Abstract
In this work, we describe the design, synthesis, and structure-activity relationship of 6-(tetrazol-5-yl)-7-aminoazolo[1,5-a]pyrimidines as inhibitors of Casein kinase 2 (CK2). At first, we optimized the reaction conditions for the azide-nitrile cycloaddition in the series of 6-cyano-7-aminoazolopyridimines and sodium azide. The regioselectivity of this process has been shown, as the cyano group of the pyrimidine cycle was converted to tetrazole while the nitrile of the azole fragment did not react. The desired tetrazolyl-azolopyrimidines were obtained in a moderate to excellent yields (42–95%) and converted further to water soluble sodium salts by the action of sodium bicarbonate. The obtained 6-(tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidines 2a–k and their sodium salts 3a–c, 3g–k showed nano to low micromolar range of CK2 inhibition while corresponding [1,2,4]triazolopyrimidines 10a–k were less active (IC50 > 10 µM). The leader compound 3-phenyl-6-(tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine 2i as CK2 inhibitor showed IC50 45 nM.
1. Introduction
Casein Kinase 2 (CK2) is a highly conserved polyfunctional serine/threonine protein kinase that plays an important role in the regulation of the processes of several cells, such as proliferation, differentiation and survival [1]. It is considered that CK2 has been implicated in the manifestation of some diseases, including multiple sclerosis [2], inflammation [3], hypertension [4], and viral infections [5]. The role of CK2 has been extensively studied in the development of malignant tumors and it was proved as a key regulator of multiple oncogenic pathways, including the PI3K/Akt, JAK/STAT, IL-6 and NF-jB signaling cascades [6]. In turn, CK2 is a key suppressor of cell apoptosis [7], which determines its role in oncogenesis of several tumors with overexpression of CK2, including breast carcinoma, adenocarcinoma of the lung, prostate carcinoma and lymphomas [8]. It can be noted that Silmitasertib has been approved by the FDA for the treatment of cholangiocarcinoma as CK2 inhibitor [9]. Thus, the development of novel CK2 inhibitors as chemotherapeutic agents against cancer and other nosologies where this type of kinases is involved is a relevant task.
Previously, a wide variety of different molecules have been described as CK2 inhibitors, including polyhalogenated benzimidazole and benzotriazole derivatives [10], nitrogen-containing heterocycles [11,12,13] and their polycondensed analogues [14], as well as condensed coumarin derivatives [15] (Figure 1). Azoloazines heterocycles with bridge nitrogen atom are of considerable interest, since many representatives of this class are known to inhibit CK2 in the low nanomolar range. However, most of the currently available CK2 inhibitors lack the potency, physiochemical, and pharmacological properties required to be successful in clinical trials.
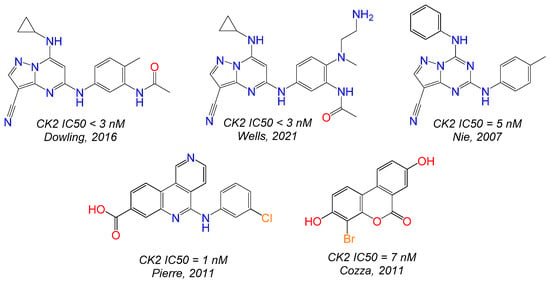
Figure 1.
Examples of pyrazoloazines and other molecules with high affinity for CK2 [11,12,13,14,15].
It should be noted that related azolopyrimidines are a privileged class of heterocycles in medicinal chemistry as they demonstrate a wide range of biological activities, in particular, anticoagulant [16], anti-inflammatory [17], antidiabetic [18], hypotensive [19], antiseptic [20].
At the same time, a nitro group or carboxylic fragment should present within heterocyclic scaffold for this useful activity to be formed (Figure 2). On the other hand, the tetrazole cycle is a metabolically stable bio-isostere of the carboxyl group and the cis-amide fragment due to the similar electronic structure [21,22,23,24,25]. The corresponding similarity for the carboxylic anion and the nitro group can be noted and one can consider the tetrazolyl fragment as an isostere for both of them (Figure 3a). However, only one example of azoloazines containing tetrazole cycle has been published to date—2-nitro-6-(1H-tetrazol-5-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine was considered as nitrogen-rich energetic material [26].
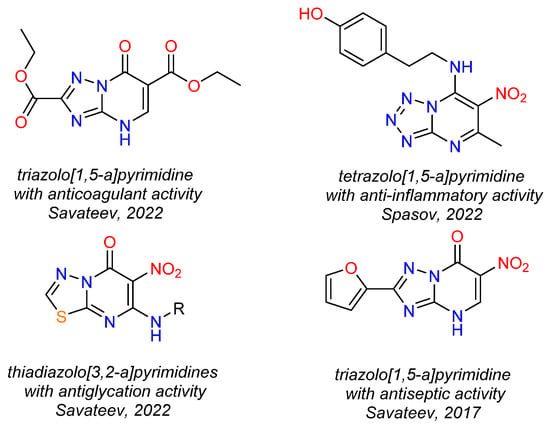
Figure 2.
Examples of biologically active azolopyrimidines [16,17,18,20].
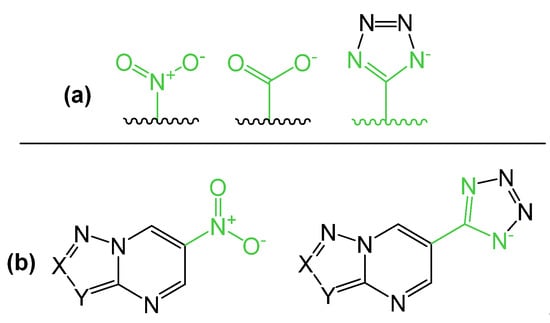
Figure 3.
(a) Isosterism of tetrazole ring, carboxylic fragment and nitro group. (b) Potent nitro-azolopyridimidines and tetrazolyl-containing analogues as perspective alternative.
In this work we propose the introduction of tetrazolyl fragment into azolopyrimidine scaffold as promising structural modification to search for novel CK2 inhibitors (Figure 3b).
2. Results
2.1. Chemistry
We have developed a versatile approach to the synthesis of 6-cyano-7-aminoazolo[1,5-a]pyrimidines and obtained a library of corresponding heterocycles [27] which are good precursors for azide-nitrile cycloaddition. Herein, 3-Ethoxycarbonyl-6-cyano-7-aminopyrazolo[1,5-a]pyrimidine 1g was used as model substrate to study this process and evaluate different reaction conditions while sodium azide served as the source of the azide fragment (Scheme 1).
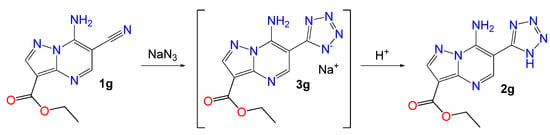
Scheme 1.
Model reaction of cyanoderivative 1g with sodium azide for condition optimization.
The mechanism of this process has been studied extensively by DFT calculations and it was shown that energy barrier for the reaction of the azide anion with nitriles is considerably lower than the barrier for the attack of the neutral hydrazoic acid [28]. At the same time, experimental data revealed that the reaction is strongly accelerated by Brønsted acids such as AcOH and ammonium salts [29,30]. Lewis acids [31], specific organocatalysts [32], and ionic liquids [33] could serve as catalyst in azide-nitrile cycloaddition as well.
It was found that formation of tetrazole cycle by reaction of sodium azide with nitrile derivative 1g proceeded smoothly in polar aprotic solvents (DMF, MeCN) in the presence of ammonium salts (entry 1–6 and 8, Table 1), AcOH (entry 7, Table 1), ZnCl2 (entry 10, Table 1), or 1-butyl-3-methylimidazolium chloride (entry 9, Table 1). The highest yield (78%) of the desired product 2g was observed in the case AcOH while control experiment where 1g reacted with sodium azide in DMF without any additive resulted in 82% yield of tetrazole 2g (entry 11, Table 1). Optimal conditions required heating at 120 °C for 8 h of a 0.25 molar solution of 1g in DMF with 1.1 equiv. of NaN3 and further treatment of water suspension of 3g with conc. HCl to provide 90% yield of 2g (entry 13, Table 1). It is worth noting that the formation of the tetrazole cycle was not observed in protic polar solvents (H2O, MeOH) both with catalysis (entry 2 and 6, Table 1) and without (entry 18, Table 1) by TLC analysis of the reaction mixture as well as by NMR analysis of the isolated products.

Table 1.
Optimization of reaction parameters in the synthesis of tetrazolyl-pyrazolopyrimidine 2g a.
With the optimized reaction conditions in hand, a series of 6-(tetrazol-5-yl)pyrazolopyrimidines 2a–k were synthesized in good to excellent yields (60–95%) (Scheme 2).
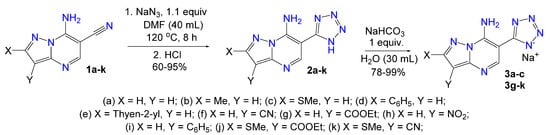
Scheme 2.
Scope of 6-(tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidines 2a–k and corresponding sodium salts 3a–c, 3g–k.
The cycloaddition of sodium azide to the C6-nitrile fragment in this series 1a–k proceeded without competing processes. Thus, in the case of dinitriles 1f and 1k it was observed that only one cyano group reacted with azide to form tetrazole cycle as there were CN characteristic absorption peaks in the region of 2217–2231 cm−1 in IR spectra of the obtained products 2f and 2k. The same results were obtained in the reaction of 1f and 1k with 3 equiv. of sodium azide by the analysis of reaction products with 1H and IR spectroscopy. We have tried to obtain 3,6-di(tetrazol-5-yl)pyrazolopyrimidine 6 by independent synthesis via two steps starting from 3-amino-4-cyanopyrazole 4 (Scheme 3). It was showed that the heating of compound 4 with sodium azide in DMF both with ammonium chloride and in the absence of it did not lead to tetrazolyl heterocycle 5. Subsequently, pyrazole 4 has been converted to 3-cyano-7-aminopyrazolopyrimidine 7 [34], but the latter also did not react with sodium azide under different conditions and starting material 7 was isolated after workup of the reaction mixture (Scheme 3). These findings support regioselectivity of the azide-nitrile cycloaddition process in the series of 3,6-dicyanopyrazolopyrimidines as only nitrile group in the pyrimidine ring converts into tetrazole fragment.
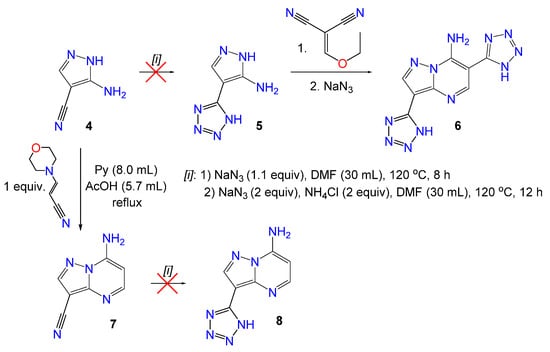
Scheme 3.
Regioselectivity of nitrile-azide cycloaddition.
The azide-nitrile cycloaddition proceeded smoothly in the case of [1,2,4]-triazolo[1,5-a]pyrimidines 9a–k under optimized conditions and resulted in a series of 2-R-6-(tetrazol-5-yl)-7-amino[1,2,4]triazolo[1,5-a]pyrimidines 10a–k with good yields (42–89%) (Scheme 4). The unidentified oily products were isolated when thiopropargyl containing derivative 9l was introduced in the reaction, probably due to the side azide-alkyne cycloaddition to form 1,2,3-triazole.
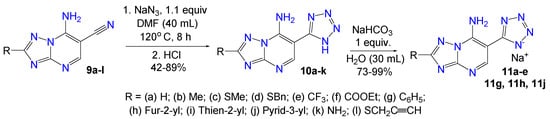
Scheme 4.
Scope of 6-(tetrazol-5-yl)-7-amino[1,2,4]triazolo[1,5-a]pyrimidines 10a–k and corresponding sodium salts 11a–e, 11g, 11h, 11j.
The structure of the obtained heterocycles 2a–k and 10a–k was confirmed by 1H NMR spectroscopy (the signal of C5H proton was shifted downfield (∆δ ≈ 0.5 ppm) in comparison with starting material), 13C NMR technic (characteristic signal around δ ≈ 85–91 ppm, probably, it can be attributed to C6 atom, while other aromatic carbons located in the region of δ ≈ 145–160 ppm), IR spectroscopy (absence of CN absorption peak in the region of 2100–2300 cm−1 in comparison with starting material), mass-spectrometry (a molecular ion peaks were detected) and elemental analysis (see Supporting Information).
We converted obtained 6-(1H-tetrazol-5-yl)-7-aminoazolo[1,5-a]pyrimidines 2 and 10 to the corresponding sodium salts by the reaction with sodium bicarbonate (Scheme 2 and Scheme 4) based on the NH-acidity of the tetrazole ring [35]. These sodium salts 3a–c, 3g–k and 11a–e, 11g, 11h, 11j possess high water solubility which is an undoubted advantage for testing its biological activity in the CK2 assay and further experiments.
2.2. CK2 Inhibition
Once in hand target compounds were evaluated against human recombinant CK2 using luminescent ADP-GloTM platform. Initial screening performed at 50 µM compound concentration revealed scaffold as a rich source of CK2 inhibitors. Confirmation experiments were run in a concentration-dependent manner to obtain IC50 values (Table 2).

Table 2.
CK2 inhibition of obtained tetrazolyl-azolopyrimidines 2,3 and their sodium salts 10,11.
Structure-activity relationship analysis (Figure 4) suggests that 6-(tetrazol-5-yl)-[1,2,4]triazolo[1,5-a]pyrimidines 10a–j generally have lower activity than corresponding pyrazolopyrimidines 2a–k reflecting in IC50 values in higher micromolar range. Notably, in the triazolopyrimidine series, compounds 10a and 10h are the most active (IC50 23.78 and 11.81 µM, correspondingly), while any other substituents in the triazole ring resulted in the decrease of affinity towards CK2.
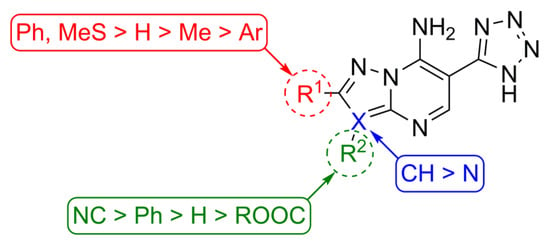
Figure 4.
Structure-activity relationship for 6-(tetrazol-5-yl)azolo[1,5-a]pyrimidines as CK2 inhibitors.
In turn, derivatives of 6-(tetrazol-5-yl)pyrazolo[1,5-a]pyrimidine series 2a–k and 3a–c, 3f, 3g, 3i demonstrated rather improved potency. Compounds 2a and its sodium salt 3a are micromolar inhibitors. Introduction of SMe (2c) or Ph (2d) group in the C2-position of heterocyclic scaffold is beneficial, while the smaller Me-substituent at this position led to less active compound 2b. Evaluation of substituents in position C3 of the pyrazolopyrimidine system indicates non-additive SAR. Thus, both electron-withdrawing and electron-donating groups resulted in low micromolar inhibitors 2f–i with leader compound 2i demonstrated IC50 = 45 nM. At the same time, the combination of 2-methylsulfanyl group with 3-ethoxycarbonyl or 3-nitrile substituents also revealed compounds 2j and 2k with good affinity to CK2. It is worth noting that in most cases sodium salts 3 were surprisingly less active than NH-form of tetrazolyl containing heterocycles 2 excluding potent sodium 5-(7-amino-3-cyanopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide 3f with IC50 = 65 nM.
3. Materials and Methods
3.1. Chemistry
Commercial reagents were obtained from Sigma-Aldrich, Acros Organics, or Alfa Aesar and used without any further purification. All workup and purification procedures were carried out using analytical grade solvents. One-dimensional 1H, 19F, and 13C NMR spectra were acquired on a Bruker DRX-400 instrument (400, 376, and 101 MHz, respectively), utilizing DMSO-d6 as solvent and as an external reference. The following abbreviations are used for multiplicity of NMR signals: s—singlet, d—doublet, t—triplet, q—quartet, dd—doublet of doublets, dt–doublet of triplets, m—multiplet, br—broaded. Mass spectroscopy studies were performed on a Shimadzu GCMS-QP2010 Ultra (EI, 70 eV). IR spectra were recorded on a Bruker Alpha spectrometer equipped with a ZnSe ATR accessory. Elemental analysis was performed on a PerkinElmer PE 2400 elemental analyzer. Melting points were determined on a Stuart SMP3 and are uncorrected. The monitoring of the reaction progress was performed by using TLC on Silufol UV254 plates (eluent is AcOEt). All synthesized compounds are >95% pure by elemental analysis.
General procedure for the synthesis of 6-(tetrazol-5-yl)-7-aminoazolo[1,5-a]pyrimidines (2a–k, 10a–k).
A suspension of 0.01 mol (1 equiv.) of the corresponding 6-cyano-7-aminoazolo[1,5-a]pyrimidine (1a-k, 9a-k) and 0.011 mol (1.1 equiv.) of sodium azide in 40 mL of DMF was stirred at 120 °C for 8 h under air atmosphere (TLC control, AcOEt as eluent, starting material Rf ≈ 0.6–0.7, tetrazole products Rf ≈ 0.0). The reaction mixture was cooled to 25 °C, evaporated at reduced pressure, residue was dissolved in 30 mL of H2O and acidified with conc. HCl to pH≈1. The obtained precipitate was filtered off and washed with 100 mL of H2O to give the corresponding product.
6-(1H-tetrazolTetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2a).
Brown solid. Yield 1.67 g, 83%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 6.58 (H, d, C3H, J = 2.2), 8.24 (H, d, C2H, J = 2.2), 8.70 (2H, s, NH2), 8.76 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 152.7, 148.4, 147.6, 146.0, 145.4, 96.4, 85.7. IR, ν, cm−1: 3259 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (100), 77 (18), 105 (15), 145 (32), 159 (15), 174 (50), 202 (73), [M]+). Anal. Calcd. for C7H6N8: C 41.59, H 2.99, N 55.42; found: C 41.65, H 3.06, N 55.33.
2-methylMethyl-6-(1H-tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2b).
Beige solid. Yield 1.84 g, 85%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 2.44 (3H, s, CH3), 6.39 (H, s, C3H), 8.61 (2H, s, NH2), 8.69 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 155.0, 152.6, 148.8, 147.3, 145.5, 96.0, 85.2, 14.4. IR, ν, cm−1: 3173 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (100), 81 (19), 159 (28), 108 (50), 173 (18), 188 (53), 216 (85), [M]+). Anal. Calcd. for C8H8N8: C 44.44, H 3.73, N 51.83; found: C 44.39, H 3.76, N 51.83.
2-(methylthioMethylthio)-6-(1H-tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2c).
Brown solid. Yield 1.95 g, 79%. Mp = 273–275 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 2.64 (3H, s, CH3), 6.41 (H, s, C3H), 8.56 (2H, s, NH2), 8.69 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 156.0, 152.4, 149.1, 147.8, 144.9, 94.5, 85.5, 13.9. IR, ν, cm−1: 3238 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (70), 131 (20), 159 (16), 173 (87), 220 (42), 248 (100), [M]+). Anal. Calcd. for C8H8N8S: C 38.70, H 3.25, N 45.14; found: C 38.79, H 3.11, N 45.23.
2-phenylPhenyl-6-(1H-tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2d).
Yellow solid. Yield 2.64 g, 95%. Mp = 291–293 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 6.95 (H, s, C2H), 7.41 (H, t, C4’H, J = 7.6), 7.48 (2H, t, C3’H, C5’H, J = 7.6), 8.07 (2H, d, C2’H, C6’H, J = 7.6), 8.64 (2H, s, NH2), 8.76 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 155.6, 152.4, 149.1, 147.6, 145.9, 132.2, 129.3, 128.8, 126.4, 93.4, 85.9. IR, ν, cm−1: 3280 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (38), 77 (100), 116 (32), 170 (10), 208 (8), 221 (23), 250 (65), 278 (92), [M]+). Anal. Calcd. for C13H10N8: C 56.11, H 3.62, N 40.27; found: C 56.03, H 3.62, N 40.23.
2-(thiophenThiophen-2-yl)-6-(1H-tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2e).
Brown solid. Yield 2.56 g, 90%. Mp = 297–298 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 6.83 (H, s, C3H), 7.15 (H, dd, C4’H, J1 = 5.0, J2 = 3.5), 7.54 (H, d, C3’H, J = 3.5), 7.68 (H, d, C5’H, J = 3.5), 8.56 (2H, s, NH2), 8.74 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 152.4, 151.3, 149.3, 148.0, 145.6, 135.2, 128.1, 127.5, 127.2, 93.3, 86.0. IR, ν, cm−1: 3348 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52(55), 93 (25), 148 (29), 227 (23), 241 (17), 256(70), 284 (100), [M]+). Anal. Calcd. for C11H8N8S: C 46.47, H 2.84, N 39.41; found: C 46.47, H 2.90, N 39.41.
3-carbonitrileCarbonitrile-6-(1H-tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2f).
Brown solid. Yield 1.93 g, 82%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 8.64 (H, s, C2H), 8.83 (H, s, NH), 8.97 (H, s, C5H), 9.35 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 152.2, 150.7, 150.6, 147.6, 146.6, 113.5, 89.4, 80.8. IR, ν, cm−1: 2231 (CN); 3321 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (100), 144 (92), 170 (18), 184 (15), 199 (52), 227 (63), [M]+). Anal. Calcd. for C8H5N9x1/2H2O: C 40.67, H 2.54, N 53.39; found: C 40.75, H 2.52, N 53.38.
3-ethoxycarbonylEthoxycarbonyl-6-(1H-tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2g).
Beige solid. Yield 2.54 g, 90%. Mp = 288–290 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 1.36 (3H, t, CH3, J = 7.2), 4.30 (2H, q, CH2, J = 7.2), 8.44 (H, s, C2H), 8.70 (H, s, NH), 9.06 (H, s, C5H), 9.12 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 161.9, 153.8, 150.1, 147.5, 147.0, 146.0, 101.2, 91.1, 59.4, 14.5. IR, ν, cm−1: 1683 (COOEt); 3315 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (100), 94 (70), 159 (100), 202 (46), 230 (27), 246 (11), 274 (50), [M]+). Anal. Calcd. for C10H10N8O2xH2O: C 41.10, H 4.11, N 38.36; found: C 41.00, H 4.15, N 38.37.
3-nitroNitro-6-(1H-tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2h).
Yellow solid. Yield 1.48 g, 60%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 8.93 (H, s, C2H), 8.93 (H, s, NH), 9.08 (H, s, C5H), 9.52 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 152.4, 152.1, 146.6, 143.3, 142.9, 122.4, 91.6. IR, ν, cm−1: 1557 (NO2); 1267 (NO2); 3325 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (100), 92 (18), 144 (14), 204 (5), 219 (63), 247 (50), [M]+). Anal. Calcd. for C7H5N9O2: C 34.01, H 2.04, N 51.00; found: C 33.98, H 1.89, N 51.13.
3-phenylPhenyl-6-(1H-tetrazol-5-yl)pyrazolo[1,5-a]pyrimidin-7-amine (2i).
Yellow solid. Yield 1.92 g, 69%. Mp = 277–279 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 7.20 (H, t, C4’H, J = 7.8), 7.39 (2H, t, C3’H, C5’H, J = 7.8), 8.11 (2H, dd, C2’H, C6’H, J1 = 7.8, J2 = 1.2), 8.63 (H, s, C2H), 8.74 (2H, s, NH2), 8.85 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 152.40, 147.88, 146.17, 144.43, 143.39, 131.98, 128.59, 125.88, 125.60, 109.31, 86.07. IR, ν, cm−1: 3292 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (50), 142 (25), 235 (11), 250 (42), 278 (100), [M]+). Anal. Calcd. for C13H10N8: C 56.11, H 3.62, N 40.27; found: C 56.02, H 3.66, N 40.26.
2-(methylthioMethylthio)-3-ethoxycarbonlyl-6-(1H-tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2j).
Yellow solid. Yield 2.91 g, 86%. Mp = 281–284 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 1.30 (3H, t, CH3, J = 7.2), 2.64 (3H, s, SCH3), 4.27 (2H, q, CH2, J = 7.2), 8.72 (2H, s, NH2), 8.84 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 162.0, 158.7, 152.2, 149.9, 148.9, 144.9, 98.8, 88.8, 59.5, 14.5, 12.8. IR, ν, cm−1: 1639 (COOEt); 3309 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (100), 144 (23), 292 (30), 320 (83), [M]+). Anal. Calcd. for C11H12N8O2SxH2O: C 39.05, H 4.14, N 33.14; found: C 38.99, H 4.15, N 33.10.
2-(methylthioMethylthio)-3-carbonitrile-6-(1H-tetrazol-5-yl)-7-aminopyrazolo[1,5-a]pyrimidine (2k).
Orange solid. Yield 2.02 g, 74%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 2.75 (3H, s, CH3), 8.85 (H, s, NH), 8.90 (H, s, C5H), 9.01 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 157.4, 153.2, 151.4, 150.3, 145.3, 113.1, 91.6, 78.9, 13.2. IR, ν, cm−1: 2217 (CN); 3364 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (100), 77 (37), 92 (49), 230 (31), 245 (43), 273 (52), [M]+). Anal. Calcd. for C9H7N9S: C 39.56, H 2.58, N 46.13; found: C 39.56, H 2.60, N 46.23.
6-(1H-tetrazolTetrazol-5-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10a).
Pale yellow solid. Yield 1.50 g, 74%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 8.47 (H, s, C2H), 8.82 (s, NH), 9.02 (H, s, C5H), 9.05 (s, NH). 13C NMR (150 MHz, DMSO-d6), δ, ppm.: 155.5, 155.4, 152.4, 146.8, 89.4. IR, ν, cm−1: 3322 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (98), 146 (32), 160 (10), 175 (100), 203 (60), [M]+). Anal. Calcd. for C6H5N9: C 35.47, H 2.48, N 62.05; found: C 35.55, H 2.38, N 62.01.
2-methylMethyl-6-(1H-tetrazol-5-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10b).
Beige solid. Yield 1.45 g, 64%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 3.08 (3H, s, CH3), 9.47 (2H, s, NH2), 9.54 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 164.5, 155.6, 153.7, 151.6, 145.9, 91.1, 14.8. IR, ν, cm−1: 3399 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (55), 83 (15), 174 (6), 189 (50), 217 (33), [M]+). Anal. Calcd. for C6H5N9x1/2H2O: C 37.17, H 3.54, N 55.75; found: C 37.17, H 3.55, N 55.71.
2-(methylthioMethylthio)-6-(1H-tetrazol-5-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10c).
Beige solid. Yield 1.94 g, 78%. Mp = 275–280 °C. 1H NMR (600 MHz, DMSO-d6), δ, ppm. (J, Hz): 2.7 (3H, s, CH3), 8.7 (H, s, NH), 8.91 (H, s, C5H), 8.96 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 166.9, 155.7, 152.0, 152.0, 145.6, 89.2, 13.2. IR, ν, cm−1: 3404 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (95), 176 (20), 192 (8), 221 (66), 249 (100), [M]+). Anal. Calcd. for C7H7N9S: C 33.73, H 2.83, N 50.58; found: C 33.77, H 2.81, N 50.53.
2-(benzylthioBenzylthio)-6-(1H-tetrazol-5-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10d).
Yellow solid. Yield 2.76 g, 85%. Mp = 263–266 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 4.55 (2H, s, CH2), 7.25 (H, t, C4’H, J = 6.4), 7.32 (2H, t, C3’H, C5H, J = 6.4), 7.51 (2H, d, C2’H, C6’H, J = 7.2), 8.86 (2H, s, NH2), 8.90 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 165.9, 155.8, 152.4, 152.1, 145.7, 137.7, 129.0, 128.5, 127.3, 89.9, 34.6. IR, ν, cm−1: 3323 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (10), 91 (100), 282 (3), 325 (20), [M]+). Anal. Calcd. for C13H11N9S: C 47.99, H 3.41, N 38.75; found: C 47.98, H 3.40, N 38.83.
6-(1H-tetrazolTetrazol-5-yl)-2-(trifluoromethyl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10e).
Orange solid. Yield 1.14 g, 42%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 8.89 (H, s, NH), 9.12 (H, s, C5H), 9.67 (H, s, NH). 19F NMR (376 MHz, DMSO-d6), δ, ppm.: −64.96. 13C NMR (100 MHz, DMSO-d6), δ, ppm. (J, Hz): 155.9, 155.0, 154.0 (q, J = 38.6), 152.2, 147.5, 119.3 (q, J = 269.6), 91.1. IR, ν, cm−1: 3383 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (100), 69 (50), 214 (12), 243 (80), 271 (30), [M]+). Anal. Calcd. for C7H4F3N9S: C 31.01, H 1.49, N 21.02; found: C 30.90, H 1.33, N 21.19.
2-ethoxycarbonylEthoxycarbonyl-6-(1H-tetrazol-5-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10f).
Yellow solid. Yield 1.70 g, 58%. Mp = 226–228 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 1.44 (3H, t, CH3, J = 7.2), 4.46 (2H, q, CH2, J = 7.2), 8.86 (H, s, NH), 9.08 (H, s, C5H), 9.59 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 159.7, 156.2, 155.6, 153.5, 152.3, 147.2, 90.3, 61.8, 14.1. IR, ν, cm−1: 1650 (COOEt); 3255 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 160 (45), 232 (12), 247 (8), 275 (32), [M]+). Anal. Calcd. for C9H9N9O2xH2O: C 36.86, H 3.75, N 43.00; found: C 36.99, H 3.73, N 43.20.
2-phenylPhenyl-6-(1H-tetrazol-5-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10g).
Yellow solid. Yield 1.73 g, 62%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 7.5 (2H, d, J = 6.8, C3’ H, C5’H), 7.52 (H, s, C4’H), 8.26 (2H, dd, J1 = 7.6, J2 = 2.8, C2’H, C6’H), 8.88 (2H, s, NH2), 8.99 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 164.1, 156.1, 152.4, 152.2, 146.6, 130.6, 130.3, 128.9, 126.9, 89.2. IR, ν, cm−1: 3361 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (39), 77 (65), 251 (55), 279 (32), [M]+). Anal. Calcd. for C12H9N9: C 51.61, H 3.25, N 45.14; found: C 51.61, H 3.23, N 45.28.
2-(furanFuran-2-yl)-6-(1H-tetrazol-5-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10h).
Beige solid. Yield 1.67 g, 60%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 6.65 (H, dd, C4’H, J = 2.4), 7.18 (H, d, C3’H, J = 3.2), 7.82 (H, s, C5’H), 8.99 (2H, s, NH2), 9.04 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 157.1, 155.8, 152.6, 152.2, 146.6, 145.6, 145.3, 112.56, 112.2, 89.5. IR, ν, cm−1: 3255 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (41), 94 (100), 160 (23), 212 (20), 226 (18), 241(65), 269 (65), [M]+). Anal. Calcd. for C10H7N9O2x1/2H2O: C 43.16, H 2.88, N 45.32; found: C 43.15, H 2.92, N 45.31.
2-(thiophenThiophen-2-yl)-6-(1H-tetrazol-5-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10i).
Beige solid. Yield 2.39 g, 83%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 7.24 (H, t, C4’H, J = 4.4), 7.78 (H, d, C3’H, J = 4.8), 7.86 (H, d, C5’H, J = 3.6), 8.89 (2H, s, NH2), 8.92 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm 160.4, 155.9, 152.5, 152.2, 146.4, 133.0, 129.5, 128.4, 128.3, 89.5. IR, ν, cm−1: 3250 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (53), 110 (100), 228 (15), 242 (33), 257 (48), 285 (52), [M]+). Anal. Calcd. for C10H7N9S: C 42.10, H 2.47, N 44.19; found: C 42.01, H 2.55, N 44.20.
2-(pyridinPyridin-3-yl)-6-(1H-tetrazol-5-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidine (10j).
Beige solid. Yield 2.49 g, 83%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 7.56 (H, t, C5’H, J = 6.4), 8.55 (H, d, C6’H, J = 7.6), 8.69 (H, s, C4’H), 8.96 (2H, s, NH2), 9.03 (H, s, C5H), 9.41 (H, s, C2’H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 161.9, 156.0, 152.6, 152.3, 150.9, 147.5, 146.5, 134.3, 126.2, 123.9, 89.6. IR, υ, cm−1: 3240 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (63), 105 (100), 223 (12), 237 (74), 252 (53), 280 (35), [M]+). Anal. Calcd. for C11H8N10: C 47.14, H 2.88, N 49.98; found: C 47.16, H 2.87, N 50.00.
6-(1H-tetrazolTetrazol-5-yl)-2,7-diamino-[1,2,4]triazolo[1,5-a]pyrimidine (10k).
Yellow solid. Yield 1.44 g, 66%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 6.10 (2H, s, C2-NH2), 8.34 (2H, s, C7-NH2), 8.72 (H, s, C5H). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 166.5, 155.3, 152.3, 150.3, 144.7, 88.6. IR, ν, cm−1: 3331 (NH2); 3425 (NH2). MS (EI, 70 eV), m/z, (Irel), %: 52 (35), 120 (40), 161 (7), 175 (13), 190 (21), 218 (18), [M]+). Anal. Calcd. for C6H6N10: C 33.03, H 2.77, N 64.20; found: C 32.90, H 2.77, N 64.38.
General procedure for the synthesis of sodium 5-(7-aminoazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ides (3,11).
A suspension of 0.005 mol (1 equiv.) of the corresponding 6-(tetrazol-5-yl)azolo[1,5-a]pyrimidin-7-amines and 0.005 mol (0.42 g, 1 equiv.) of sodium bicarbonate in 30 mL of deionized H2O was refluxed for 5 min under air atmosphere. The resulting solution was cooled to 25 °C, evaporated at reduced pressure to dryness to give the corresponding product.
Sodium 5-(7-aminopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (3a).
Beige solid. Yield 1.19 g, 99%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 6.34 (H, d, J = 2.0, C3H), 7.76 (H, s, NH), 7.98 (H, d, J = 2.0, C2H), 8.91 (H, s, C5H), 9.20 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 157.2, 148.3, 147.8, 144.9, 144.1, 94.7, 92.5. IR, ν, cm–1: 3333 (NH2). Anal. Calcd. for C7H5N8NaxH2O: C 34.72, H 2.91, N 46.27, found: C 34.72, H 3.00, N 46.39.
Sodium 5-(2-methyl-7-aminopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (3b).
Brown solid. Yield 1.21 g, 95%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 2.44 (3H, s, CH3), 6.11 (H, s, C3H), 7.52 (H, s, NH), 8.85 (H, s, C5H), 9.13 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 157.3, 153.3, 148.9, 147.4, 144.4, 94.1, 92.3, 14.4. IR, ν, cm–1: 3397 (NH2). Anal. Calcd. for C8H7N8NaxH2O: C 37.50, H 3.54, N 43.74, found: C 37.55, H 3.59, N 43.66.
Sodium 5-(2-(methylthio)-7-aminopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (3c).
Brown solid. Yield 1.38 g, 96%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 2.62 (3H, s, CH3), 6.24 (H, s, C3H), 7.68 (H, s, NH), 8.84 (H, s, C5H), 9.20 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 157.1, 154.0, 149.1, 147.7, 144.0, 92.8, 92.7, 14.1. IR, ν, cm–1: 3397 (NH2). Anal. Calcd. for C8H7N8NaSxH2O: C 33.33, H 3.15, N 38.87, found: C 33.21, H 3.10, N 38.86.
Sodium 5-(3-cyano-7-aminopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (3f).
Brown solid. Yield 1.32 g, 99%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 8.44 (H, s, C3H), 8.44 (H, s, NH), 9.08 (H, s, C5H), 9.64 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 156.25, 149.81, 149.78, 147.02, 145.81, 114.33, 96.28, 78.88. IR, ν, cm–1: 2241 (CN). Anal. Calcd. for C8H4N9NaxH2O: C 35.96, H 2.26, N 47.18, found: C 36.02, H 2.26, N 47.11.
Sodium 5-(3-(ethoxycarbonyl)-7-aminopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (3g).
Beige solid. Yield 1.55 g, 99%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 1.32 (3H, t, J = 7.2, CH3), 4.28 (2H, q, J = 7.2, CH2), 8.52 (H, s, C2H), 8.53 (H, s, NH), 9.10 (H, s, C5H), 9.47 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 162.26, 156.43, 149.68, 147.20, 146.58, 145.42, 100.14, 95.67, 59.19, 14.53. IR, ν, cm−1: 1608 (COOEt), 3326 (NH2). Anal. Calcd. for C10H9N8NaO2xH2O: C 38.22, H 3.53, N 35.66, found: C 38.20, H 3.57, N 35.66.
Sodium 5-(3-nitro-7-aminopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (3h).
Yellow solid. Yield 1.51 g, 99%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 8.95 (H, s, C2H), 9.01 (H, s, NH), 9.18 (H, s, C5H), 9.76 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 156.0, 150.9, 145.9, 142.6, 142.5, 121.5, 98.5. IR, ν, cm−1: 1373 (NO2), 1645 (NO2), 3316 (NH2). Anal. Calcd. for C7H4N9NaO2x2H2O: C 27.55, H 2.64, N 41.31, found: C 27.69, H 2.75, N 41.49.
Sodium 5-(3-phenyl-7-aminopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (3i).
Yellow solid. Yield 1.42 g, 95%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 7.15 (H, t, C4’H, J = 7.6), 7.37 (2H, t, C3’H, C5’H, J = 7.6), 7.90 (H, s, NH), 8.14 (2H, d, C2’H, C6’H, J = 7.6), 8.47 (H, s, C2H), 9.02 (H, s, C5H), 9.32 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 156.94, 147.89, 145.03, 144.95, 144.47, 141.92, 132.89, 128.52, 125.23, 125.20, 107.53, 93.34, 93.30. IR, ν, cm−1: 3366 (NH2). Anal. Calcd. for C13H9N8Na: C 52.00, H 3.02, N 37.32, found: C 52.03, H 2.99, N 37.20.
Sodium 5-(2-(methylthio)-3-(ethoxycarbonyl)-7-aminopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (3j).
Beige solid. Yield 1.78 g, 99%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 1.40 (3H, t, CH3, J = 7.2), 2.63 (3H, s, CH3), 4.31 (2H, q, CH2, J = 7.2), 7.99 (H, s, NH2), 9.07 (H, s, C5H), 9.50 (H, s, NH2). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 162.5, 157.7, 156.4, 149.2, 148.4, 144.3, 97.1, 95.9, 59.2, 14.6, 12.7. IR, ν, cm−1: 1670 (COOEt). Anal. Calcd. for C11H11N8NaO2SxH2O: C 36.67, H 3.64, N 31.10, found: C 36.67, H 3.69, N 30.95.
Sodium 5-(2-(methyilthio)-3-cyano-7-aminopyrazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (3k).
Orange solid. Yield 1.55 g, 99%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 2.75 (3H, s, CH3), 8.62 (H, s, NH), 8.98 (H, s, CH), 9.58 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 156.8, 156.2, 150.9, 149.5, 145.0, 113.7, 96.6, 77.7, 13.3. IR, ν, cm−1: 2224 (CN), 3382 (NH2). Anal. Calcd. for C9H6N9NaSxH2O: C 34.51, H 2.57, N 40.24, found: C 34.39, H 2.60, N 40.07.
Sodium 5-(7-amino-[1,2,4]triazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (11a).
Yellow solid. Yield 1.16 g, 95%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 8.49 (H, s, C2H), 8.60 (H, s, NH), 9.13 (H, s, C5H), 9.44 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 156.4, 154.8, 151.3, 145.9, 95.7. IR, ν, cm−1: 3342 (NH2). Anal. Calcd. for C6H4N9NaxH2O: C 29.64, H 2.49, N 51.84, found: C 29.66, H 2.51, N 51.96.
Sodium 5-(2-methyl-7-amino-[1,2,4]triazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (11b).
Beige solid. Yield 1.22 g, 95%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 2.48 (3H, s, CH3), 8.35 (H, s, NH), 9.01 (H, s, C5H), 9.36 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 163.9, 156.5, 155.3, 150.8, 145.4, 95.5, 14.8. IR, ν, cm−1: 3364 (NH2). Anal. Calcd. for C7H6N9NaxH2O: C 32.69, H 3.14, N 49.01, found: C 32.70, H 3.01, N 49.15.
Sodium 5-(2-(methylthio)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (11c).
Orange solid. Yield 1.43 g, 99%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 2.68 (3H, s, CH3), 8.36 (H, s, NH), 9.00 (H, s, C5H), 9.40 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 165.9, 156.3, 155.3, 150.6, 144.9, 96.0, 13.4. IR, ν, cm−1: 3376 (NH2). Anal. Calcd. for C7H6N9NaSxH2O: C 29.07, H 2.79, N 43.58, found: C 29.09, H 2.66, N 43.63.
Sodium 5-(2-(benzylthio)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (11d).
Pale yellow solid. Yield 1.65 g, 95%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 4.52 (2H, s, CH2), 7.22 (H, t, C4’H, J = 7.2), 7.29 (2H, t, C3’H, C5’H, J = 7.2), 7.48 (2H, d, C2’H, C6’H, J = 7.2), 8.23 (H, s, NH), 9.04 (H, s, C5H), 9.43 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 164.8, 156.3, 155.1, 150.6, 144.9, 137.9, 129.0, 128.5, 127.3, 96.2, 34.5. IR, ν, cm−1: 3363 (NH2). Anal. Calcd. for C13H10N9NaS: C 44.95, H 2.90, N 36.29, found: C 45.01, H 3.05, N 36.27.
Sodium 5-(2-(trifluoromethyl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (11e).
Orange solid. Yield 1.45 g, 93%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 8.88 (H, s, NH), 9.22 (H, s, C5H), 9.71 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm, (J, Hz): 156.1, 155.0, 154.5 (q, J = 38.6), 152.5, 146.4, 119.6 (q, J = 269.6), 97.6. IR, ν, cm−1: 3242 (NH2). Anal. Calcd. for C7H3F3N9NaxH2O: C 27.02, H 1.62, N 40.51, found: C 26.91, H 1.68, N 40.34.
Sodium 5-(2-phenyl-7-amino-[1,2,4]triazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (11g).
Yellow solid. Yield 1.43 g, 95%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 7.50 (H, m, C4’H), 7.52 (2H, m, C3’H, C5’H), 8.21 (H, s, NH), 8.26 (2H, d, C2’H, C6’H, J = 6.8), 9.10 (H, s, C5H), 9.49 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 163.5, 156.5, 155.6, 151.4, 145.7, 130.9, 130.3, 128.9, 126.9, 96.1. IR, ν, cm−1: 3258 (NH2). Anal. Calcd. for C12H8N9Na: C 47.84, H 2.68, N 41.85, found: C 47.84, H 2.66, N 41.90.
Sodium 5-(2-(furan-2-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (11h).
Orange solid. Yield 1.48 g, 96%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 6.65 (H, dd, C4’H, J1 = 2.0, J2 = 1.6), 7.15 (H, d, C3’H, J = 2.8), 7.80 (H, s, C5’H), 8.47 (H, s, NH), 9.10 (H, s, C5H), 9.50 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 156.7, 156.4, 155.2, 151.4, 146.2, 145.7, 144.9, 112.1, 111.8, 96.2. IR, ν, cm−1: 3228 (NH2). Anal. Calcd. for C10H6N9NaxH2O: C 38.84, H 2.61, N 40.77, found: C 38.90, H 2.66, N 40.59.
Sodium 5-(2-(pyridin-3-yl)-7-amino-[1,2,4]triazolo[1,5-a]pyrimidin-6-yl)tetrazol-1-ide (11j).
Pale yellow solid. Yield 1.49 g, 93%. Mp > 300 °C. 1H NMR (400 MHz, DMSO-d6), δ, ppm. (J, Hz): 7.53 (H, dd, C5’H, J1 = 8.0, J2 = 3.2), 8.37 (H, s, NH), 8.54 (H, dt, C4’H, J1 = 8.0, J2 = 1.6), 8.66 (H, dd, C6’H, J1 = 3.2, J2 = 1.6), 9.13 (H, s, C5H), 9.40 (H, d, C2’H, J = 2.4), 9.54 (H, s, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm.: 161.8, 156.8, 156.0, 151.9, 151.5, 148.3, 146.2, 134.6, 127.3, 124.5, 96.8. IR, ν, cm−1: 3348 (NH2). Anal. Calcd. for C11H7N10NaxH2O: C 41.26, H 2.83, N 43.74, found: C 41.21, H 2.71, N 43.88.
3.2. CK2 Assay
Kinase activity was determined using the CK2a1 enzyme system (Promega V4482, Madison, WI, USA) and the ADP-GloTM kit (Promega V9101, Madison, USA) in white 384-well plates (ThermoFisher). The assay was carried out using 10 ng/well of N-GST labeled human recombinant CK2a1 expressed in Sf9 cells, 0.1 µg/µL casein, 10 µM ATP in a 40 mM Tris buffer (pH 7.50) containing 20 mM MgCl2, 0.1 mg/mL BSA and 50 µM DTT. Compounds were introduced in 1.25% DMSO and preincubated with kinase at 450 rpm. within 10 min. The reaction was carried out during 60 min. at 25 °C in PST-60HL shaker (Biosan, Latvia). ATP-dependent luminescence was measured at an integration time of 1000 ms using Infinite M200 PRO microplate reader (Tecan GmbH, Grödig, Austria). The experiments were run in two replicates. The activity of CK2 in sample wells was normalized against control and enzyme-blank wells, and IC50 values were calculated using 3-parameter log-logistic nonlinear regression with Prism 8.0 (GraphPad Software, San Diego, CA, USA).
4. Conclusions
We have explored the chemical space around azolo[1,5-a]pyrimidines as a valuable scaffold for the design of potent CK2 inhibitors. Tetrazolyl-containing azolopyrimidines have been proposed as perspective structural analogues of nitroazoloazines with a wide range of useful biological activity. A method for the synthesis of 6-(tetrazol-5-yl)-7-aminoazolo[1,5-a]pyrimidines based on azide-nitrile cycloaddition was developed. Optimized conditions allowed us to obtain a library of tetrazolyl-containing azolopyrimidines and screened it for CK2 inhibitory activity. Some SAR have been revealed as azolo[1,5-a]pyrimidines of this series, which showed a higher affinity to CK2 then corresponding [1,2,4]triazolo[1,5-a]pyrimidines. We have found several low micromolar and nanomolar CK2 inhibitors and leader compound 2i demonstrated IC50 = 45 nM. These findings are going to be used for further optimization of azoloazines as promising bioactive compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27248697/s1, NMR, IR, mass spectra of compounds 2, 3, 10, 11.
Author Contributions
Synthesis, G.V.U. and K.V.S., studying of the CK2 activity, D.A.B. and E.V.S., methodology, V.L.R., S.K.K. and A.A.S., writing—original draft preparation, K.V.S. and D.A.B., writing—review and editing, K.V.S., V.L.R., S.K.K. and A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation, State Contract № FEUZ-2020–0058 (H687.42B.223/20).
Institutional Review Board Statement
All animal procedures were carried out under the generally accepted ethical standards for the manipulations on animals adopted by the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (1986) and taking into account the International Recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimental research (1997). The study was approved by the Local Ethics Committee of the Volgograd State Medical University (registration No. IRB 00005839 IORG 0004900, OHRP), Certificate No. 2021/056, 15 June 2021. All sections of this study adhere to the ARRIVE Guidelines for reporting animal research.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The team of authors would like to thank the Laboratory for Comprehensive Research and Expert Evaluation of Organic Materials under the direction of O. S. Eltsov.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1–3, 9, 11 are available from the authors.
References
- Pinna, L.-A. Protein kinase CK2: A challenge to canons. J. Cell Sci. 2002, 115, 3873–3878. [Google Scholar] [CrossRef] [PubMed]
- Axtell, R.-C.; Xu, L.; Barnum, S.-R.; Raman, C. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: Protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J. Immunol. 2006, 177, 8542–8549. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.-N.; Ramji, D.-P. Protein kinase CK2, an important regulator of the inflammatory response? J. Mol. Med. 2008, 86, 887–897. [Google Scholar] [CrossRef]
- Murtaza, I.; Wang, H.-X.; Feng, X.; Alenina, N.; Bader, M.; Prabhakar, B.S.; Li, P.-F. Down-regulation of catalase and oxidative modification of protein kinase CK2 lead to the failure of apoptosis repressor with caspase recruitment domain to inhibit cardiomyocyte hypertrophy. J. Biol. Chem. 2008, 283, 5996–6004. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.I.; Puustinen, P.; Gabrenaite, R.; Vihinen, H.; Rönnstrand, L.; Valmu, L.; Kalkkinen, N.; Mäkinen, K. Phosphorylation of the potyvirus capsid protein by protein kinase CK2 and its relevance for virus infection. J. Plant Cell. 2003, 15, 2124–2139. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.S.; Kim, S.; Leung, P.C.; Auersperg, N.; Pelech, S.L. Profiling of protein kinases in the neoplastic transformation of human ovarian surface epithelium. J. Gynecol. Oncol. 2001, 82, 305. [Google Scholar] [CrossRef]
- Ahmad, K.A.; Wang, G.; Unger, G.; Slaton, J.; Ahmed, K. Protein kinase CK2--a key suppressor of apoptosis. J. Adv. Enzym. Regul. 2008, 48, 179–187. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, H.W. Casein Kinase 2 Is Activated and Essential for Wnt/β-Catenin Signaling. J. Biol. Chem. 2006, 281, 18394. [Google Scholar] [CrossRef] [PubMed]
- CX-4945 granted orphan drug designation. Oncol. Times 2017, 39, 23. [CrossRef]
- Cozza, G.; Pinna, L.A. Casein kinases as potential therapeutic targets. Expert Opin. Ther. Targets 2016, 20, 319–340. [Google Scholar] [CrossRef]
- Dowling, J.E.; Alimzhanov, M.; Bao, L.; Chuaqui, C.; Denz, C.R.; Jenkins, E.; Larsen, N.A.; Lyne, P.D.; Pontz, T.; Ye, Q.; et al. Potent and Selective CK2 Kinase Inhibitors with Effects on Wnt Pathway Signaling in Vivo. ACS Med. Chem. Lett. 2016, 7, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.I.; Drewry, D.H.; Pickett, J.E.; Tjaden, A.; Krämer, A.; Müller, S.; Gyenis, L.; Menyhart, D.; Litchfield, D.W.; Knapp, S.; et al. Development of a Potent and Selective Chemical Probe for the Pleiotropic Kinase CK2. J. Cell Chem. Biol. 2021, 28, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Perretta, C.; Erickson, P.; Margosiak, S.; Almassy, R.; Lu, J.; Averill, A.; Yager, K.M.; Chu, S. Structure-based design, synthesis, and study of pyrazolo[1,5-a][1,3,5]triazine derivatives as potent inhibitors of protein kinase CK2. Bioorg. Med. Chem. Lett. 2007, 17, 4191–4195. [Google Scholar] [CrossRef] [PubMed]
- Pierre, F.; Chua, P.C.; O’Brien, S.E.; Siddiqui-jain, A.; Bourbon, P.; Haddach, M.; Michaux, J.; Nagasawa, J.; Schwaebe, M.K.; Stefan, E.; et al. Discovery and SAR of 5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-8-carboxylic Acid (CX-4945), the First Clinical Stage Inhibitor of Protein Kinase CK2 for the Treatment of Cancer. J. Med. Chem. 2011, 54, 635–654. [Google Scholar] [CrossRef]
- Cozza, G.; Gianoncelli, A.; Bonvini, P.; Zorzi, E.; Pasquale, R.; Rosolen, A.; Pinna, L.A.; Meggio, F.; Zagotto, G.; Moro, S. Urolithin as a converging scaffold linking ellagic acid and coumarin analogues: Design of potent protein kinase CK2 inhibitors. ChemMedChem 2011, 6, 2273–2278. [Google Scholar] [CrossRef]
- Savateev, K.V.; Fedotov, V.V.; Rusinov, V.L.; Kotovskaya, S.K.; Spasov, A.A.; Kucheryavenko, A.F.; Vasiliev, P.M.; Kosolapov, V.A.; Sirotenko, V.S.; Gaidukova, K.A.; et al. Azolo[1,5-a]pyrimidines and Their Condensed Analogs with Anticoagulant Activity. Molecules 2022, 27, 274. [Google Scholar] [CrossRef]
- Spasov, A.; Kosolapov, V.; Babkov, D.; Klochkov, V.; Sokolova, E.; Miroshnikov, M.; Borisov, A.; Velikorodnaya, Y.; Smirnov, A.; Savateev, K.; et al. Discovery of Nitro-azolo[1,5-a]pyrimidines with Anti-Inflammatory and Protective Activity against LPS-Induced Acute Lung Injury. Pharmaceuticals 2022, 15, 537. [Google Scholar] [CrossRef] [PubMed]
- Savateev, K.V.; Spasov, A.A.; Rusinov, V.L. Small synthetic molecules with antiglycation activity. Structure-activity relationship. Russ. Chem. Rev. 2022, 91, 5041. [Google Scholar] [CrossRef]
- Savateev, K.V.; Rusinov, V.L.; Kotovskaya, S.K.; Spasov, A.A.; Naumenko, L.V.; Taran, A.S.; Brigadirova, A.A.; Yakovlev, D.S.; Sultanova, K.T.; Shcherbakova, N.M. The Effects of Nitroazolopyrimidines on the A1 Adenosine Receptor and Intraocular Pressure in Rats. Russ. J. Bioorg. Chem. 2022, 48, 777. [Google Scholar] [CrossRef]
- Savateev, K.V.; Ulomsky, E.N.; Fedotov, V.V.; Rusinov, V.L.; Sivak, K.V.; Lyubishin, M.M.; Kuzmich, N.N.; Aleksandrov, A.G. 6-Nitrotriazolo[1,5-a]pyrimidines as promising structures for pharmacotherapy of septic conditions. Russ. J. Bioorg. Chem. 2017, 43, 421. [Google Scholar] [CrossRef]
- Ostrovskii, V.A.; Popova, E.A.; Trifonov, R.E. Developments in Tetrazole Chemistry. Adv. Heterocycl. Chem. 2017, 123, 1–63. [Google Scholar] [CrossRef]
- Herr, J.R. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: Medicinal chemistry and synthetic methods. Bioorg. Med. Chem. 2002, 10, 3379. [Google Scholar] [CrossRef] [PubMed]
- Pegklidou, K.; Koukoulitsa, C.; Nicolaou, I.; Demopoulos, V.J. Design and synthesis of novel series of pyrrole based chemotypes and their evaluation as selective aldose reductase inhibitors. A case of bioisosterism between a carboxylic acid moiety and that of a tetrazole. Bioorg. Med. Chem. 2010, 18, 2107. [Google Scholar] [CrossRef] [PubMed]
- Pinter, T.; Jana, S.; Courtemanche, R.J.M.; Hof, F. Recognition Properties of Carboxylic Acid Bioisosteres: Anion Binding by Tetrazoles, Aryl Sulfonamides, and Acyl Sulfonamides on a Calix[4]arene Scaffold. J. Org. Chem. 2011, 76, 3733. [Google Scholar] [CrossRef] [PubMed]
- Allen, F.H.; Groom, C.R.; Liebeschuetz, J.W.; Bardwell, D.A.; Olsson, T.S.G.; Wood, P.A. The Hydrogen Bond Environments of 1H-Tetrazole and Tetrazolate Rings: The Structural Basis for Tetrazole–Carboxylic Acid Bioisosterism. J. Chem. Inf. Model. 2012, 52, 857. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Lu, Z.-J.; Dong, W.-S.; Zhang, J.-G.; Sinditskii, V.P. Detonation performance enhancement through a positional isomerism modification strategy. New J. Chem. 2022, 46, 13874–13879. [Google Scholar] [CrossRef]
- Urakov, G.V.; Savateev, K.V.; Rusinov, V.L. A versatile method for the synthesis of 7-aminoazolo[1,5-a]pyrimidine-6-carbonitriles. Dokl. Chem. 2022, in press. [Google Scholar]
- Cantillo, D.; Gutmann, B.; Kappe, C.O. An Experimental and Computational Assessment of Acid-Catalyzed Azide-Nitrile Cycloadditions. J. Org. Chem. 2012, 77, 10882. [Google Scholar] [CrossRef]
- Roh, J.; Artamonova, T.V.; Vávrová, K.; Koldobskii, G.I.; Hrabálek, A. Practical synthesis of 5-substituted tetrazoles under microwave irradiation. Synthesis 2009, 13, 2175–2178. [Google Scholar] [CrossRef]
- Gutmann, B.; Roduit, J.-P.; Roberge, D.; Kappe, C.O. Synthesis of 5-Substituted 1H-Tetrazoles from Nitriles and Hydrazoic Acid by Using a Safe and Scalable High-Temperature Microreactor Approach. Angew. Chem. Int. Ed. 2010, 49, 7101–7105. [Google Scholar] [CrossRef] [PubMed]
- Demko, Z.P.; Sharpless, K.B. Preparation of 5-Substituted 1H-Tetrazoles from Nitriles in Water. J. Org. Chem. 2001, 66, 7945–7950. [Google Scholar] [CrossRef]
- Cantillo, D.; Gutmann, B.; Kappe, C.O. Mechanistic Insights on Azide−Nitrile Cycloadditions: On the Dialkyltin Oxide−Trimethylsilyl Azide Route and a New Vilsmeier−Haack-Type Organocatalyst. J. Am. Chem. Soc. 2011, 133, 4465–4475. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Roy, A.; Ghosh, M.; Mitra, S.; Majee, A.; Hajra, A. Organocatalysis by an aprotic imidazolium zwitterion: A dramatic anion–cation cooperative effect on azide–nitrile cycloaddition. RSC Adv. 2014, 4, 6116. [Google Scholar] [CrossRef]
- Gazizov, D.A.; Fedotov, V.V.; Gorbunov, E.B.; Ulomskiy, E.N.; Yeltsov, O.S.; Rusinov, G.L.; Rusinov, V.L. Effective method for the synthesis of azolo[1,5-a]pyrimidin-7-amines. Chem. Heterocycl. Compd. 2019, 55, 573–577. [Google Scholar] [CrossRef]
- Trifonov, R.E.; Ostrovskii, V.A. Protolytic equilidria in tetrasoles. Russ. J. Org. Chem. 2006, 42, 1585. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).