UPLC-MS-ESI-QTOF Analysis and Antifungal Activity of Aqueous Extracts of Spondias tuberosa
Abstract
1. Introduction
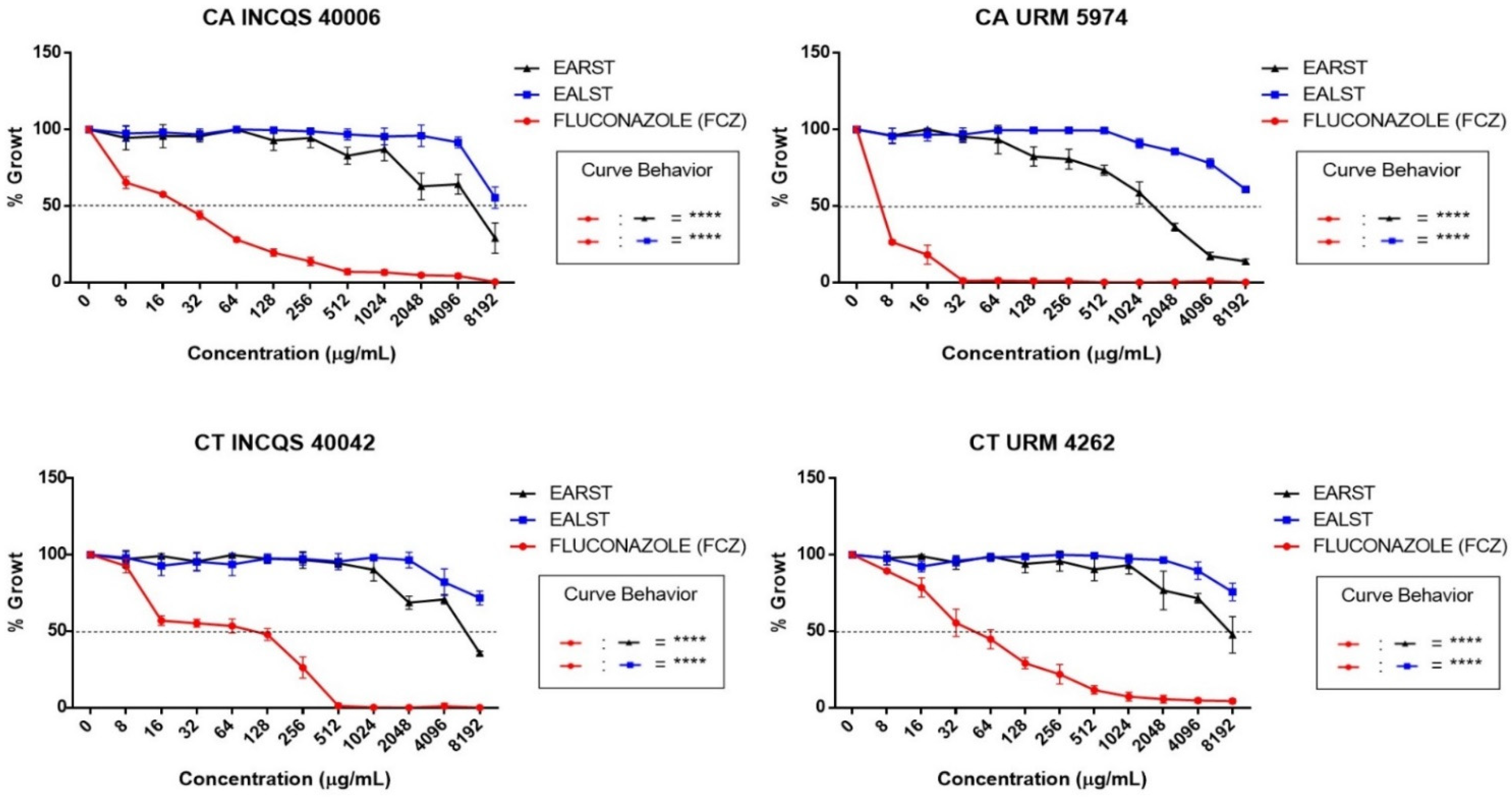
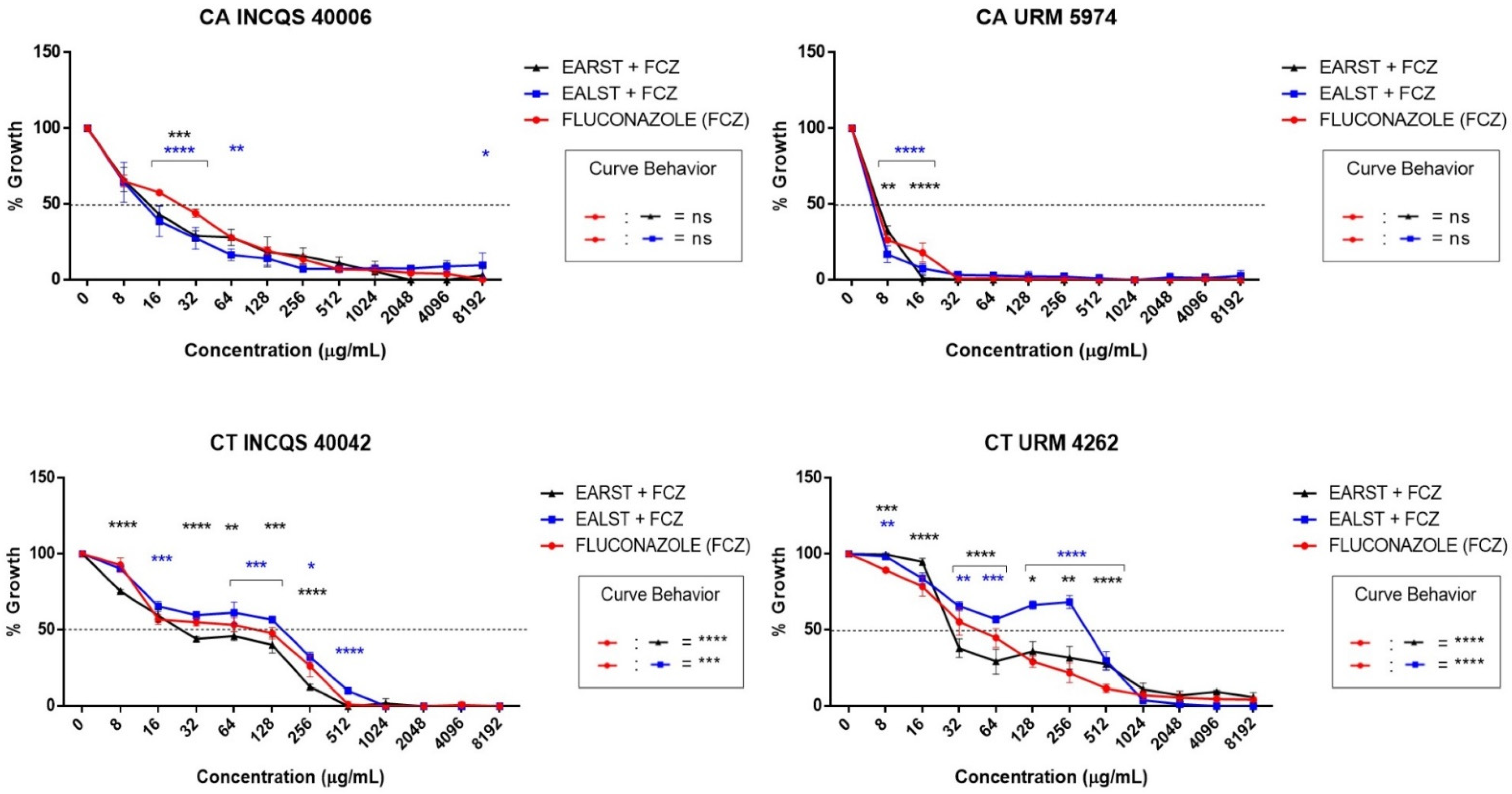
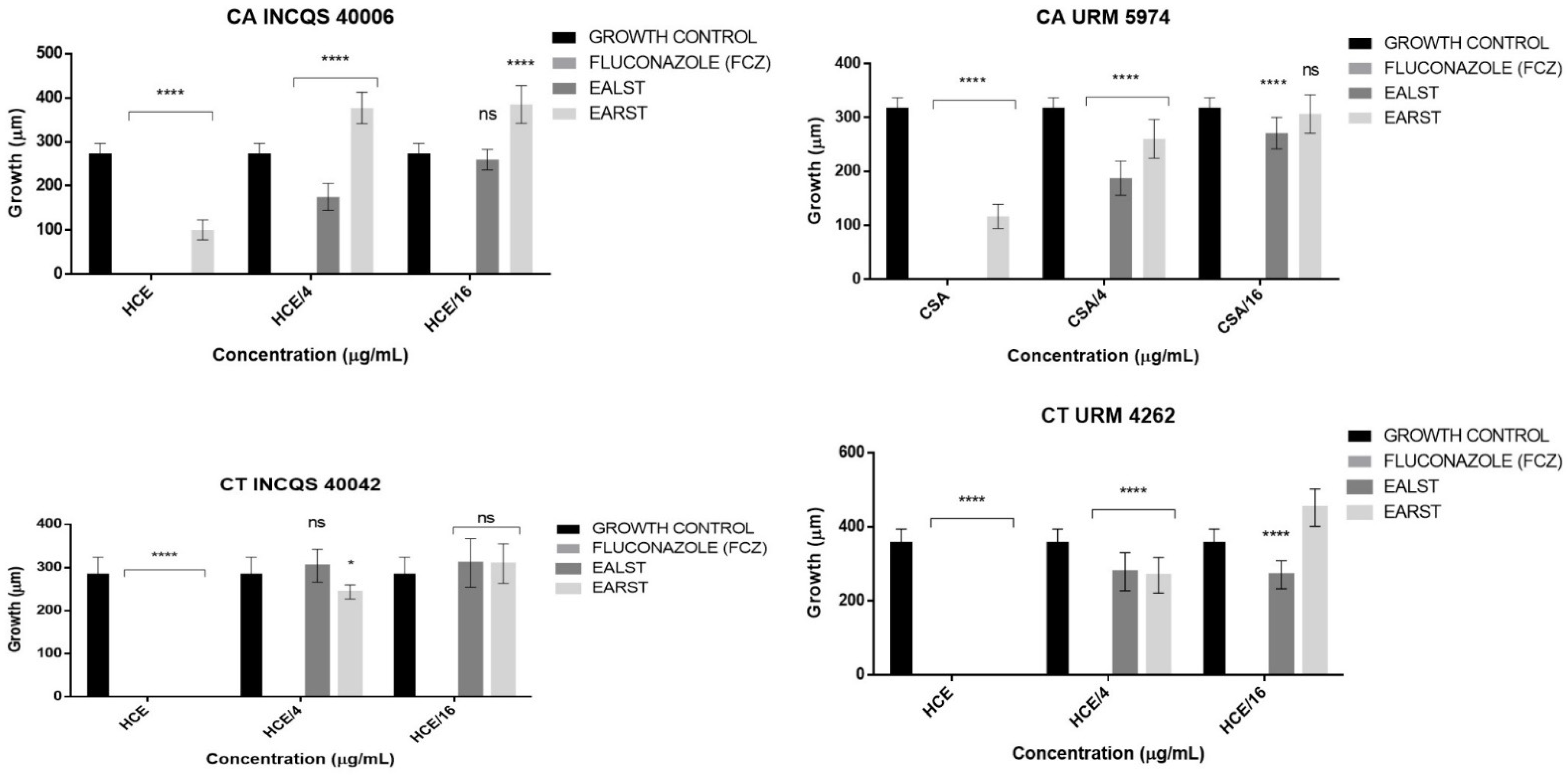
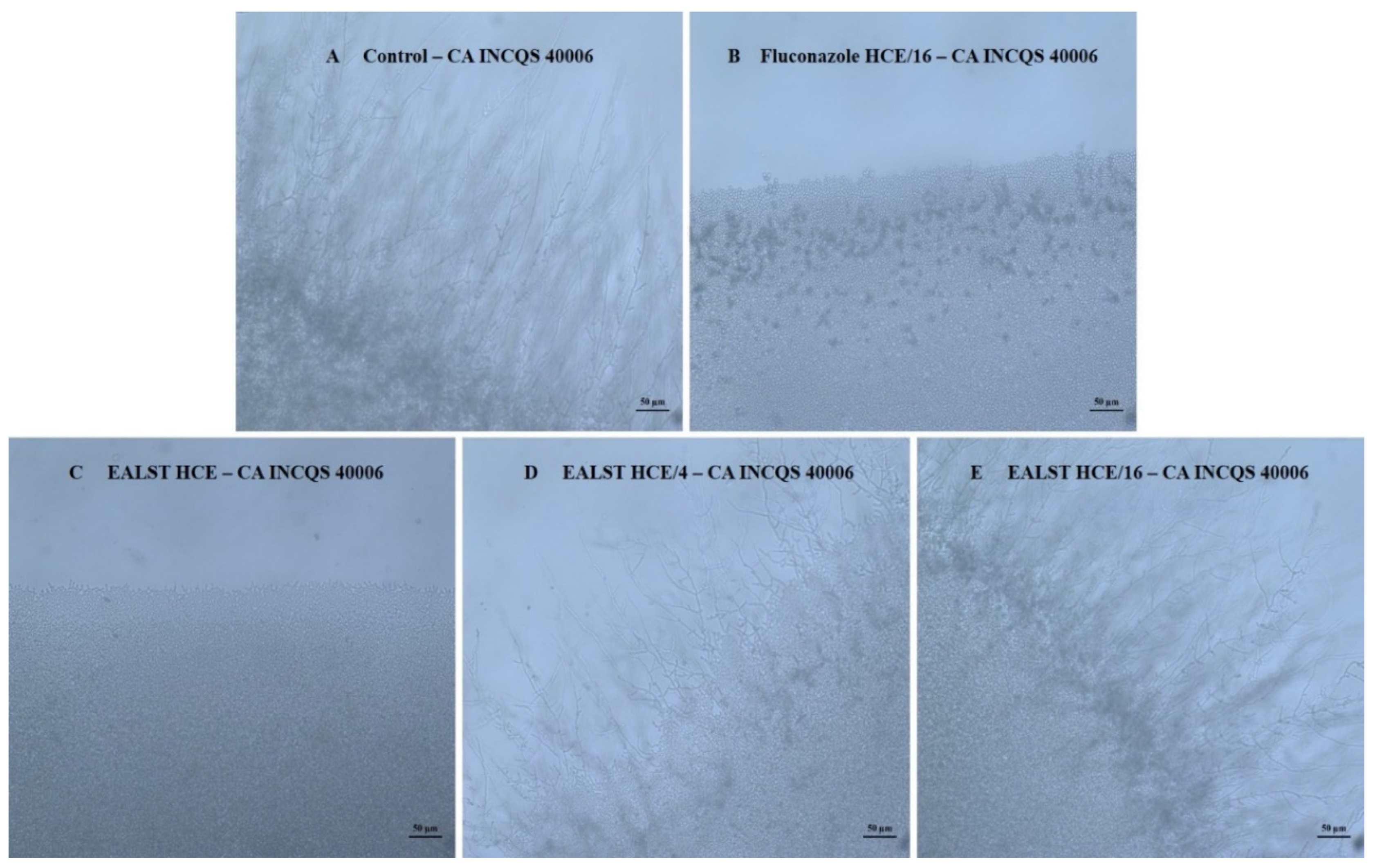
2. Results
3. Discussion
4. Materials and Methods
4.1. Botanical Material Collection and Identification
4.2. Extract Acquisition and Chemical Analysis
4.3. Antifungal Assays
4.3.1. Microorganisms
4.3.2. Culture Media
4.3.3. Inoculum Preparation
4.3.4. Used Drugs and Reagents
4.3.5. IC50 Determination and Cell Viability Curve
4.3.6. Determination of Minimum Fungicidal Concentration (MFC)
4.3.7. Evaluation of the Modifying Effect on Fluconazole Action
4.3.8. Effect of the Extracts and Fluconazole on the Micromorphology of Candida spp.
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- da Silva, T.R. A etnobiologia utilizada como ferramenta para a prática da educação ambiental. Rev. Sergipana Educ. Ambient. 2016, 1, 142–152. [Google Scholar] [CrossRef]
- Queiroz, J.M.G.; Suzuki, M.C.M.; Motta, A.P.R.; Nogueira, J.M.R.; Carvalho, E.M. Popular and scientific aspects of Eugenia species use as herbal. Rev. Fitos. 2015, 9, 73–159. [Google Scholar]
- da Silva, N.F.; Hanazaki, N.; Albuquerque, U.P.; Campos, A.; Loureiro, J.; Feitosa, I.S.; Araújo, E.d.L. Local Knowledge and Conservation Priorities of Medicinal Plants near a Protected Area in Brazil. Evid. Based Complement. Altern. Med. 2019, 2019, 8275084. [Google Scholar] [CrossRef]
- Soldati, G.T.; de Albuquerque, U.P. A new application for the optimal foraging theory: The extraction of medicinal plants. Evid. Based Complement. Altern. Med. 2012, 2012, 364564. [Google Scholar] [CrossRef] [PubMed]
- López, C.A.A. Considerações gerais sobre plantas medicinais. Ambient. Gestão E Desenvolv. 2006, 1, 19–27. [Google Scholar]
- Heinzmann, B.M.; de Barros, F.M.C. Potencial das plantas nativas brasileiras para o desenvolvimento de fitomedicamentos tendo como exemplo Lippia alba (Mill.) NE Brown (Verbenaceae). Saúde St. Maria 2007, 33, 43–48. [Google Scholar]
- de Freitas Lins Neto, E.M.; Peroni, N.; de Albuquerque, U.P. Traditional knowledge and management of Umbu (Spondias tuberosa, Anacardiaceae): An endemic species from the semi-arid region of Northeastern Brazil. Econ. Bot. 2010, 64, 11–21. [Google Scholar] [CrossRef]
- Mertens, J.; Germer, J.; Siqueira Filho, J.A.; Sauerborn, J. Spondias tuberosa Arruda (Anacardiaceae), a threatened tree of the Brazilian Caatinga? Braz. J. Biol. 2017, 77, 542–552. [Google Scholar] [CrossRef]
- da Silva Siqueira, E.M.; Félix-Silva, J.; de Araújo, L.M.L.; Fernandes, J.M.; Cabral, B.; Gomes, J.A.d.S.; de Araújo Roque, A.; Tomaz, J.C.; Lopes, N.P.; de Freitas Fernandes-Pedrosa, M. Spondias tuberosa (Anacardiaceae) leaves: Profiling phenolic compounds by HPLC-DAD and LC–MS/MS and in vivo anti-inflammatory activity. Biomed. Chromatogr. 2016, 30, 1656–1665. [Google Scholar] [CrossRef]
- Jorge, E.C.; Borges, S.V.; De Castro, F.T.; Amorim, E.; Luchese, R.; Cavalcanti, N.B. Preservation of “Umbu”(Spondias Tuberosa Arruda Camara) pulp in the green stage of maturation by combined methods. J. Food Process. Preserv. 2007, 31, 286–297. [Google Scholar] [CrossRef]
- Uchôa, A.D.A.; Oliveira, W.F.; Pereira, A.P.C.; Silva, A.G.; Cordeiro, B.M.P.C.; Malafaia, C.B.; Almeida, C.M.A.; Silva, N.H.; Albuquerque, J.F.C.; Silva, M.V.; et al. Antioxidant Activity and Phytochemical Profile of Spondias tuberosa Arruda Leaves Extracts. Am. J. Plant Sci. 2015, 06, 3038–3044. [Google Scholar] [CrossRef][Green Version]
- Júnior, F.; Soares, W.; Siqueira, C.F.Q.; de Albuquerque, U.P. Plant stem bark extractivism in the northeast semiarid region of Brazil: A new aport to utilitarian redundancy model. Evid. Based Complement. Altern. Med. 2012, 2012, 543207. [Google Scholar]
- Junior, P.; Resende, L.; De Andrade, A.P.; Araújo, K.D.; Barbosa, A.d.S.; Barbosa, F.M. Espécies da caatinga como alternativa para o desenvolvimento de novos fitofármacos. Floresta Ambient. 2014, 21, 509–520. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Macêdo, D.G.; Oliveira, L.G.S.; Saraiva, M.E.; Oliveira, S.F.; Souza, M.M.A.; Menezes, I.R.A. Potencial terapêutico e uso de plantas medicinais em uma área de Caatinga no estado do Ceará, nordeste do Brasil. Rev. Bras. Plantas Med. 2014, 16, 912–930. [Google Scholar] [CrossRef]
- Macêdo, M.S.; Ribeiro, D.A.; de Almeida Souza, M.M. Uso de plantas medicinais cultivadas em uma área de Caatinga em Assaré-Ceará. Cad. Cult. Ciência. 2013, 12, 36–45. [Google Scholar] [CrossRef]
- Da Rocha, P.D.S.; de Araújo Boleti, A.P.; do Carmo Vieira, M.; Carollo, C.A.; da Silva, D.B.; Estevinho, L.M.; dos Santos, E.L.; de Picoli Souza, K. Microbiological quality, chemical profile as well as antioxidant and antidiabetic activities of Schinus terebinthifolius Raddi. Comp. Biochem. Phys. C 2019, 220, 36–46. [Google Scholar]
- Cunha, A.G.; Brito, E.S.; Moura, C.F.H.; Ribeiro, P.R.V.; Miranda, M.R.A. UPLC–qTOF-MS/MS-based phenolic profile and their biosynthetic enzyme activity used to discriminate between cashew apple (Anacardium occidentale L.) maturation stages. J. Chromatogr. B 2017, 1051, 24–32. [Google Scholar] [CrossRef]
- Sayed, E.A.; Martiskainen, O.; Sinkkonen, J.; Pihlaja, K.; Ayoub, N.; Singab, A.-E.N.; El-Azizi, M. Chemical composition and bioactivity of Pleiogynium timorense (Anacardiaceae). Nat. Prod. Commun. 2010, 5, 545–550. [Google Scholar] [CrossRef]
- Lasano, N.F.; Hamid, A.H.; Karim, R.; Dek, M.S.P.; Shukri, R.; Shazini Ramli, N. Nutritional composition, anti-diabetic properties and identification of active compounds using UHPLC-ESI-orbitrap-MS/MS in Mangifera odorata L. peel and seed kernel. Molecules 2019, 24, 320. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.D.L.L.; Lozano-Sánchez, J.; Fernández-Ochoa, Á.; Segura-Carretero, A. Enhancing the Yield of Bioactive Compounds from Sclerocarya birrea Bark by Green Extraction Approaches. Molecules 2019, 24, 966. [Google Scholar] [CrossRef]
- Galvão, W.A.; Braz Filho, R.; Canuto, K.M.; Ribeiro, P.R.V.; Campos, A.R.; Moreira, A.C.O.M.; Silva, S.O.; Mesquita Filho, F.A.; Santos, S.A.A.R.; Junior, J.M.; et al. Gastroprotective and anti-inflammatory activities integrated to chemical composition of Myracrodruon urundeuva Allemão-A conservationist proposal for the species. J. Ethnopharmacol. 2018, 222, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, H.M.; Nascimento, J.N.; Araújo, T.A.S.; Duarte, F.S.; Albuquerque, U.P.; Vieira, J.R.C.; Santana, E.R.B.D.E.; Yara, R.; Lima, C.S.A.; Gomes, D.A. Acute toxicity and cytotoxicity effect of ethanolic extract of Spondias tuberosa arruda bark: Hematological, biochemical and histopathological evaluation. An. Acad. Bras. Cienc. 2016, 88, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- De Lima, E.Q.; Ferreira, C.F.S.L.; Oliveira, E.D.; Costa, V.C.d.O.; Dantas, M.K.L. Phytochemical characterization of Spondias sp. and Spondias tuberosa Arruda Câmera extracts of occurrence in Paraiba semiarid. J. Exp. Biol. Agric. Sci. 2017, 5, 713–717. [Google Scholar] [CrossRef]
- Thassya Luca dos Santos, A.; Pereira Carneiro, J.N.; Pereira da Cruz, R.; Lima Sales, D.; Cosmo Andrade, J.; de Oliveira Almeida, W.; Martins da Costa, J.G.; Riceli Vasconcelos Ribeiro, P.; Sousa de Brito, E.; Alves Batista, F.L.; et al. UPLC-MS-ESI-QTOF Analysis and Antifungal Activity of the Spondias tuberosa Arruda Leaf and Root Hydroalcoholic Extracts. Antibiotics 2019, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.B.; Zuchetto, M.; Oliveira, C.F.; Paula, C.S.; Duarte, A.F.S.; Miguel, M.D.; Miguel, O.G. Efeito de diferentes técnicas extrativas no rendimento, atividade antioxidante, doseamentos totais e no perfil por clae-dad de Dicksonia sellowiana (presl.). Hook, dicksoniaceae. Rev. Bras. Plan. Med. 2016, 18, 230–239. [Google Scholar] [CrossRef]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Jones, W.P.; Kinghorn, A.D. Extraction of plant secondary metabolites. Nat. Prod. Isol. 2012, 20, 341–366. [Google Scholar]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Acharyya, S.; Dash, G.K.; Mondal, S.; Dash, S.K. Antioxidative and antimicrobial study of Spondias mangifera willd root. Int. J. Pharm. Pharm. Sci. 2010, 2, 68–71. [Google Scholar]
- Saha, S. Phytochemical screening and comparative study of antioxidative properties of the fruits and leaves of Spondias mombin in Bangladesh. J. Pharmacogn. Phytochem. 2019, 8, 379–383. [Google Scholar]
- Santhirasegaram, V.; Razali, Z.; George, D.S.; Somasundram, C. Effects of thermal and non-thermal processing on phenolic compounds, antioxidant activity and sensory attributes of chokanan mango (Mangifera indica L.) juice. Food Bioprocess Technol. 2015, 8, 2256–2267. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, M.; Wang, T.; Li, Y.; Wang, C. The roles of CDR1, CDR2, and MDR1 in kaempferol-induced suppression with fluconazole-resistant Candida albicans. Pharm. Biol. 2016, 54, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, B.M.P.C.; Junior, A.C.; Santos, J.R.A.; Araújo, A.D.; Silva, A.G.; Correia, M.T.S.; Silva, M.; Napoleão, T.; Silva, L.; Santos, N.; et al. Anticryptococcal activity of hexane extract from Spondias tuberosa Arruda and associated cellular events. J. Mycol. Medic. 2020, 30, 100965. [Google Scholar] [CrossRef]
- Da Costa Cordeiro, B.M.P.; de Lima Santos, N.D.; Ferreira, M.R.A.; de Araújo, L.C.C.; Junior, A.R.C.; da Conceição Santos, A.D.; De Oliveira, A.P.; Da Silva, A.G.; Falcão, E.P.D.S.; Correia, M.T.D.S.; et al. Hexane extract from Spondias tuberosa (Anacardiaceae) leaves has antioxidant activity and is an anti-Candida agent by causing mitochondrial and lysosomal damages. BMC Complem. Altern. Medic. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- de Brito Costa, E.M.M.; Barbosa, A.S.; Florentino, V.G.B.; da Silva, J.D.F.; Trovão, D.M.d.B.M.; de Medeiros, A.C.D. In vitro antimicrobial activity of plant extracts of semi-arid region of Paraíba, PB, Brazil. Rev. Odonto Ciência. 2013, 28, 101–104. [Google Scholar]
- Manik, M.K.; Islam, S.M.A.; Wahid, M.A.; Morshed, M.M.; Kamal, S.; Islam, M.S.; Ahmed, K.T. Investi-gation of in vitro antioxidant, antimicrobial and thrombolytic activity of the exocarp of Spondias pinnata (Anacardiaceae). Can. Chem. Trans. 2013, 1, 191–201. [Google Scholar]
- Islam, S.M.A.; Ahmed, K.T.; Manik, M.K.; Wahid, M.A.; Kamal, C.S.I. A comparative study of the antioxidant, antimicrobial, cytotoxic and thrombolytic potential of the fruits and leaves of Spondias dulcis. Asian Pac. J. Trop. Biomed. 2013, 3, 682–691. [Google Scholar] [CrossRef]
- Umeh, E.; Igoli, J.; Agada, E.; Usman, S. Evaluating extracts of Spondias mombin for antimicrobial activitie. Bio-Research 2009, 7, 505–508. [Google Scholar] [CrossRef]
- Okwuosa, O.M.; Chukwura, E.I.; Chukwuma, G.O.; Okwuosa, C.N.; Enweani, I.B.; Agbakoba, N.R.; Chukwuma, C.M.; Manafa, P.O.; Umedum, C.U. Phytochemical and antifungal activities of Uvaria. chamae leaves and roots, Spondias mombin leaves and bark and Combretum racemosum leaves. Afri. J. Medic. Med. Sci. 2012, 41, 99–103. [Google Scholar]
- De Freitas, M.A.; Da Cruz, R.P.; Dos Santos, A.T.L.; Almeida-Bezerra, J.W.; Machado, A.J.T.; Dos Santos, J.F.S.; Rocha, J.E.; Boligon, A.A.; Bezerra, C.F.; de Freitas, T.S.; et al. HPLC–DAD analysis and antimicrobial activities of Spondias mombin L.(Anacardiaceae). 3 Biotech. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Alves, L.A.; Freires, I.D.A.; Pereira, T.M.; Souza, A.D.; Lima, E.D.O.; Castro, R.D.D. Effect of Schinus terebinthifolius on Candida albicans growth kinetics, cell wall formation and micromorphology. Acta Odontol. Scand. 2013, 71, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.D.; Wilson, D.; Wächtler, B.; Brunke, S.; Naglik, J.R.; Hube, B. Candida albicans dimorphism as a therapeutic target. Expert Rev. Anti. Infect. Ther. 2012, 10, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.L. Candida Yeasts Isolated from the Mouth of Children with and without Down Syndrome: Phenotypic Aspects, Intra-Family Relationship and Immunoglobulin Profile. Ph.D. Thesis, University of Brasília, Brasília, Brazil, 2008. [Google Scholar]
- Arif, T.; Bhosale, J.D.; Kumar, N.; Mandal, T.K.; Bendre, R.S.; Lavekar, G.S.; Dabur, R. Natural products–antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009, 11, 621–638. [Google Scholar] [CrossRef]
- Salas, M.P.; Céliz, G.; Geronazzo, H.; Daz, M.; Resnik, S.L. Antifungal activity of natural and enzymatically-modified flavonoids isolated from Citrus species. Food Chem. 2011, 124, 1411–1415. [Google Scholar] [CrossRef]
- Ansari, M.A.; Anurag, A.; Fatima, Z.; Hameed, S. Natural phenolic compounds: A potential antifungal agent. Microb. Pathog. Strateg. Combat. Sci. Technol. Educ. 2013, 1, 1189–1195. [Google Scholar]
- Inci, M.; Atalay, M.A.; KOÇ, A.N.; Yula, E.; Evirgen, Ö.; Durmaz, S.; Demir, G. Investigating virulence factors of clinical Candida isolates in relation to atmospheric conditions and genotype. Turkish J. Med. Sci. 2012, 42, 1476–1483. [Google Scholar]
- Muthamil, S.; Pandian, S.K. Inhibitory effect of Murraya koenigii against Candida albicans virulence and biofilm development. Biologia 2016, 71, 256–264. [Google Scholar] [CrossRef]
- Candiracci, M.; Citterio, B.; Piatti, E. Antifungal activity of the honey flavonoid extract against Candida albicans. Food Chem. 2012, 131, 493–499. [Google Scholar] [CrossRef]
- Matos, F.J.A. Farmácias Vivas, 4th ed.; Editora UFC: Fortaleza, Brazil, 2020; pp. 36–40. [Google Scholar]
- Masters, K. Spray Drying Handbook, 5th ed.; Longman Scientific & Technical: New York, NY, USA, 1991. [Google Scholar]
- Sousa, E.O.; Miranda, C.M.; Nobre, C.B.; Boligon, A.A.; Athayde, M.L.; Costa, J.G. Phytochemical analysis and antioxidant activities of Lantana camara and Lantana montevidensis extracts. Ind. Crops Prod. 2015, 70, 7–15. [Google Scholar] [CrossRef]
- Andrade, J.C.; Silva, A.R.P.; Santos, A.T.L.; Freitas, M.A.; Carneiro, J.N.P.; Gonçalo, M.I.P.; de Souza, A.; Freitas, T.; Ribeiro, P.; Brito, E.; et al. UPLC-MS-ESI-QTOF characterization and evaluation of the antibacterial and modulatory antibiotic activity of Ziziphus joazeiro Mart. aqueous extracts. Sou. Afri. J. Bot. 2019, 123, 105–112. [Google Scholar] [CrossRef]
- Souza, E.L.; Stamford, T.L.M.; Lima, E.O.; Trajano, V.N. Effectiveness of Origanum vulgare L. essential oil to inhibit the growth of food spoiling yeasts. Food Control 2007, 18, 409–413. [Google Scholar] [CrossRef]
- Javadpour, M.M.; Juban, M.M.; Lo, W.C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De Novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Morais-Braga, M.F.B.; Sales, D.L.; Carneiro, J.N.P.; Machado, A.J.T.; dos Santos, A.T.L.; de Freitas, M.A.; Martins, G.M.D.A.B.; Leite, N.F.; de Matos, Y.M.L.; Tintino, S.R.; et al. Psidium guajava L. and Psidium brownianum Mart ex DC.: Chemical composition and anti-Candida effect in association with fluconazole. Microb. Pathog. 2016, 95, 200–207. [Google Scholar] [CrossRef]
- Ernst, E.J.; Klepser, M.E.; Ernst, M.E.; Messer, S.A.; Pfaller, M.A. In Vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 1999, 33, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, H.D.M.; Costa, J.G.M.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Sidrim, J.J.C.; Rocha, M.F.G. Micologia Médica à Luz de Autores Contemporâneos; Guanabara Koogan: Rio de Janeiro, Brazil, 2010; p. 388. [Google Scholar]
- Mendes, J.M. Investigação da Atividade Antifúngica do Óleo Essencial de Eugenia Caryophyllata Thunb. Sobre Cepas de Candida Tropicalis; UFPB: João Pessoa, Brasil, 2011; p. 74. [Google Scholar]
- Pereira Carneiro, J.N.; da Cruz, R.P.; da Silva, J.C.P.; Rocha, J.E.; de Freitas, T.S.; Sales, D.L.; Bezerra, C.F.; de Oliveira Almeida, W.; da Costa, J.G.M.; da Silva, L.E.; et al. Piper diospyrifolium Kunth.: Chemical analysis and antimicrobial (intrinsic and combined) activities. Microb. Pathog. 2019, 136, 103700. [Google Scholar] [CrossRef]
| Special Metabolite Classes (SMC) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SMC1 | SMC2 | SMC3 | SMC4 | SMC5 | SMC6 | SMC7 | SMC8 | SMC9 | SMC 10 | |
| EALST | + | - | - | + | + | + | - | - | + | + |
| EARST | + | - | - | + | - | + | - | - | + | + |
| Peak no. | Rt min | [M + H]+ Observed | Product Ions (MS/MS) | Molecular Formula | Ppm Error | Putative Name | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 2.52 | 214 | 198 | C4H6S5 | 4.6 | No identified | |
| 2 | 2.67 | 185 | - | C8H8O5 | 1.5 | Methyl digallate | [16] |
| 3 | 2.74 | 445 | 283 | C21H32O10 | 2.5 | Dihydrophaseic acid hexose | [17] |
| 4 | 4.19 | 449 | 303 | C21H20O11 | 0.4 | Quercetin rhamnoside | [17] |
| 5 | 4.49 | 167 | 120 | C9H12NO2 | −4.2 | No identified | - |
| 6 | 4.35 | 449 | 287 | C21H20O11 | 0.2 | Kaempferol hexoside | [18] |
| 7 | 4.55 | 463 | 287 | C21H18O12 | 0.4 | Kaempferol glucuronide | [18] |
| 8 | 4.62 | 477 | 287 | C22H20O12 | −4.4 | Kaempferol-methyl-glucuronide | [18] |
| 9 | 4.80 | 287 | - | C15H10O6 | −2.5 | Kaempferol | [18] |
| 10 | 5.49 | 681 | 303 | C34H48O14 | −1.6 | No identified | - |
| Peak no. | Rt min | [M − H]− Observed | Product Ions (MS/MS) | Molecular Formula | Ppm Error | Putative Name | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 2.89 | 271 | - | C15H12O5 | 3.8 | No identified | - |
| 2 | 3.16 | 377 | 237, 189 | C25H14O4 | 2.1 | No identified | - |
| 3 | 3.20 | 331 | 169 | C9H16O13 | 2.8 | Monogalloyl-glucose | [17] |
| 4 | 3.51 | 197 | - | C9H10O5 | 1.8 | Hydroxy-dimethoxybenzoic acid | [16] |
| 5 | 4.55 | 461 | 285 | C22H22O11 | 2.4 | Kaempferol-glucuronide | [17] |
| 6 | 4.66 | 433 | 301 | C20H18O11 | 3.0 | Quercetin-pentoside | [19,20] |
| 7 | 4.89 | 339 | - | C22H28O3 | −3.4 | No identified | - |
| 8 | 5.07 | 361 | 317 | C20H26O6 | 2.1 | Giberellin GA19 | [17] |
| 9 | 5.37 | 537 | 479, 375 | C30H18O10 | 4.5 | Agathisflavone | [21] |
| 10 | 5.55 | 315 | - | C16H12O7 | 4.1 | Rhamnetin | [19,20] |
| 11 | 5.92 | 341 | 297 | C22H30O3 | 4.4 | Anacardic acid 1 | [17] |
| 12 | 7.00 | 343 | 299 | C22H32O3 | −4.7 | Anacardic acid 2 | [17] |
| 13 | 7.59 | 345 | 301 | C22H34O3 | −4.1 | Anacardic acid 3 | [17] |
| CA INCQS 40006 | CA URM 5974 | CT INCQS 40042 | CT URM 4262 | |
|---|---|---|---|---|
| EALST | 8305.3 | 11,090.9 | 16,288.03 | 13,335.7 |
| EARST | 5344.8 | 1306.6 | 6678.9 | 7785.9 |
| FLUCONAZOLE | 22.74 | 3.97 | 88.08 | 47.30 |
| EALST + FCZ | 11.80 | 2.62 | 177.41 | 308.96 |
| EARST + FCZ | 12.68 | 5.46 | 30.42 | 35.62 |
| CA INCQS 40006 | CA URM 5974 | CT INCQS 40042 | CT URM 4262 | |
|---|---|---|---|---|
| EALST | ≥16,384 | ≥16,384 | ≥16,384 | ≥16,384 |
| EARST | ≥16,384 | ≥16,384 | ≥16,384 | ≥16,384 |
| FLUCONAZOLE | 8192 | 8192 | ≥16,384 | ≥16,384 |
| EALST + FCZ | 8192 | 2048 | ≥16,384 | 8192 |
| EARST + FCZ | ≥16,384 | 1024 | ≥16,384 | 8192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.T.L.d.; Carneiro, J.N.P.; da Cruz, R.P.; Sales, D.L.; Andrade-Pinheiro, J.C.; de Freitas, M.A.; Kerntopf, M.R.; Delmondes, G.d.A.; Ribeiro, P.R.V.; de Brito, E.S.; et al. UPLC-MS-ESI-QTOF Analysis and Antifungal Activity of Aqueous Extracts of Spondias tuberosa. Molecules 2023, 28, 305. https://doi.org/10.3390/molecules28010305
Santos ATLd, Carneiro JNP, da Cruz RP, Sales DL, Andrade-Pinheiro JC, de Freitas MA, Kerntopf MR, Delmondes GdA, Ribeiro PRV, de Brito ES, et al. UPLC-MS-ESI-QTOF Analysis and Antifungal Activity of Aqueous Extracts of Spondias tuberosa. Molecules. 2023; 28(1):305. https://doi.org/10.3390/molecules28010305
Chicago/Turabian StyleSantos, Antonia Thassya Lucas dos, Joara Nályda Pereira Carneiro, Rafael Pereira da Cruz, Débora Lima Sales, Jacqueline Cosmo Andrade-Pinheiro, Maria Audilene de Freitas, Marta Regina Kerntopf, Gyllyandeson de Araújo Delmondes, Paulo Riceli Vasconcelos Ribeiro, Edy Sousa de Brito, and et al. 2023. "UPLC-MS-ESI-QTOF Analysis and Antifungal Activity of Aqueous Extracts of Spondias tuberosa" Molecules 28, no. 1: 305. https://doi.org/10.3390/molecules28010305
APA StyleSantos, A. T. L. d., Carneiro, J. N. P., da Cruz, R. P., Sales, D. L., Andrade-Pinheiro, J. C., de Freitas, M. A., Kerntopf, M. R., Delmondes, G. d. A., Ribeiro, P. R. V., de Brito, E. S., Batista, F. L. A., Magalhães, F. E. A., Pita Neto, I. C., Morais-Braga, M. F. B., Kowalski, R., Kowalska, G., Szopa, A., Baj, T., & Coutinho, H. D. M. (2023). UPLC-MS-ESI-QTOF Analysis and Antifungal Activity of Aqueous Extracts of Spondias tuberosa. Molecules, 28(1), 305. https://doi.org/10.3390/molecules28010305









