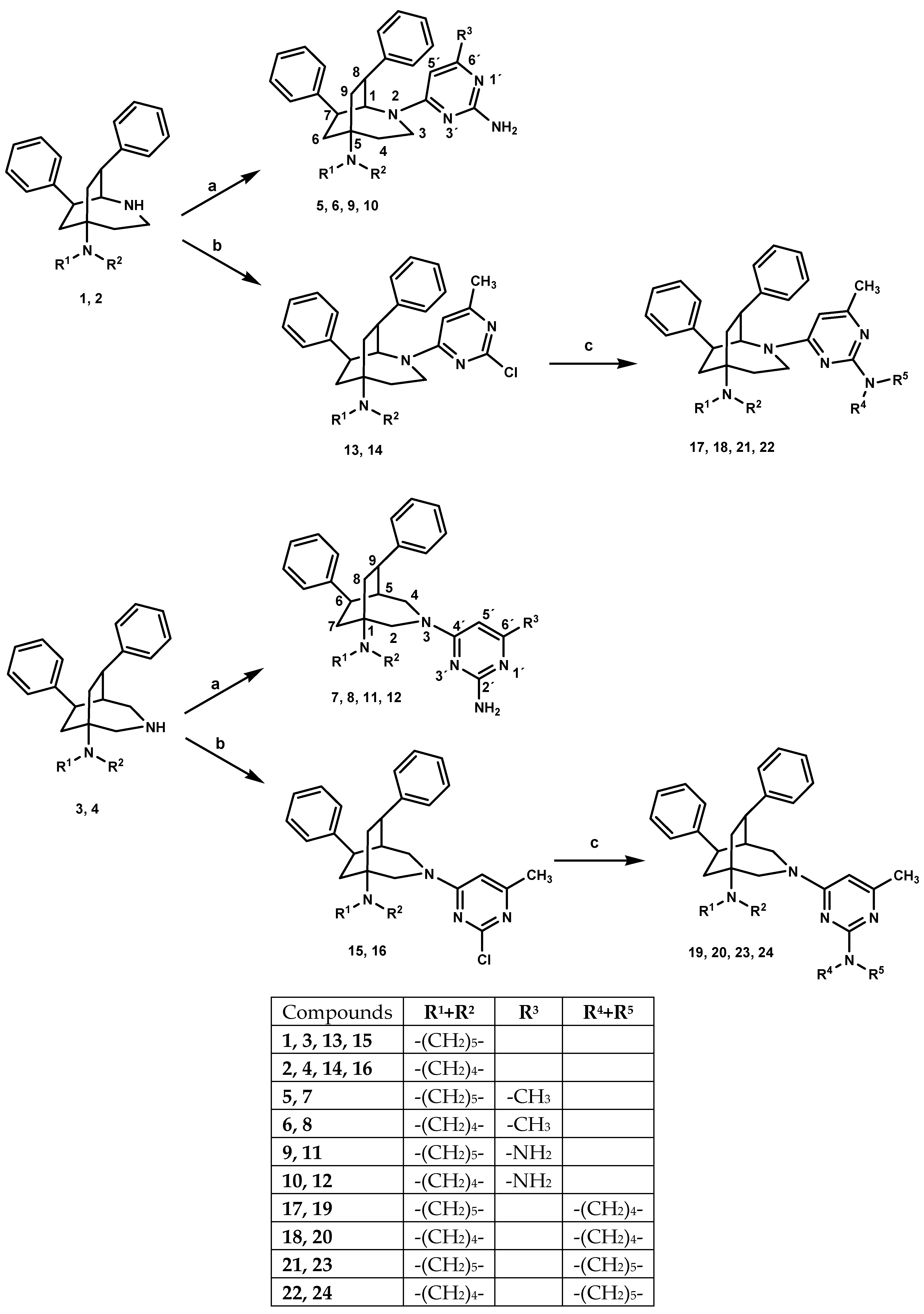
The 2-azabicyclo[3.2.2]nonanes 1, 2 were prepared from bicyclo[2.2.2]octan-2-ones [21] via a Beckmann rearrangement and the subsequent reduction of the lactam intermediates [22]. Alternatively, bicyclo[2.2.2]octan-2-ones were converted to lactams via a Schmidt reaction. Their reduction yielded 3-azabicyclo[2.2.2]nonanes 3, 4 [12,23]. A scheme is given in the Supplemental Materials.
The azabicyclo-nonanes 1–4 were refluxed with the corresponding 4-chloropyrimidine in boiling butan-1-ol for 2 d giving pyrimidine hybrids 5–12. DIPEA served as a proton catcher. Regioselective reactions with 2,4-dichloro-6-methylpyrimidine yielding 13–16 required milder conditions. The experiments were carried out at room temperature and were more time-consuming, accordingly. The second chloro substituent was finally replaced by reaction with excess secondary amine in boiling acetonitrile giving 17–24. (Scheme 1)
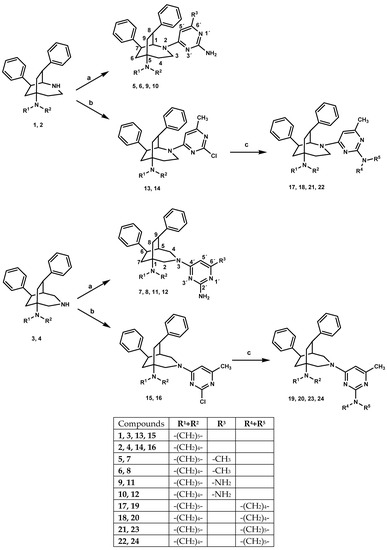
Scheme 1.
Preparation of compounds 5–24. Reagents and conditions: (a) corresp. 4-chloropyrimidine, DIPEA, 1-BuOH, 145 °C, 48h; (b) 2,4-dichloro-6-methylpyrimidine, DIPEA, EtOH, 0 °C→RT, 44–188 h; (c) sec. amine, DIPEA, ACN, 105 °C, 20–48h.
The structures of all newly synthesized compounds were clarified by one- and two-dimensional NMR spectroscopy. Successful N-arylation was detected by typical shifts of resonances and remarkable signal broadening, particularly of the bicyclic ring atoms in the vicinity of the new bonding sites. The most striking change was a 17–19 ppm upfield shift of C-5′ of the pyrimidine moiety due to the mesomeric effect of the new nitrogen substituent. In consequence of restricted rotation, the signals of each of the two protons H-2 and H-4 of the 3-azabicyclo-nonanes were strongly broadened. The corresponding signals of H-1 and one of the two H-3 protons of 2-azabicyclo-nonanes were even invisible in the 1H NMR spectrum. The signals of the adjacent ring carbon atoms of both azabicyclo-nonanes were massively broadened and typically shifted about 1.5–5.5 ppm to lower frequencies. Their chemical shifts were usually deduced from 2D spectra, and 1H- and 13C-nmr spectra of the new compounds are given in the supplementary material.
2.2. Antiprotozoal Activity and Cytotoxicity
Compounds were tested for their activities against Plasmodium falciparum NF54 (sensitive to chloroquine) and Trypanosoma brucei rhodesiense STIB900, via serial dilution assays. Furthermore, selected compounds were tested against the K1 strain of Plasmodium falciparum (resistant against chloroquine, sulfadoxine and pyrimethamine). Their cytotoxicity was determined with rat skeletal myoblasts (L-6 cells). Chloroquine, melarsoprol and podophyllotoxin served as standards; additional data for pyrimethamine are included (Table 1).

Table 1.
Activities of compounds 1–24 against P. falciparum NF54, P. falciparum K1, T. brucei rhodesiense (STIB900) and L-6 cells, expressed as IC50 (µM) a.
3. Discussion
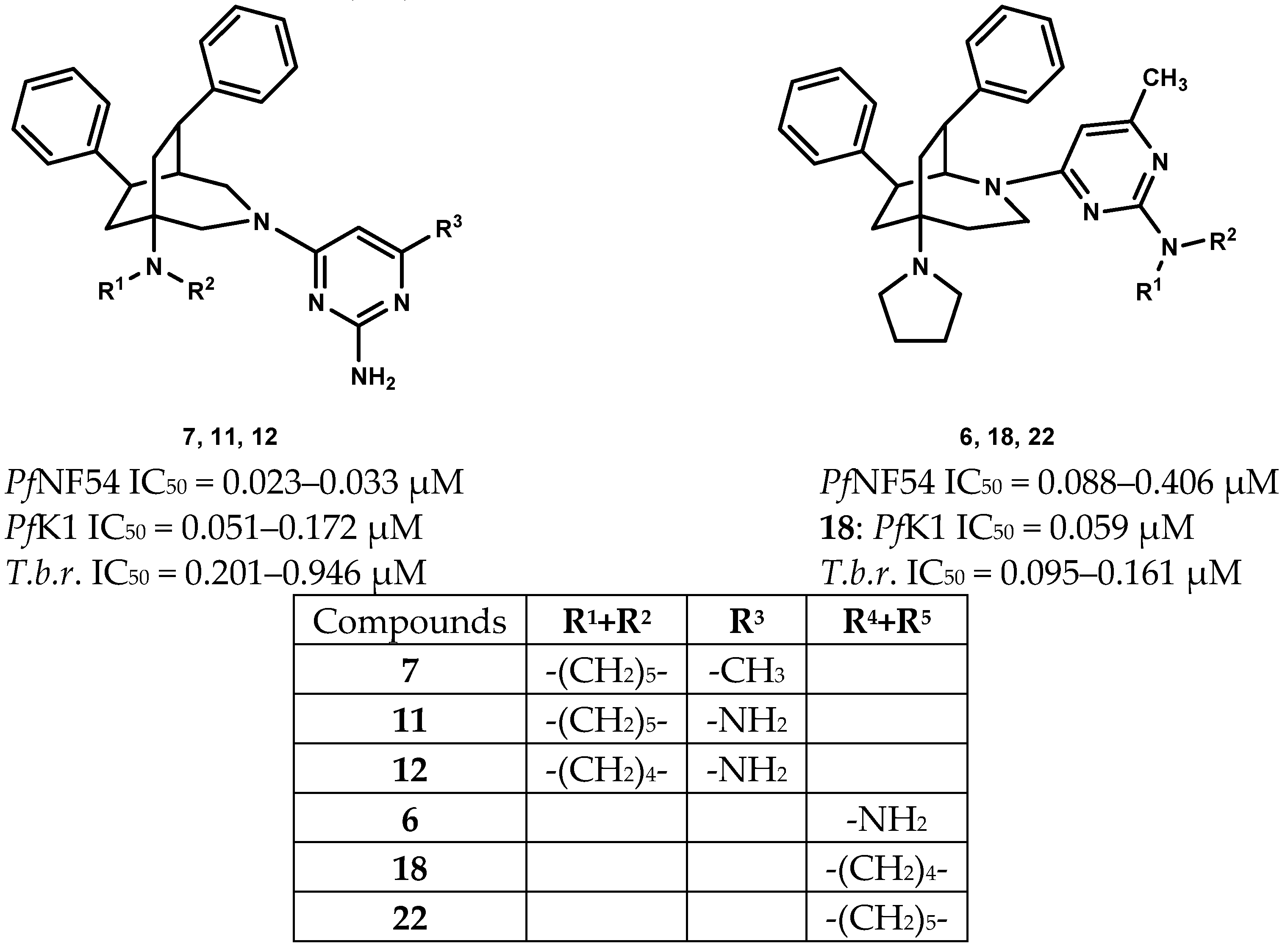
Many of the newly prepared compounds showed activity against P.falciparum NF54 (PfNF54 IC50 = 0.023–0.694 µM) in submicromolar concentration (Table 1). Their activity was highly influenced by the substituents of the pyrimidine scaffold. Compounds 6–12 (PfNF54 IC50 = 0.023–0.088 µM) with a 2,6-diamino or a 2-amino-6-methyl substitution were the most active ones. Within this group the 3-azabicyclo-nonanes (7, 11, 12 (PfNF54 IC50 = 0.023–0.033 µM) were more active than their 2-azabicyclo-nonane analogues 5, 9, 10 (PfNF54 IC50 = 0.051–0.212 µM) (Figure 2). They showed roughly the same activity as pyrimethamine (PfNF54 IC50 = 0.017 µM). The parent N-unsubstituted 3-azabicyclo-nonane 4 was less active (PfNF54 IC50 = 0.072 µM) but highly selective (SI = 1013). In comparison with their pyrrolidino analogues, compounds with piperidino substitution of the bridgehead atom showed higher activity. With the exception of 8 (SI = 72.3), their selectivity indices were very good due to their low cytotoxicity (6, 7, 9–12: SI = 142.5–337.3). Compounds with a 2-dialkylamino- 17–24 (PfNF54 IC50 = 0.087–5.29 µM) or a 2-chloropyrimidine moiety 13–16 (PfNF54 IC50 = 0.281–1.49 µM) were less active and less selective (SI = 4.47–110.7).
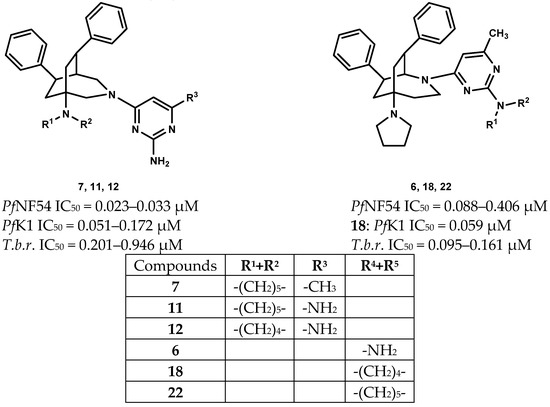
Figure 2.
Most active compounds.
In addition, selected compounds were tested against the multiresistant K1 strain of Plasmodium falciparum. Pyrimethamine was inactive against this strain (PfK1 IC50 = 10.00 µM). In comparison to the activities against the NF54 strain, the observed activities were lower for the 2,6-diamino or 2-amino-6-methyl substituted compounds 7 and 9–12 (PfK1 IC50 = 0.051–0.216 µM). However, compounds 5–7 and 9–11 were more active than their parent azabicyclo-nonanes 1–3. Compounds 7 and 9 exhibited good activity (PfK1 IC50 = 0.051–0.094 µM) and selectivity (SI = 103.6–155.6). In contrast, compounds with a 2-dialkylamino or a 2-chloropyrimidine moiety were by trend more active against the K1 than against the NF54 strain. Highest activities were observed for the bis(pyrrolidino) substituted compounds 18 and 20. The 3-azabicyclo-nonane 20 showed equal activity against both strains (PfK1 IC50 = 0.087 µM). Its activity was comparable to its parent 3-azabicyclo-nonane 4 (PfK1 IC50 = 0.095 µM), but the selectivity was considerably lower. Compared to its parent 2-azabicyclo-nonane 2 (PfK1 IC50 = 0.56 µM; SI = 215.0), compound 18 showed improved activity (PfK1 IC50 = 0.059 µM) and selectivity (SI = 254.5). A further promising issue is its increased activity against the multiresistant strain.
The antitrypanosomal activity of the parent azabicyclo-nonanes 1–4 was moderate (T.b.r. IC50 = 1.00–6.57 µM). Their activity was changed by the substitution with the pyrimidine substituent, but the impact of the substitution of the pyrimidine ring was varying. Compounds with a 2-azabicyclo-nonane skeleton were in general more active than their 3-azabicyclo-nonane analogues, and pyrrolidino substitution of the bridgehead atom furnished higher activity compared to piperidino substitution. The 2-(6-methylpyrimidin-4-yl)-5-pyrrolidino-2-azabicyclo[3.2.2]nonanes 6, 18, 22 showed the highest activity (T.b.r. IC50 = 0.095–0.161 µM) and selectivity (SI = 62.90–158.4). All the other test compounds were by far less selective (SI ≤ 42.63). (Table 1)
The molecular targets and the mechanisms of the antiplasmodial and the antitrypanosomal action of azabicyclo-nonanes are not yet known. The interpretation of test results relies on phenotypic screening, considering that inhibitors act against their antitrypanosomal targets in approximately physiological circumstances.
4. Materials and Methods
4.1. Instrumentation and Chemicals
IR spectra were recorded using a Bruker Alpha Platinum ATR FTIR spectrometer; frequencies are reported in cm−1. NMR spectra: Varian Unity Inova 400 (298 K) 5 mm tubes, TMS as internal standard; 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra are reported in ppm; 1H- and 13C-resonances were assigned using 1H,1H-; and 1H,13C-correlation spectra and are numbered as given in the formulas. Signal multiplicities are abbreviated as follows: br broad, d doublet, dd doublet of doublets, ddd doublet of doublet of doublets, m multiplet, s singlet, t triplet, td triplet of doublets; resonances marked with a single quote belong to the pyrimidine substituent of the compounds (Scheme 1). HRMS: GCT-Premier, Waters (EI, 70 eV). Materials: column chromatography (CC): aluminium oxide 90 (neutral, Merck, Rahway, NJ, USA), aluminium oxide 60 (pH: 9.5, Merck), silica gel 60 (Merck 70–230 mesh, pore-diameter 60 Å), thin-layer chromatography (TLC): TLC plates silica gel 60 F254 (Merck), aluminium oxide 60 F254 (neutral, Merck).
4.2. Syntheses
The syntheses of compounds 1–4 have already been reported elsewhere [12,22,23].
4.2.1. General Procedure for the Syntheses of 5–12
The corresponding azabicyclo[3.2.2]nonane 1, 2, 3, 4, the 4-chloro-6-methylpyrimidin-2-amine, respectively, 6-chloropyrimidine-2,4-diamine and DIPEA were suspended in butan-1-ol. The reaction batch was refluxed for 48h at 145 °C in an atmosphere of Ar. After cooling to room temperature, the mixture was diluted with diethyl ether or CH2Cl2 and alkalized with 2N NaOH. The combined organic phases were washed with water, dried over anhydrous sodium sulfate, filtered and finally the solvent was removed in vacuo giving crude products which were purified by column chromatography.
rac-4-[(7R,8R)-7,8-diphenyl-5-(piperidin-1-yl)-2-azabicyclo[3.2.2]nonan-2-yl]-6-methylpyrimidin-2-amine 5
The reaction of 1.3g 5-(piperidin-1-yl)-2-azabicyclo-nonane 1 (3.61 mmol), 0.535 g 4-chloro-6-methylpyrimidin-2-amine (3.73 mmol) and 0.982 g DIPEA (7.60 mmol) in 50 mL butan-1-ol gave after 48 h a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 19 + 1) yielding 0.715 g 5 (42%, 1.53 mmol) as amorphous solid. IR = 2929, 1584, 1543, 1448, 1416, 1255, 1103, 792, 698; 1H NMR (CDCl3) δ = 1.42–1.50 (m, 2H, CH2), 1.56–1.68 (m, 4H, 2CH2), 1.83 (br, td, J = 13.5, 4.6 Hz, 1H, 4-H), 1.91–2.02 (m, 2H, 4-H, 6-H), 2.10–2.19 (m, 1H, 9-H), 2.16 (s, 3H, CH3), 2.33–2.44 (m, 2H, 6-H, 9-H), 2.51–2.68 (m, 4H, N(CH2)2), 3.02–3.11 (m, 1H, 3-H), 3.22 (t, J = 9.6 Hz, 1H, 7-H), 3.43–3.52 (m, 1H, 8-H), 4.68 (s, 2H, NH2), 5.71 (br, s, 1H, 5’-H), 7.09–7.51 (m, 10H, aromatic H); 13C NMR (CDCl3) δ = 24.06 (CH3), 24.99 (CH2), 26.73 (2CH2), 31.37 (C-4), 36.26 (C-6), 36.60 (C-9), 37.08 (C-8), 41.17 (br, C-3), 46.05 (C-7), 46.30 (N(CH2)2), 56.56 (br, C-1), 58.32 (C-5), 93.30 (C-5′), 126.22, 126.61, 126.79, 127.54, 128.41, 128.59 (aromatic C), 143.47, 144.35 (aromatic Cq), 162.31 (C-4′), 162.52 (C-2′), 165.94 (C-6′); HRMS (EI+) calcd for C30H37N5: 467.3049; found: 467.3050.
rac-4-[(7R,8R)-7,8-diphenyl-5-(pyrrolidin-1-yl)-2-azabicyclo[3.2.2]nonan-2-yl]-6-methylpyrimidin-2-amine 6.
The reaction of 1.32 g 5-(pyrrolidin-1-yl)-2-azabicyclononane 2 (3.81 mmol), 0.547 g 4-chloro-6-methylpyrimidin-2-amine (3.81 mmol) and 0.985 g DIPEA (7.62 mmol) in 50 mL butan-1-ol gave after 48 h 1.54 g 6 (89%, 3.39 mmol) as amorphous solid. IR = 3087, 2952, 2872, 2807, 1639, 1586, 1541, 1497, 1485, 1448, 1417, 1355, 1254, 1213, 1175, 1122, 1032, 1011, 966, 934, 881, 790, 750, 698; 1H NMR (CDCl3) δ = 1.76–1.82 (m, 4H, (CH2)2), 1.92 (br, td, J = 14.2, 4.2 Hz, 1H, 4-H), 1.93–1.99 (m, 1H, 4-H), 2.07–2.20 (m, 2H, 6-H, 9-H), 2.17 (s, 3H, CH3), 2.30–2.37 (m, 1H, 6-H), 2.49 (t, J = 12.3 Hz, 1H, 9-H), 2.69–2.79 (m, 4H, N(CH2)2), 3.09 (ddd, J = 14.6, 11.7, 4.5 Hz, 1H, 3-H), 3.28 (t, J = 9.5 Hz, 1H, 7-H), 3.48–3.56 (m, 1H, 8-H), 4.69 (s, 2H, NH2), 5.73 (br, s, 1H, 5′-H), 7.11-7.50 (m, 10H, aromatic H); 13C NMR (CDCl3) δ = 23.54 ((CH2)2), 24.08 (CH3), 31.63 (C-4), 36.38 (C-8), 37.16 (C-9), 37.38 (C-6), 40.55 (br, C-3), 45.21 (N(CH2)2), 45.71 (C-7), 56.87 (C-1, C-5), 93.28 (C-5′), 126.20, 126.63, 126.74, 127.62, 128.40, 128.62 (aromatic C), 143.42, 144.33 (aromatic Cq), 162.37 (C-4′), 162.50 (C-2′), 166.02 (C-6′); HRMS (EI+) calcd for C29H35N5: 453.2892; found: 453.2914.
rac-4-[(7R,8R)-7,8-diphenyl-1-(piperidin-1-yl)-3-azabicyclo[3.2.2]nonan-3-yl]-6-methylpyrimidin-2-amine 7.
The reaction of 1.17 g 1-(piperidin-1-yl)-3-azabicyclononane 3 (3.25 mmol), 0.480 g 4-chloro-6-methylpyrimidin-2-amine (3.34 mmol) and 0.947 g DIPEA (7.33 mmol) in 45 mL butan-1-ol gave after 48 h a product of 1.35 g 7 (89%, 2.89 mmol) as amorphous solid. IR = 3153, 3027, 2927, 1585, 1494, 1453, 1422, 1307, 1252, 1221, 1182, 1152, 1104, 1011, 963, 792, 755, 733, 700; 1H NMR (CDCl3) δ = 1.45–1.52 (m, 2H, CH2), 1.58–1.66 (m, 4H, 2CH2), 1.90 (dd, J = 13.0, 9.8 Hz, 1H, 8-H), 2.00 (t, J = 11.8 Hz, 1H, 7-H), 2.09–2.17 (m, 2H, 5-H, 8-H), 2.22 (s, 3H, CH3), 2.24–2.33 (m, 1H, 7-H), 2.57–2.65 (m, 2H, 2NCH), 2.81–2.90 (m, 2H, 2NCH), 3.08 (d, J = 12.8 Hz, 1H, 4-H), 3.16 (d, J = 14.6 Hz, 1H, 2-H), 3.35–3.43 (m, 2H, 6-H, 9-H), 3.77–3.85 (m, 1H, 4-H), 4.73 (s, 2H, NH2), 5.04-5.14 (br, 1H, 2-H), 5.75 (s, 1H, 5′-H), 7.07 (d, J = 7.0 Hz, 2H, aromatic H), 7.15–7.43 (m, 8H, aromatic H); 13C NMR (CDCl3) δ = 24.12 (CH3), 25.06 (CH2), 26.82 (2CH2), 30.78 (C-8), 38.19 (C-7), 39.07 (C-9), 43.78 (C-6), 44.91 (C-5), 46.89 (N(CH2)2), 49.14 (C-2), 50.37 (C-4), 60.07 (C-1), 93.20 (C-5′), 126.36, 126.44, 126.68, 128.18, 128.38, 128.77 (aromatic C), 143.46, 146.12 (aromatic Cq), 162.16 (C-2′), 163.80 (C-4′), 166.25 (C-6′); HRMS (EI+) calcd for C30H37N5: 467.3049; found: 467.3051.
rac-4-[(7R,8R)-7,8-diphenyl-1-(pyrrolidin-1-yl)-3-azabicyclo[3.2.2]nonan-3-yl]-6-methylpyrimidin-2-amine 8.
The reaction of 0.935 g 1-(pyrrolidin-1-yl)-3-azabicyclononane 4 (2.70 mmol), 0.410 g 4-chloro-6-methylpyrimidin-2-amine (2.86 mmol) and 0.750 g DIPEA (5.80 mmol) in 45 mL butan-1-ol gave after 48 h a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 19 + 1) yielding 1.00 g 8 (82%, 2.21 mmol) as amorphous solid. IR = 2960, 1583, 1494, 1450, 1419, 1308, 1252, 1219, 1180, 1155, 1032, 965, 792, 753, 699; 1H NMR (CDCl3) δ = 1.78–1.84 (m, 4H, (CH2)2), 1.96 (dd, J = 12.8, 9.9 Hz, 1H, 8-H), 2.06–2.15 (m, 2H, 5-H, 7-H), 2.18–2.25 (m, 1H, 8-H), 2.21 (s, 3H, CH3), 2.36 (ddd, J = 13.0, 8.4, 1.7 Hz, 1H, 7-H), 2.74–2.82 (m, 2H, 2NCH), 2.91–2.99 (m, 2H, 2NCH), 3.14 (br, d, J = 13.2 Hz, 1H, 4-H), 3.26 (d, J = 13.9 Hz, 1H, 2-H), 3.38–3.47 (m, 2H, 6-H, 9-H), 3.77–3.86 (m, 1H, 4-H), 4.73 (s, 2H, NCH2), 5.07 (br, 1H, 2-H), 5.75 (s, 1H, 5′-H), 7.07 (d, J = 7.0 Hz, 2H, aromatic H), 7.15–7.45 (m, 8H, aromatic H); 13C NMR (CDCl3) δ = 23.62 ((CH2)2), 24.12 (CH3), 31.81 (C-8), 38.71 (C-9), 38.82 (C-7), 43.74 (C-6), 45.36 (C-5), 45.70 (N(CH2)2), 49.16 (C-2), 50.35 (C-4), 58.32 (C-1), 93.23 (C-5′), 126.35, 126.46, 126.76, 128.15, 128.34, 128.77 (aromatic C), 143.38, 146.05 (aromatic Cq), 162.19 (C-2′), 163.77 (C-4′), 166.24 (C-6′); HRMS (EI+) calcd for C29H35N5: 453.2892; found: 453.2894.
rac-6-[(7R,8R)-7,8-diphenyl-5-(piperidin-1-yl)-2-azabicyclo[3.2.2]nonan-2-yl]pyrimidine-2,4-diamine 9.
The reaction of 0.160 g 5-(piperidin-1-yl)-2-azabicyclononane 1 (0.44 mmol), 0.098 g 6-chloropyrimidine-2,4-diamine (0.68 mmol) and 0.129 g DIPEA (0.10 mmol) in 7 mL butan-1-ol gave after 48 h a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 19 + 1) yielding 0.027 g 9 (14%, 0.06 mmol) as amorphous solid. IR = 3397, 2929, 1579, 1423, 1240, 1103, 1032, 793, 699; 1H NMR (CDCl3) δ = 1.46–1.53 (m, 2H, CH2), 1.65–1.76 (m, 4H, 2CH2), 1.88 (td, J = 13.5, 4.0 Hz, 1H, 4-H), 1.98 (br, d, J = 13.5 Hz, 1H, 4-H), 2.03–2.19 (m, 2H, 6-H, 9-H), 2.39 (ddd, J = 12.4, 8.9, 2.6 Hz, 1H, 6-H), 2.52 (t, J = 12.0 Hz, 1H, 9-H), 2.58–2.79 (m, 4H, N(CH2)2), 2.98–3.08 (m, 1H, 3-H), 3.25 (t, J = 9.5 Hz, 1H, 7-H), 3.45-3.54 (m, 1H, 8-H), 4.31 (br, s, 2H, NH2), 4.52 (s, 2H, NH2), 5.00 (s, 1H, 5′-H), 7.13–7.36 (m, 8H, aromatic H), 7.48 (d, J = 7.5 Hz, 2H, aromatic H); 13C NMR (CDCl3) δ = 24.66 (CH2), 26.14 (2CH2), 31.62 (C-4), 36.13 (C-6), 36.35 (C-9), 37.09 (C-8), 40.53 (C-3), 45.90 (C-7), 46.44 (N(CH2)2), 56.51 (C-1), 59.79 (C-5), 75.32 (C-5′), 126.30, 126.65, 126.84, 127.53, 128.48, 128.69 (aromatic C), 143.40, 144.29 (aromatic Cq), 162.11 (C-6′), 163.15 (C-4′), 164.17 (C-2′); HRMS (EI+) calcd for C29H36N6: 468.3001; found: 468.2985.
rac-6-[(7R,8R)-7,8-diphenyl-5-(pyrrolidin-1-yl)-2-azabicyclo[3.2.2]nonan-2-yl]pyrimidine-2,4-diamine 10.
The reaction of 0.350 g 5-(pyrrolidin-1-yl)-2-azabicyclononane 2 (2.70 mmol), 0.172 g 6-chloropyrimidine-2,4-diamine (1.19 mmol), 0.278 g DIPEA (2.15 mmol) in 14 mL butan-1-ol gave after 48h a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 19 + 1→9 + 1) yielding 0.100 g 10 (22%, 0.22 mmol) as amorphous solid. IR = 3405, 2936, 1577, 1424, 1237, 1123, 1032, 792, 699; 1H NMR (CDCl3) δ = 1.79 (br, s, 4H, (CH2)2), 1.90–1.95 (m, 2H, 4-H), 2.05–2.17 (m, 2H, 6-H, 9-H), 2.29–2.37 (m, 1H, 6-H), 2.49 (br, t, J = 12.3 Hz, 1H, 9-H), 2.70–2.79 (m, 4H, N(CH2)2), 3.00–3.08 (m, 1H, 3-H), 3.29 (t, J = 9.5 Hz, 1H, 7-H), 3.47–3.55 (m, 1H, 8-H), 4.25 (s, 2H, NH2), 4.47 (s, 2H, NH2), 5.01 (s, 1H, 5′-H), 7.12-7.36 (m, 8H, aromatic H), 7.48 (d, J = 7.6 Hz, 2H, aromatic H); 13C NMR (CDCl3) δ = 23.60 ((CH2)2), 31.73 (C-4), 36.67 (C-8), 37.26 (C-9), 37.68 (C-6), 40.53 (C-3), 45.27 (N(CH2)2), 45.79 (C-7), 56.60 (C-1), 57.07 (C-5), 75.36 (C-5′), 126.17, 126.56, 126.82, 127.59, 128.43, 128.65 (aromatic C), 143.64, 144.65 (aromatic Cq), 162.48 (C-6′), 163.22 (C-4′), 164.52 (C-2′); HRMS (EI+) calcd for C28H34N6: 454.2845; found: 454.2846.
rac-6-[(7R,8R)-7,8-diphenyl-1-(piperidin-1-yl)-3-azabicyclo[3.2.2]nonan-3-yl]pyrimidine-2,4-diamine 11.
The reaction of 0.293 g 1-(piperidin-1-yl)-3-azabicyclononane 3 (0.81 mmol), 0.143 g 6-chloropyrimidine-2,4-diamine (0.99 mmol) and 0.211 g DIPEA (1.69 mmol) in 12 mL butan-1-ol gave after 48 h a crude product which was purified by column chromatography (1. alox neutral, CH + EtOAc + MeOH = 18 + 1 + 1; 2. silica, CH2Cl2 + MeOH = 9 + 1) yielding 0.025 g 11 (6%, 0.05 mmol) as amorphous solid. IR = 3403, 2929, 1578, 1425, 1235, 1152, 1103, 1032, 793, 753, 699; 1H NMR (CDCl3) δ = 1.44–1.52 (m, 2H, CH2), 1.57-1.66 (m, 4H, 2CH2), 1.90 (dd, J = 12.7, 9.9 Hz, 1H, 8-H), 1.98 (t, J = 11.9 Hz, 1H, 7-H), 2.08–2.22 (m, 2H, 5-H, 8-H), 2.24–2.31 (m, 1H, 7-H), 2.47–2.55 (m, 2H, 2NCH), 2.82–2.90 (m, 2H, 2NCH), 3.03 (d, J = 12.5 Hz, 1H, 4-H), 3.13 (d, J = 14.5 Hz, 1H, 2-H), 3.34–3.42 (m, 2H, 6-H, 9-H), 3.67–3.75 (m, 1H, 4-H), 4.35 (s, 2H, NH2), 4.56 (s, 2H, NH2), 5.04 (s, 1H, 5′-H), 5.03–5.07 (m, 1H, 2-H), 7.11 (d, J = 7.0 Hz, 2H, aromatic H), 7.17 (t, J = 7.2 Hz, 1H, aromatic H), 7.22–7.42 (m, 7H, aromatic H); 13C NMR (CDCl3) δ = 25.07 (CH2), 26.79 (2CH2), 30.91 (C-8), 38.04 (C-7), 39.26 (C-9), 43.77 (C-6), 44.86 (C-5), 46.90 (N(CH2)2), 49.44 (C-2), 50.51 (C-4), 60.09 (C-1), 75.38 (C-5′), 126.28, 126.40, 126.69, 128.30, 128.37, 128.75 (aromatic C), 143.69, 146.27 (aromatic Cq), 162.18 (C-6′), 164.56 (C-2′), 164.62 (C-4′); HRMS (EI+) calcd for C29H36N6: 468.3001; found: 468.2999.
rac-6-[(7R,8R)-7,8-diphenyl-1-(pyrrolidin-1-yl)-3-azabicyclo[3.2.2]nonan-3-yl]pyrimidine-2,4-diamine 12.
The reaction of 0.424 g 1-(pyrrolidin-1-yl)-3-azabicyclononane 4 (1.22 mmol), 0.354 g 6-chloropyrimidine-2,4-diamine (2.45 mmol) and 0.403 g DIPEA (3.12 mmol) in 10 mL butan-1-ol gave after 68 h a crude product which was purified by column chromatography (alox basic, CH2Cl2 + MeOH = 29 + 1→19 + 1) yielding 0.147 g 12 (26%, 0.323 mmol) as amorphous solid. IR = 3398, 2933, 1579, 1494, 1428, 1237, 793, 753, 699; 1H NMR (CDCl3) δ = 1.78–1.83 (m, 4H, (CH2)2), 1.96 (dd, J = 13.0, 9.9 Hz, 1H, 8-H), 2.06-2.12 (m, 2H, 5-H, 7-H), 2.27 (ddd, J = 12.3, 10.1, 1.8 Hz, 1H, 8-H), 2.34 (ddd, J = 12.9, 8.6, 1.8 Hz, 1H, 7-H), 2.75–2.81 (m, 2H, NCH2), 2.92–2.97 (m, 2H, NCH2), 3.08 (br d, J = 12.7 Hz, 1H, 4-H), 3.23 (d, J = 14.1 Hz, 1H, 2-H), 3.38-3.45 (m, 2H, 6-H, 9-H), 3.72 (dd, J = 12.8, 4.3 Hz, 1H, 4-H), 4.35 (s, 2H, NH2), 4.56 (s, 2H, NH2), 5.01-5.08 (m, 1H, 2-H), 5.04 (s, 1H, 5′H), 710–7.43 (m, 10H, aromatic H); 13C NMR (CDCl3) δ = 23.63 ((CH2)2), 32.00 (C-8), 38.76 (C-7), 38.92 (C-9), 43.75 (C-6), 45.34 (C-5), 45.71 (N(CH2)2), 49.36 (C-2), 50.49 (C-4), 58.34 (C-1), 75.41 (C-5′), 126.26, 126.40, 126.77, 128.28, 128.36, 128.74 (aromatic C), 143.62, 146.20 (aromatic Cq), 162.24 (C-6′), 164.59 (C-2′, C-4′); HRMS (EI+) calcd for C28H34N6: 454.2845; found: 454.2852.
4.2.2. General Procedure for the Synthesis of 13–16
The respective bicyclononane 1,2,3,4, 2,4-dichloro-6-methylpyrimidine and DIPEA were suspended in ethanol cooling with an ice bath for 2 h. Afterwards the reaction batch was warmed up to 20 °C and stirred until the reaction was completed. The reaction progress was monitored by thin-layer chromatography (silica, CH2Cl2 + MeOH = 19 + 1). When the reaction was completed, the mixture was diluted with CH2Cl2 and alkalized with 2N NaOH. The organic phase was washed with water, dried over anhydrous sodium sulfate, filtered and finally the solvent was removed in vacuo giving crude products which were purified by column chromatography.
rac-(7R,8R)-2-(2-chloro-6-methylpyrimidin-4-yl)-7,8-diphenyl-5-(piperidin-1-yl)-2-azabicyclo[3.2.2]nonane 13.
The reaction of 1.07 g 5-(piperidin-1-yl)-2-azabicyclononane 1 (2.97 mmol), 0.532 g 2,4-dichloro-6-methylpyrimidine (3.27 mmol) and 0.768 g DIPEA (5.94 mmol) in 17 mL ethanol gave after 188 h a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 29 + 1) yielding 0.920 g 13 (64%, 1.89 mmol) as a pale yellow oil. IR = 2931, 1587, 1536, 1495, 1289, 1165, 1089, 757, 699; 1H NMR (CDCl3) δ = 1.43–1.51 (m, 2H, CH2), 1.56–1.68 (m, 4H, 2CH2), 1.88 (td, J = 13.8, 4.7 Hz, 1H, 4-H), 1.98–2.09 (m, 2H, 4-H, 6-H), 2.13–2.31 (m, 4H, 9-H, CH3), 2.33–2.43 (m, 2H, 6-H, 9-H), 2.52–2.70 (m, 4H, N(CH2)2), 3.17–3.28 (m, 2H, 3-H, 7-H), 3.38–3.57 (m, 1H, 8-H), 5.97 (br, 1H, 5′-H), 7.08–7.40 (m, 10H, aromatic H); 13C NMR (CDCl3) δ = 23.96 (CH3), 24.90 (CH2), 26.64 (2CH2), 31.41 (C-4), 35.99 (C-6, C-9), 37.39 (C-8), 41.24 (C-3), 46.05 (C-7), 46.36 (N(CH2)2), 58.29 (C-5), 99.98 (C-5′), 126.54, 126.79, 126.95, 127.42, 128.56, 128.82 (aromatic C), 142.65, 143.58 (aromatic Cq), 160.10 (C-2′), 162.65 (C-4′), 167.48 (C-6′); HRMS (EI+) calcd for C30H35ClN4: 486.2550; found: 486.2554.
rac-(7R,8R)-2-(2-chloro-6-methylpyrimidin-4-yl)-7,8-diphenyl-5-(pyrrolidin-1-yl)-2-azabicyclo[3.2.2]nonane 14.
The reaction of 0.949 g 5-(pyrrolidin-1-yl)-2-azabicyclononane 2 (2.74 mmol), 0.490 g 2,4-dichloro-6-methylpyrimidine (3.01 mmol) and 0.701 g DIPEA (5.42mmol) in 15 mL ethanol gave after 44 h a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 29 + 1) yielding 0.610 g 14 (47%, 1.29 mmol) as a pale yellow oil. IR = 2955, 1588, 1485, 1358, 1290, 1208, 1165, 1049, 757, 700; 1H NMR (CDCl3) δ = 1.76–1.85 (m, 4H, (CH2)2), 1.91–2.07 (m, 2H, 4-H), 2.13–2.28 (m, 5H, 6-H, 9-H, CH3), 2.29–2.39 (m, 1H, 6-H), 2.49 (t, J = 12.3 Hz, 1H, 9-H), 2.70–2.81 (m, 4H, N(CH2)2), 3.23–3.34 (m, 2H, 3-H, 7-H), 3.47–3.59 (m, 1H, 8-H), 6.01 (br, 1H, 5′-H), 7.08–7.65 (m, 10H, aromatic H); 13C NMR (CDCl3) δ = 23.58 ((CH2)2), 23.99 (CH3), 31.73 (C-4), 35.04 (C-8), 36.61 (C-6, C-9), 40.82 (C-3), 45.08 (C-7), 45.28 (N(CH2)2), 56.82 (C-1), 59.08 (C-5), 100.02 (C-5′), 126.53, 126.78, 126.98, 127.61, 128.58, 128.88 (aromatic C), 142.65, 143.36 (aromatic Cq), 160.15 (C-2′), 162.66 (C-4′), 167.51 (C-6′); HRMS (EI+) calcd for C29H33ClN4: 472.2394; found: 472.2390.
rac-(7R,8R)-3-(2-chloro-6-methylpyrimidin-4-yl)-6,9-diphenyl-1-(piperidin-1-yl)-3-azabicyclo[3.2.2]nonane 15.
The reaction of 1.05 g 1-(piperidin-1-yl)-3-azabicyclononane 3 (2.91 mmol), 0.522 g 2,4-dichloro-6-methylpyrimidine (3.20 mmol) and 0.764 g DIPEA (5.91 mmol) in 17 mL ethanol gave after 140h a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 29 + 1) yielding 0.400 g 15 (28%, 0.82 mmol) as a pale yellow oil. IR = 2929, 1589, 1495, 1308, 1247, 1229, 1174, 1061, 753, 699; 1H NMR (CDCl3) δ = 1.46–1.54 (m, 2H, CH2), 1.61–1,69 (m, 4H, 2CH2), 1.94 (br, t, J = 11.2 Hz, 1H, 8-H), 2.00–2.12 (m, 2H, 7-H, 8-H), 2.22 (br, s, 1H, 5-H), 2.32 (s, 3H, CH3), 2.28–2.37 (m, 1H, 7-H), 2.58–2.68 (m, 2H, 2NCH), 2.84–2.95 (m, 2H, 2NCH), 3.17 (br, d, J = 12.2. Hz, 1H, 4-H), 3.27 (br, d, J = 14.5 Hz, 1H, 2-H), 3.37–3.46 (m, 2H, 6-H, 9-H), 3.75 (br, 1H, 4-H), 5.13 (br, 1H, 2-H), 6.10 (br, s, 1H, 5′-H), 7.02 (d, J = 7.2 Hz, 2H, aromatic H), 7.16–7.43 (m, 8H, aromatic H); 13C NMR (CDCl3) δ = 24.02 (CH3), 24.96 (CH2), 26.74 (2CH2), 30.20 (C-8), 38.16 (C-7), 38.57 (C-9), 43.70 (C-6), 44.92 (C-5), 46.94 (N(CH2)2), 49.81 (C-2), 50.68 (C-4), 60.20 (C-1), 99.85 (C-5′), 126.58, 126.65, 126.66, 127.87, 128.50, 128.88 (aromatic C), 142.86, 145.71 (aromatic Cq), 159.94 (C-2′), 163.69 (C-4′), 167.71 (C-6′); HRMS (EI+) calcd for C30H35ClN4: 486.2550; found: 486.2571.
rac-(7R,8R)-3-(2-chloro-6-methylpyrimidin-4-yl)-6,9-diphenyl-1-(pyrrolidin-1-yl)-3-azabicyclo[3.2.2]nonane 16.
The reaction 0.385 g of 1-(pyrrolidin-1-yl)-3-azabicyclononane 4 (1.11 mmol), 0.199 g 2,4-dichloro-6-methylpyrimidine (1.22 mmol) and 0.291 g DIPEA (2.25 mmol) in 6 mL ethanol gave after 44 h a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 19 + 1) yielding 0.225 g 16 (45%, 0.50 mmol) as a pale yellow oil. IR = 2926, 1589, 1494, 1173, 1056, 753, 700; 1H NMR (CDCl3) δ = 1.80–1.87 (m, 4H, (CH2)2), 2.00 (br, t, J = 11.5 Hz, 1H, 8-H), 2.09–2.23 (m, 3H, 5-H, 7-H, 8-H), 2.32 (s, 3H, CH3), 2.35–2.42 (m, 1H, 7-H), 2.74–2.82 (m, 2H, 2NCH), 2.93–3.00 (m, 2H, 2NCH), 3.18–3.51 (m, 4H, 2-H, 4-H, 6-H, 9-H), 3.77 (br, 1H, 4-H), 5.08 (br, 1H, 2-H), 6.10 (br, s, 1H, 5′-H), 7.02 (d, J = 7.1 Hz, 2H, aromatic H), 7.16–7.43 (m, 8-H, aromatic H); 13C NMR (CDCl3) δ = 23.67 ((CH2)2), 24.00 (CH3), 31.55 (C-8), 38.20 (C-9), 38.90 (C-7), 43.64 (C-6), 45.26 (C-5), 45.74 (N(CH2)2), 49.58 (C-2), 50.69 (C-4), 58.35 (C-1), 99.90 (C-5′), 126.56, 126.66, 126.75, 127.85, 128.49, 128.88 (aromatic C), 142.84, 145.68 (aromatic Cq), 159.94 (C-2′), 163.65 (C-4′), 167.65 (C-6′); HRMS (EI+) calcd for C29H33ClN4: 472.2394; found: 472.2397.
4.2.3. General Procedure for the Synthesis of 17–20
The respective bicyclononane derivate 19–22 was mixed with excess pyrrolidine and acetonitrile and was refluxed at 105 °C for 20 h. The reaction progress was monitored by thin-layer chromatography (silica, CH2Cl2 + MeOH = 19 + 1 and 9 + 1). When the reaction was completed, the mixture was diluted with CH2Cl2 and alkalized with 2N NaOH. The organic phase was washed with water, dried over anhydrous sodium sulfate, filtered and finally the solvent was removed in vacuo giving crude products which were purified by column chromatography.
rac-(7R,8R)-2-[6-methyl-2-(pyrrolidin-1-yl)pyrimidin-4-yl]-7,8-diphenyl-5-(piperidin-1-yl)-2-azabicyclo[3.2.2]nonane 17.
The reaction of 0.150 g 13 (0.31 mmol) and 0.291 g pyrrolidine (3.10 mmol) in 10 mL acetonitrile gave a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 29 + 1) yielding 0.110 g 17 (68%, 0.21 mmol) as amorphous solid. IR = 2930, 2854, 1571, 1449, 1414, 1342, 1251, 1220, 1174,1104, 1032, 787, 698; 1H NMR (CDCl3) δ = 1.42–1.50 (m, 2H, CH2), 1.56–1.66 (m, 4H, 2CH2), 1.79–1.87 (m, 4H, (CH2)2), 1.85–2.05 (m, 3H, 4-H, 6-H), 2.12 (br, t, J = 10.8 Hz, 1H, 9-H), 2.20 (s, 3H, CH3), 2.31–2.44 (m, 2H, 6-H, 9-H), 2.51–2.70 (m, 4H, N(CH2)2), 3.07 (br, t, J = 12.3 Hz, 1H, 3-H), 3.22 (t, J = 9.5 Hz, 1H, 7-H), 3.34–3.50 (m, 5H, 8-H, N(CH2)2), 5.63 (s, 1H, 5′-H), 7.09–7.25 (m, 6H, aromatic H), 7.31 (t, J = 7.4 Hz, 2H, aromatic H), 7.45 (d, J = 7.5 Hz, 2H, aromatic H); 13C NMR (CDCl3) δ = 24.61 (CH3), 25.02 (CH2), 25.46 ((CH2)2), 26.73 (2CH2), 31.55 (C-4), 36.57 (C-6, C-9), 37.41 (C-8), 41.00 (C-3), 46.21 (C-7), 46.36 (N(CH2)2), 46.40 (N(CH2)2), 55.84 (C-1), 58.48 (C-5), 90.98 (C-5′), 126.08, 126.40, 126.88, 127.52, 128.37, 128.43 (aromatic C), 143.83, 144.78 (aromatic Cq), 160.43 (C-2′), 162.00 (C-4′), 165.80 (C-6′); HRMS (EI+) calcd for C34H43N5: 521.3519; found: 521.3530.
rac-(7R,8R)-2-[6-methyl-2-(pyrrolidin-1-yl)pyrimidin-4-yl]-7,8-diphenyl-5-(pyrrolidin-1-yl)-2-azabicyclo[3.2.2]nonane 18.
The reaction of 0.120 g 14 (0.25 mmol) and 0.178 g pyrrolidine (2.50 mmol) in 8mL acetonitrile gave a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 29 + 1) yielding 0.072 g 18 (56%, 0.14 mmol) as amorphous solid. IR = 2946, 2869, 1571, 1447, 1413, 1341, 1251, 1173, 787, 698; 1H NMR (CDCl3) δ = 1.79–1.90 (m, 8H, 2(CH2)2), 1.96–2.02 (m, 2H, 4-H), 2.13–2.22 (m, 2H, 6-H, 9-H), 2.21 (s, 3H, CH3), 2.30–2.38 (m, 1H, 6-H), 2.52–2.61 (m, 1H, 9-H), 2.77–2.88 (m, 4H, N(CH2)2), 3.05–3.15 (m, 1H, 3-H), 3.29 (t, J = 9.5 Hz, 1H, 7-H), 3.40–3.57 (m, 5H, 8-H, N(CH2)2), 5.64 (s, 1H, 5′-H), 7.10–7.25 (m, 6H, aromatic H), 7.34 (t, J = 7.5 Hz, 2H, aromatic H), 7.47 (d, J = 7.5 Hz, 2H, aromatic H); 13C NMR (CDCl3) δ = 23.67 ((CH2)2), 24.58 (CH3), 25.47 ((CH2)2), 31.94 (C-4), 36.60 (C-8), 36.80 (C-9), 37.31 (C-6), 40.18 (C-3), 45.53 (N(CH2)2), 45.81 (C-7), 46.45 (N(CH2)2), 55.77 (C-1), 57.86 (C-5), 91.01 (C-5′), 126.15, 126.54, 126.82, 127.61, 128.42, 128.52 (aromatic C), 143.52, 144.45 (aromatic Cq), 160.38 (C-2′), 161.96 (C-4′), 165.85 (C-6′); HRMS (EI+) calcd for C33H41N5: 507.3362; found: 507.3366.
rac-(7R,8R)-3-[6-methyl-2-(pyrrolidin-1-yl)pyrimidin-4-yl]-6,9-diphenyl-1-(piperidin-1-yl)-3-azabicyclo[3.2.2]nonane 19.
The reaction of 0.139 g 15 (0.29 mmol), 0.244 g pyrrolidine (3.38 mmol) and 0.088 g DIPEA (0.68 mmol) in 8mL acetonitrile gave a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 19 + 1) yielding 0.130 g 19 (86%, 0.25 mmol) as amorphous solid. IR = 2928, 1572, 1453, 1419, 1306, 1247, 1218, 1179, 787, 753, 699; 1H NMR (CDCl3) δ = 1.44–1.52 (m, 2H, CH2), 1.58–1.68 (m, 4H, 2CH2), 1.84–1.99 (m, 5H, 8-H, (CH2)2), 2.00–2.16 (m, 3H, 5-H, 7-H, 8-H), 2.18–2.23 (m, 1H, 7-H), 2.24 (s, 3H, CH3), 2.54–2.64 (m, 2H, 2NCH), 2.91–3.01 (m, 2H, 2NCH), 3.05 (d, J = 12.8 Hz, 1H, 4-H), 3.17 (br, d, J = 14.6 Hz, 1H, 2-H), 3.38 (br, t, J = 9.3 Hz, 1H, 9-H), 3.45 (t, J = 9.4 Hz, 1H, 6-H), 3.50–3.67 (m, 4H, N(CH2)2), 3.80 (br, 1H, 4-H), 5.39 (br, 1H, 2-H), 5.62 (s, 1H, 5′-H), 7.09 (d, J = 7.2 Hz, 2H, aromatic H), 7.15–7.44 (m, 8H, aromatic H); 13C NMR (CDCl3) δ = 24.61 (CH3), 25.08 (CH2), 25.60 ((CH2)2), 26.64 (2CH2), 29.53 (C-8), 38.92 (C-7), 39.43 (C-9), 44.02 (C-6), 45.03 (C-5), 46.55 (N(CH2)2), 47.14 (N(CH2)2), 49.32 (C-2), 50.31 (C-4), 60.48 (C-1), 90.85 (C-5′), 126.38, 126.44, 126.69, 128.36, 128.39, 128.79 (aromatic C), 143.62, 146.25 (aromatic Cq), 160.27 (C-2′), 163.07 (C-4′), 166.06 (C-6′); HRMS (EI+) calcd for C34H43N5: 521.3519; found: 521.3555.
rac-(7R,8R)-3-[6-methyl-2-(pyrrolidin-1-yl)pyrimidin-4-yl]-6,9-diphenyl-1-(pyrrolidin-1-yl)-3-azabicyclo[3.2.2]nonane 20.
The reaction of 0.080 g 16 (0.17 mmol), 0.051 g pyrrolidine (0.72 mmol) and 0.071 g DIPEA (0.55 mmol) in 3mL acetonitrile gave a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 19 + 1) yielding 0.029 g 20 (33%, 0.06 mmol) as amorphous solid. IR = 2961, 1572, 1453, 1419, 1177, 753, 699; 1H NMR (CDCl3) δ = 1.80–1.87 (m, 4H, (CH2)2), 1.91–2.00 (m, 5H, 8-H, N(CH2)2), 2.10 (br, s, 1H, 5-H), 2.12–2.21 (m, 1H, 7-H), 2.24 (s, 3H, CH3), 2.27 (br, t, J = 12.0 Hz, 1H, 8-H), 2.34–2.42 (m, 1H, 7-H), 2.80–2.88 (m, 2H, 2NCH), 3.01–3.09 (m, 2H, 2NCH), 3.10 (d, J = 13.2 Hz, 1H, 4-H), 3.25 (d, J = 14.4 Hz, 1H, 2-H), 3.39–3.48 (m, 2H, 6-H, 9-H), 3.52–3.64 (m, 4H, N(CH2)2), 3.84 (br, 1H, 4-H), 5.34 (br, 1H, 2-H), 5.64 (s, 1H, 5′-H), 7.08 (d, J = 7.3 Hz, 2H, aromatic H), 7.15–7.44 (m, 8H, aromatic H); 13C NMR (CDCl3) δ = 23.99 ((CH2)2), 24.63 (CH3), 25.60 ((CH2)2), 32.07 (C-8), 38.97 (C-9), 39.11 (C-7), 43.98 (C-6), 45.59 (C-5), 46.00 (N(CH2)2), 46.51 (N(CH2)2), 48.24 (C-2), 50.43 (C-4), 59.07 (C-1), 90.94 (C-5′), 126.36, 126.47, 126.80, 128.32, 128.38, 128.79 (aromatic C), 143.51, 146.02 (aromatic Cq), 160.30 (C-2′), 163.11 (C-4′), 166.16 (C-6′); HRMS (EI+) calcd for C33H41N5: 507.3362; found: 507.3346.
4.2.4. General Procedure for the Synthesis of 21–24
The respective bicyclononane derivate 13–16 was mixed with excess piperidine and acetonitrile and was refluxed at 105 °C for 48 h. The reaction progress was monitored by thin-layer chromatography (silica, CH2Cl2 + MeOH = 9+1). When the reaction was completed, the mixture was diluted with CH2Cl2 and alkalized with 2N NaOH. The organic phase was washed with water, dried over anhydrous sodium sulfate, filtered and finally the solvent was removed in vacuo giving crude products which were purified by column chromatography.
rac-(7R,8R)-2-[6-methyl-2-(piperidin-1-yl)pyrimidin-4-yl]-7,8-diphenyl-5-(piperidin-1-yl)-2-azabicyclo[3.2.2]nonane 21.
The reaction of 0.125 g 13 (0.26 mmol) and 0.219 g piperidine (2.57 mmol) in 7 mL acetonitrile gave a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 49 + 1) yielding 0.095 g 21 (69%, 0.18 mmol) as amorphous solid. IR = 2930, 2850, 1572, 1445, 1414, 1245, 1031, 787, 698; 1H NMR (CDCl3) δ = 1.41–1.72 (m, 12H, 6CH2), 1.82–2.03 (m, 3H, 4-H, 6-H), 2.07–2.17 (m, 1H, 9-H), 2.18 (s, 3H, CH3), 2.33–2.44 (m, 2H, 6-H, 9-H), 2.52–2.71 (m, 4H, N(CH2)2), 3.08 (br, t, J = 12.4 Hz, 1H, 3-H), 3.23 (t, J = 9.5 Hz, 1H, 7-H), 3.44–3.52 (m, 1H, 8-H), 3.60 (br, s, 4H, N(CH2)2), 5.61 (s, 1H, 5′-H), 7.10–7.34 (m, 8H, aromatic H), 7.43 (d, J = 7.5 Hz, 2H, aromatic H); 13C NMR (CDCl3) δ = 24.65 (CH3), 25.03, 25.90, 26.71 (6CH2), 31.65 (C-4), 36.39 (C-9), 36.57 (C-6), 37.37 (C-8), 40.95 (C-3), 44.76 (N(CH2)2), 46.20 (C-7), 46.41 (N(CH2)2), 55.73 (C-1), 58.44 (C-5), 91.15 (C-5′), 126.12, 126.42, 126.90, 127.51, 128.40, 128.51 (aromatic C), 143.76, 144.77 (aromatic Cq), 161.71 (C-2′), 162.15 (C-4′), 165.94 (C-6′); HRMS (EI+) calcd for C35H45N5: 535.3675; found: 535.3716.
rac-(7R,8R)-2-[6-methyl-2-(piperidin-1-yl)pyrimidin-4-yl]-7,8-diphenyl-5-(pyrrolidin-1-yl)-2-azabicyclo[3.2.2]nonane 22.
The reaction of 0.100 g 14 (0.21 mmol) and 0.186 g piperidine (2.18 mmol) in 7 mL acetonitrile gave a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 29 + 1) yielding 0.067 g 22 (62%, 0.13 mmol) as amorphous solid. IR = 2929, 2850, 1572, 1496, 1445, 1414, 1355, 1244, 1029, 787, 699; 1H NMR (CDCl3) δ = 1.45–1.54 (m, 4H, (CH2)2), 1.55–1.62 (m, 2H, CH2), 1,76–1.84 (m, 4H, 2CH2), 1.94–2.01 (m, 2H, 4-H), 2.06–2.17 (m, 2H, 6-H, 9-H), 2.18 (s, 3H, CH3), 2.28–2.38 (m, 1H, 6-H), 2.51 (t, J = 12.3 Hz, 1H, 9-H), 2.72–2.84 (m, 4H, N(CH2)2), 3.04–3.15 (m, 1H, 3-H), 3.29 (t, J = 9.6 Hz, 1H, 7-H), 3.49–3.57 (m, 1H, 8-H), 3.58–3.66 (m, 4H, N(CH2)2), 5.62 (s, 1H, 5′-H), 7.10–7.25 (m, 6H, aromatic H), 7.32 (t, J = 7.5 Hz, 2H, aromatic H), 7.45 (d, J = 7.5 Hz, 2H, aromatic H); 13C NMR (CDCl3) δ = 23.64 (2CH2), 24.66 (CH3), 25.03 (CH2), 25.90 ((CH2)2), 31.92 (C-4), 36.70 (C-8), 36.92 (C-9), 37.58 (C-6), 40.81 (C-3), 44.76 (N(CH2)2), 45.36 (N(CH2)2), 45.91 (C-7), 55.74 (C-1), 57.19 (C-5), 91.15 (C-5′), 126.11, 126.46, 126.83, 127.59, 128.39, 128.53 (aromatic C), 143.67, 144.71 (aromatic Cq), 161.74 (C-2′), 162.14 (C-4′), 165.97 (C-6′); HRMS (EI+) calcd for C34H43N5: 521.3519; found: 521.3533.
rac-(7R,8R)-3-[6-methyl-2-(piperidin-1-yl)pyrimidin-4-yl]-6,9-diphenyl-1-(piperidin-1-yl)-3-azabicyclo[3.2.2]nonane 23.
The reaction of 0.126 g 15 (0.26 mmol) and 0.218 g piperidine (2.56 mmol) in 8mL acetonitrile gave a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 29 + 1) yielding 0.094 g 23 (69%, 0.18 mmol) as amorphous solid. IR = 2929, 2850, 1573, 1444, 1418, 1306, 1244, 1212, 1099, 1025, 786, 753, 699; 1H NMR (CDCl3) δ = 1.42–1.51 (m, 2H, CH2), 1.54–1.70 (m, 10H, 5CH2), 1.83–1.92 (m, 1H, 8-H), 1.98–2.16 (m, 3H, 5-H, 7-H, 8-H), 2.17–2.25 (m, 1H, 7-H), 2.22 (s, 3H, CH3), 2.54–2.64 (m, 2H, 2NCH), 2.83–2.94 (m, 2H, 2NCH), 3.03 (d, J = 12.8 Hz, 1H, 4-H), 3.17 (br, d, J = 14.4 Hz, 1H, 2-H), 3.37 (br, t, J = 9.3 Hz, 1H, 9-H), 3.45 (t, J = 9.3 Hz, 1H, 6-H), 3.74–3.87 (m, 5H, 4-H, N(CH2)2), 5.33 (br, 1H, 2-H), 5.61 (s, 1H, 5′-H), 7.10 (d, J = 7.3 Hz, 2H, aromatic H), 7.15–7.43 (m, 8H, aromatic H); 13C NMR (CDCl3) δ = 24.66 (CH3), 25.05 (2CH2), 25.92 (2CH2), 26.71 (2CH2), 29.57 (C-8), 38.85 (C-7), 39.41 (C-9), 43.99 (C-6), 44.83 (N(CH2)2), 44.98 (C-5), 47.17 (N(CH2)2), 49.38 (C-2), 50.21 (C-4), 60.42 (C-1), 90.94 (C-5′), 126.39, 126.45, 126.70, 128.36, 128.40, 128.78 (aromatic C), 143.68, 146.22 (aromatic Cq), 161.50 (C-2′), 163.04 (C-4′), 166.29 (C-6′); HRMS (EI+) calcd for C35H45N5: 535.3675; found: 535.3695.
rac-(7R,8R)-3-[6-methyl-2-(piperidin-1-yl)pyrimidin-4-yl]-6,9-diphenyl-1-(pyrrolidin-1-yl)-3-azabicyclo[3.2.2]nonane 24.
The reaction of 0.090 g 16 (0.19 mmol) and 0.173 g piperidine (2.03 mmol) in 5mL acetonitrile gave a crude product which was purified by column chromatography (silica, CH2Cl2 + MeOH = 19 + 1) yielding 0.054 g 24 (53%, 0.10 mmol) as amorphous solid. IR = 2926, 2850, 1551, 1442, 1416, 1288, 1243, 1180, 1024, 786, 751, 698; 1H NMR (CDCl3) δ = 1.56–1.69 (m, 6H, (CH2)3), 1.78–1.85 (m, 4H, (CH2)2), 1.95 (br, t, J = 11.3 Hz, 1H, 8-H), 2.11 (br, s, 1H, 5-H), 2.14 (br, t, J = 11.6 Hz, 1H, 7-H), 2.22 (s, 3H, CH3), 2.24 (br, t, J = 12.1 Hz, 1H, 8-H), 2.34–2.40 (m, 1H, 7-H), 2.77–2.86 (m, 2H, 2NCH), 2.94–3.03 (m, 2H, 2NCH), 3.10 (d, J = 12.9 Hz, 1H, 4-H), 3.25 (d, J = 14.3 Hz, 1H, 2-H), 3.38–3.47 (m, 2H, 6-H 9-H), 3.74–3.81 (m, 4H, N(CH2)2), 3.86 (br, 1H, 4-H), 5.22 (br, 1H, 2-H), 5.64 (s, 1H, 5′-H), 7.09 (d, J = 7.4 Hz, 2H, aromatic H), 7.15–7.44 (m, 8H, aromatic H); 13C NMR (CDCl3) δ = 23.99 ((CH2)2), 24.67 (CH3), 25.04 (CH2), 25.87 (2CH2), 32.09 (C-8), 38.98 (C-9), 39.17 (C-7), 43.94 (C-6), 44.84 (N(CH2)2), 45.52 (C-5), 45.91 (N(CH2)2), 48.52 (C-2), 50.34 (C-4), 58.76 (C-1), 91.07 (C-5′), 126.34, 126.44, 126.79, 128.32, 128.37, 128.77 (aromatic C), 143.58, 146.09 (aromatic Cq), 161.50 (C-2′), 163.14 (C-4′), 166.23 (C-6′); HRMS (EI+) calcd for C34H43N5: 521.3519; found: 521.3546.
4.3. Biological Tests
4.3.1. In Vitro Microplate Assay against P. falciparum
In vitro activity against erythrocytic stages of P. falciparum was determined using a 3H-hypoxanthine incorporation assay [25,26] using the drug sensitive NF54 strain [27] or the chloroquine- and pyrimethamine-resistant K1 strain [28]. Compounds were dissolved in DMSO at 10 mg/mL and further diluted in medium before being added to parasite cultures incubated in RPMI 1640 medium without hypoxanthine, supplemented with HEPES (5.94 g/L), NaHCO3 (2.1 g/L), neomycin (100 U/mL), Albumax® (5 g/L) and washed human red cells A+ at 2.5% haematocrit (0.3% parasitaemia). Serial drug dilutions of eleven 3-fold dilution steps covering a range from 100 to 0.002 μg/mL were prepared. The 96-well plates were incubated in a humidified atmosphere at 37 °C; 4% CO2, 3% O2, 93% N2. After 48 h, 0.05 mL of 3H-hypoxanthine (= 0.5 μCi) was added to each well of the plate. The plates were incubated for a further 24 h under the same conditions. The plates were then harvested with a Betaplate™ cell harvester (Wallac, Zurich, Switzerland), and the red blood cells transferred onto a glass fibre filter then washed with distilled water. The dried filters were inserted into a plastic foil with 10 mL of scintillation fluid, and counted in a Betaplate™ liquid scintillation counter (Wallac, Zurich, Switzerland). IC50 values were calculated from sigmoidal inhibition curves by linear regression [29] using Microsoft Excel. Chloroquine (Sigma C6628) was used as control.
4.3.2. In Vitro Microplate Assay against T. brucei rhodesiense
Minimum essential medium (50 µL) supplemented with 25 mM HEPES, 1g/L additional glucose, 1% MEM non-essential amino acids (100×), 0.2 mM 2-mercaptoethanol, 1mM Na-pyruvate and 15% heat-inactivated horse serum was added to each well of a 96-well microtiter plate. Serial drug dilutions of eleven 3-fold dilution steps covering a range from 100 to 0.002 μg/mL were prepared. Then 4 × 103 bloodstream forms of T. b. rhodesiense STIB 900 in 50 µL was added to each well and the plate incubated at 37 °C under a 5 % CO2 atmosphere for 70 h. Next, 10 µL resazurin solution (resazurin, 12.5 mg in 100 mL double-distilled water) was added to each well and incubation continued for a further 2–4 h [30]. Then the plates were read with a Spectramax Gemini XS microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA, USA) using an excitation wave length of 536 nm and an emission wave length of 588 nm. Data were analyzed with the graphic programme Softmax Pro (Molecular Devices Cooperation, Sunnyvale, CA, USA), which calculated IC50 values by linear regression [29] and 4-parameter logistic regression from the sigmoidal dose inhibition curves. Melarsoprol (Arsobal Sanofi-Aventis, received from WHO) was used as control.
4.3.3. In Vitro Cytotoxicity with L-6 Cells
Assays were performed in 96-well microtiter plates, each well containing 0.1 mL of RPMI 1640 medium supplemented with 1% L-glutamine (200 mM) and 10% fetal bovine serum, and 4000 L-6 cells (a primary cell line derived from rat skeletal myoblasts, ATCC CRL-1458™) [31,32]. Serial drug dilutions of eleven 3-fold dilution steps covering a range from 100 to 0.002 μg/mL were prepared. After 70 h of incubation the plates were inspected under an inverted microscope to assure growth of the controls and sterile conditions. Then, 0.01 mL resazurin solution (resazurin, 12.5 mg in 100 mL double-distilled water) was added to each well and the plates incubated for another 2 h. The plates were read with a Spectramax Gemini XS microplate fluorometer (Molecular Devices Cooperation, Sunnyvale, CA, USA) using an excitation wave length of 536 nm and an emission wave length of 588 nm. The IC50 values were calculated by linear regression [29] from the sigmoidal dose inhibition curves using SoftmaxPro software (Molecular Devices Cooperation, Sunnyvale, CA, USA). Podophyllotoxin (Sigma P4405) was used as control.
5. Conclusions
The synthesis and the evaluation of the structure–activity relationships of the antiprotozoal activities of azabicyclo-nonane pyrimidine hybrids were reported. A number of structure–activity relationships were revealed. A couple of 1-piperidino-3-azabicyclo-nonanes with a 2-aminopyrimidine moiety exhibited in vitro activity against P. falciparum NF54 in submicromolar concentration and high selectivity. A 2-azabicyclo-nonane with a pyrrolidino substituent on both the bicyclic ring system as well as on the pyrimidine ring even possessed improved activity against the multiresistant K1 strain of P. falciparum. Three 5-pyrrolidino-2-azabicyclo-nonanes have shown good trypanocidal activity and selectivity. Further modifications of the substitution pattern of the pyrimidine moiety will be investigated in a future project.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules28010307/s1: 1H-, 13C-NMR and MS-spectra; synthesis route of compounds 1–4.
Author Contributions
Conceptualization, C.H. and R.W.; Data curation, C.H., J.D., W.S., R.S., P.M., M.K. and R.W.; Investigation, C.H., R.S., P.M., M.K. and R.W.; Methodology, C.H., J.D., W.S. and R.W.; Project administration, C.H. and R.W.; Supervision, R.W.; Writing—original draft, C.H., J.D. and R.W.; Writing—review and editing, J.D. and R.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors acknowledge Open Access Funding by the University of Graz.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of selected compounds are not available from the authors.
References
- WHO. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 29 December 2022).
- Ashley, E.A.; Dhorda, M.; Fairhust, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of Artemisinin Restistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Manna, S.; Saha, B.; Hati, A.K.; Roy, S. Novel pfkelch13 Gene Polymorphism Associates with Artemisinin Resistance in Eastern India. Clin. Infect. Dis. 2019, 69, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- WHO Factsheet. Available online: https://www.who.int/news-room/fact-sheets/detail/trypanosomiasis-human-african-(sleeping-sickness) (accessed on 27 September 2022).
- DNDi. Drugs for Neglected Diseases Initiative. Available online: https://dndi.org/diseases/sleeping-sickness (accessed on 27 September 2022).
- Waller, D.G.; Sampson, A.P. 51-Chemotherapy of infections. In Medical Pharmacology and Therapeutics, 5th ed.; Waller, D.G., Sampson, A.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 581–629. [Google Scholar] [CrossRef]
- Kuhlmann, M.F.; Fleckenstein, J.M. 157-Antiparasitic Agents. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2, pp. 1345–1372. [Google Scholar] [CrossRef]
- Ouellette, M.; Drummelsmith, J.; El Fadili, A.; Kündig, C.; Richard, D.; Roy, G. Pterin Transport and Metabolism in Leishmania and Related Trypanosomatid Parasites. Int. J. Parasitol. 2002, 32, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Landi, G.; Linciano, P.; Borsari, C.; Bertolacini, C.P.; Moraes, C.B.; Cordeiro-da-Silva, A.; Gul, S.; Witt, G.; Kuzikov, M.; Costi, M.P.; et al. Structural Insights into the Development of Cycloguanil Derivates as Trypanosoma Brucei Pteridine-Reductase-1 Inhibitors. ACS Infect. Dis. 2019, 5, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Tulloch, L.B.; Martini, V.P.; Iulek, J.; Huggan, J.K.; Lee, J.H.; Gibson, C.L.; Smith, T.K.; Suckling, C.J.; Hunter, W.N. Structure-Based Design of Pteridine Reductase Inhibitors Targeting African Sleeping Sickness and the Leishmaniases. J. Med. Chem. 2010, 53, 221–229. [Google Scholar] [CrossRef]
- Tassone, G.; Landi, G.; Linciano, P.; Francesconi, V.; Tonelli, M.; Tagliazucchi, L.; Costi, M.P.; Mangani, S.; Pozzi, C. Evidence of Pyrimethamine and Cycloguanil Analogues as Dual Inhibitors of Trypanosoma brucei Pteridine Reductase and Dihydrofolate Reductase. Pharmaceuticals 2021, 14, 636. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, W.; Wolkinger, V.; Faist, J.; Kaiser, M.; Brun, R.; Saf, R.; Bucar, F.; Gröblacher, B.; Brantner, A.; Merino, V.; et al. Synthesis of 3-azabicyclo [3.2.2]nonanes and their antiprotozoal activities. Bioorganic Med. Chem. Lett. 2015, 25, 1390–1393. [Google Scholar] [CrossRef]
- Achmad, S.; Seebacher, W.; Faist, J.; Kaiser, M.; Brun, R.; Saf, R.; Weis, R. The antiprotozoal potencies of newly prepared 3-azabicyclo [3.2.2]nonanes. Arch. Pharm. Res. 2016, 39, 1391–1403. [Google Scholar] [CrossRef]
- Agarwal, D.; Gupta, R.D.; Awasthi, S.K. Are Antimalarial Hybrid Molecules a Close Reality or a Distant Dream? Antimicrob. Agents Chemother. 2017, 61, e00249-17. [Google Scholar] [CrossRef]
- Dolensky, J.; Hinteregger, C.; Leitner, A.; Seebacher, W.; Saf, R.; Belaj, F.; Mäser, P.; Kaiser, M.; Weis, R. Antiprotozoal Activity of Azabicyclo-Nonanes Linked to Tetrazole or Sulfonamide Cores. Molecules 2022, 27, 6217. [Google Scholar] [CrossRef]
- Manohar, S.; Rajesh, U.C.; Khan, S.I.; Tekwani, B.L.; Rwat, D.S. Novel 4-Aminoquinoline-Pyrimidine Based Hybrids with Improved in Vitro and in Vivo Antimalarial Activity. ACS Med. Chem. Lett. 2012, 3, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Pavan, V.S.; Taylor, D.; Kumar, D.; Ponnan, P.; Wiesner, L.; Rwat, D.S. Highly active 4-aminoquinoline-pyrimidine based molecular hybrids as potential next generation antimalarial agents. RSC Adv. 2015, 5, 28171–28186. [Google Scholar] [CrossRef]
- Pretorius, S.I.; Breytenbach, W.J.; de Kock, C.; Smith, P.J.; N′Da, D.D. Synthesis, characterization and antimalarial activity of quinoline-pyrimidine hybrids. Bioorg. Med. Chem. 2013, 21, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chaturvedi, V.; Manju, Y.K.; Bhatnagar, S.; Srivastava, K.; Puri, S.K.; Chauhan, P.M.S. Substituted quinolinyl chalcones and quinolinyl pyrimidines as a new class of anti-infective agents. Eur. J. Med. Chem. 2009, 44, 2081–2091. [Google Scholar] [CrossRef]
- Singh, K.; Kaur, H.; Chibale, K.; Balzarini, J.; Little, S.; Bharatam, P.V. 2-Aminopyrimidine based 4-aminoquinoline anti-plasmodial agents. Synthesis, biological activity, structure-activity relationship and mode of action studies. Eur. J. Med. Chem. 2012, 52, 82–97. [Google Scholar] [CrossRef]
- Weis, R.; Schweiger, K.; Seebacher, W.; Belaj, F. One-pot synthesis of 4-Aminobicyclo [2.2.2]octan-2-ones. Tetrahedron 1998, 54, 14015–14022. [Google Scholar] [CrossRef]
- Seebacher, W.; Weis, R.; Kaiser, M.; Brun, R.; Saf, R. Synthesis of 2-azabicyclo [3.2.2]nonanones from bicyclo [2.2.2]octan-2-ones and their activities against Trypanosoma brucei rhodesiense and Plasmodium falciparum K1. J. Pharm. Pharm. Sci. 2005, 8, 578–585. [Google Scholar]
- Seebacher, W.; Weis, R. Novel Antimalarial 3-azabicyclo [3.2.2]nonane derivatives. European Patent 2301627A1, 31 March 2011. [Google Scholar]
- Delves, M.; Plouffe, D.; Scheurer, C.; Meister, S.; Wittlin, S.; Winzeler, E.A.; Sinden, R.E.; Leroy, D. The Activities of Current Antimalarial Drugs on the Life Cycle Stages of Plasmodium: A Comparative Study with Human and Rodent Parasites. PLoS Med. 2012, 9, e1001169. [Google Scholar] [CrossRef]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semi-automated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef]
- Matile, H.; Pink, J.R.L. Plasmodium falciparum malaria parasite cultures and their use in immunology. In Immunological Methods; Lefkovits, I., Pernis, B., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 221–234. [Google Scholar]
- Ponnudurai, T.; Leeuwenberg, A.D.; Meuwissen, J.H. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop. Geogr. Med. 1981, 33, 50–54. [Google Scholar]
- Thaithong, S.; Beale, G.H.; Chutmongkonkul, M. Susceptibility of Plasmodium falciparum to five drugs: An in vitro study of isolates mainly from Thailand. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Koella, J.C. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993, 55, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue® assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Page, B.; Page, M.; Noel, C. A new fluorometric assay for cytotoxicity measurements in-vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H]thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).