Abstract
Honeybee products, as multicomponent substances, have been a focus of great interest. The present work aimed to perform the nutritional and chemical profiling and biochemical characterization of bee pollen (BP), bee bread (BB), and royal jelly (RJ) and study their applications in the fortification of functional fermented dairy products. Their effects on starter cultures and the physicochemical and sensorial quality of products were monitored. A molecular networking analysis identified a total of 46 compounds in the three bee products that could be potential medicines, including flavonoids, fatty acids, and peptides. BB showed the highest protein and sugar contents (22.57 and 26.78 g/100 g), which cover 45.14 and 53.56% of their daily values (DVs), with considerable amounts of the essential amino acids threonine and lysine (59.50 and 42.03%). BP, BB, and RJ can be considered sources of iron, as 100 g can cover 141, 198.5, and 94.94% of DV%, respectively. BP was revealed to have the highest phenolic and flavonoid contents (105.68 and 43.91 µg/g) and showed a synergetic effect when mixed with RJ, resulting in increased antioxidant activity, while BB showed a synergetic effect when mixed with RJ in terms of both antioxidant and proteolytic powers (IC50 7.54, 11.55, 12.15, 12.50, and 12.65 cP compared to the control (10.55 cP)), reflecting their organoleptic properties and highlighting these health-oriented products as promising natural products for human health care.
1. Introduction
The development of advanced analytical tools and their applications have opened new possibilities to expand knowledge in the food science field. Bioactive compounds are a key challenge in the development of interventions involving tailored functional diets [1]. Mass Spectrometry combined with the international molecular networking GNPS database of food metabolite data can be a powerful resource to understand the molecular landscape and enables the reporting of research results in the context of foods [2,3,4].
Honeybee colonies produce various products, such as honey, bee pollen (BP), bee bread (BB), royal jelly (RJ), propolis, beeswax, and bee venom. BP, as a source of protein for the honeybee colony, is a highly nutritious substance that is commonly used to meet the growth and development needs of honeybees, including feeding broods and supplying the protein required for RJ secretion [5]. Honeybee workers collect BP from plant anthers and then place it in baskets (corbiculae) that are situated on their hind legs. In the composition of BP, there are more than 250 identified compounds, including amino acids, vitamins, macro- and micronutrients, and flavonoids [6,7]. BB is created from collected BP that is dampened with bee workers’ saliva, fragmented, packed in honeycomb cells, and then covered with a thin layer of honey, after which it is fermented and preserved by rising lactic acid under anaerobic conditions, which increases its nutritional value, digestibility, and absorption by the human body [8]. Additionally, BB contains considerably large amounts of peptides and all essential amino acids, which make it an excellent food product that could supplement deficient nutrients and also function to help eliminate various toxins [8]. RJ is secreted by nurse bee (6–12 days old) hypopharyngeal glands, playing an important role in larval development. Worker bee larvae are fed with RJ for 3 days, and only larvae that develop into queen bees and adult honeybee queens are continuously fed with large quantities of RJ [9]. RJ has been utilized in the pharmaceutical and food industries, as it provides essential amino acids, lipids, vitamins, acetylcholine (Ach), and other nutrients and active compounds of larval and adult queen food [10].
Functional foods are one of the growing food industries, where proteases are in great demand. Proteases are master enzymes in the digestive system, anatomically beginning at the oral cavity, proceeding to the esophagus, stomach, and intestinal tract, and ending with the colon [11]. The consequence of the proteolytic action of these enzymes is a change in the molecular conformation of native proteins to produce functional bioactive products that are widely used in food systems as additives [12]. These bioactive peptides can improve the functional and nutritional properties of proteins. They may aid in flavor and reduce the milk allergens in dairy products. Studies have indicated that some dietary proteins hydrolyzed by proteases show greater biological effects; for example, RJ peptides hydrolyzed by various proteases have been reported to show functional activities. The antioxidant activities derived from Major Royal Jelly Proteins (MRJPs) and peptides include scavenging activities, cholesterol-lowering effects, and anti-hypertensive ability and support the ongoing applications of available proteases [13,14]. However, it is difficult to know whether these enzymes are produced by the bee and secreted in RJ or whether they are of vegetal origin, which encourages more investigations in this area [15].
The objective of this study was the assessment of the nutritional profiles, chemical profiles, antioxidant potentials, and functional proteolytic enzymatic activities of honeybee products (BP, BB, and RJ), in addition to the evaluation of the effect of their combinations on their activities and their application in functional fermented dairy products by monitoring their impacts on starter cultures and consumer acceptance. This would pave the way to encouraging manufacturers to use fortifications with honeybee products in healthy functional food products as part of our ongoing project on honeybees and bee products [16,17,18].
2. Results and Discussion
2.1. Chemical Composition of Honeybee Products
The chemical compositions of BP, BB, RJ, and their mixture are exhibited in Table 1. BP showed a high content of total solids (TS) at 82.59% and was rich in protein and total sugars (21.09 and 19.69 g/100 g, respectively). Its ash (2.35%) was found to consist mainly of K, P, Ca, Na, and Mg in descending order (with values of 480.41, 226.15, 138.31, 98.65, and 96.41 mg/100 g, respectively), small amounts of Fe, Zn, and Mn, and traces of Cu. The results are in agreement with [7]. The main outcome measure of nutrient-based standards is whether the food can fulfill daily macro- and micronutrient requirements in a diet [19]. In this context, BP can play role in the fulfillment of daily protein and sugar requirements as macronutrients. On the other hand, it provides generous amounts of minerals, especially Fe and Mn [20].

Table 1.
Nutritional profiles and daily values (%) of bee products and their mixture.
Since BB is bee-collected pollen with a mixture of honey and bee salivary enzymes stored inside the beehive [21], its protein content was found to be comparable to that of BP (22.57 g/100 g), with higher contents of moisture and sugars (25.92 and 26.78 g/100 g, respectively). Compared to BP, BB contains more reducing sugars due to the effect of glycolytic enzymes, which convert complex sugars to simple sugars [22]. The potassium content in BB can provide 3.25% of DV with a value of 518.77 mg/100 g, followed by Ca, P, Na, Mg, and Fe. According to the required daily values of minerals, BB can be considered a rich source of Fe, Mn, and Cu since it provides 198.5, 91.5, and 70% of DV, respectively. Naturally, BB has various compositions depending on its BP source; however, the obtained nutrient composition is in agreement with that reported by [23].
Water was found to be the main component of RJ with a value of 63.81%, while its dry matter is composed mainly of carbohydrates (sugars) and proteins (14.78 and 12.89 g/100 g, respectively), which fulfill 29.56 and 25.78% of the daily requirements (Table 1). The predominant elements in descending order are K, P, Na, Ca, and Mg, with values of 124.01, 104.44, 74.42, 52.44, and 30.13 mg/100 g, respectively (Table 1). In addition, it can provide 17.09 and 0.50 mg/100 g of iron and copper, which cover 94.94 and 25% of the daily requirements, respectively. These results agree with [10,24], who also stated that the quantitative presence of RJ metals is related either to factors outside the colony, including the environment, food, and production period, or to internal factors, such as physiological factors tied to the nurse worker bees. From the nutritional perspective, which was supported by the obtained results, the combination of the three tested honeybee products (BP, BB, and RJ in a tri-mix) is considered useful by providing balanced contents of protein and sugars (37.38 and 39.38% of DV, respectively) and compensating for the lack of some minerals in the individual components, which encourages its application in functional foods’ fortification. The differences in the concentration levels of macro- and micronutrients in BP, BB, and RJ may be due to the nature of the sample and different environmental and physiological factors, such as the source of pollen, the nature of bee feeding, enzymes, and metabolic reactions in bee organs. In all cases, BP, BB, and RJ are considered good sources of proteins, sugars, and minerals.
2.2. Amino Acid Composition and Score (AAS%)
The amino acid profiles and amino acid scores (%) of BP, BB, and RJ are mentioned in Table 2. The term “complete protein” refers to foods that contain all nine essential amino acids in the proportions required to build proteins in the body. In contrast, “incomplete protein” refers to foods that have all essential amino acids but not in the correct proportions and is termed “limiting amino acid” [25]. The amino acid profiles of the three tested honeybee products (Table 2) reflect the high quality of protein, as they are shown to be significant sources of complete protein with a good balance of essential amino acids that achieve full protein adequacy in adults, especially in vegetarian/vegan diets. The main essential amino acids in BP were leucine, lysine, phenylalanine, + tyrosine, and threonine, with contents of 5.34, 5.04, 5.02, and 4.17 mg/g, covering 38.13, 42.03, 35.86, and 59.50%, respectively, for adult daily requirements, according to [26].

Table 2.
Amino acid profiles of bee pollen, bee bread, and royal jelly.
Additionally, BP contained considerable amounts of glutamic acid and proline (9.01 and 6.63 mg/g). Although neither glutamine nor proline is traditionally considered essential in the human diet, they are required in increased amounts in some pathological conditions and thus are usually classified as conditionally essential. Glutamine supplementation has been investigated in clinical work and sports nutrition; consequently, glutamine is readily available in many forms, ranging from pure glutamine powder to glutamine-fortified drinks and energy bars. Proline, however, has received very little attention; proline supplements (up to 488 mg/kg has been used to treat patients with gyrate atrophy due to a lack of ornithine aminotransferase) are not associated with reports of any deleterious side effects. However, it is generally accepted that the majority of proline synthesis in the body occurs via the glutamate/P5C synthase pathway [29]. BP is well-known as a rich source of proteins and essential amino acids with many pharmacological functions [30]. BB had high contents of phenylalanine + tyrosine, leucine, lysine, and methionine + cysteine (9.53, 9.46, 9.28, and 5.44 mg/g protein) that cover nearly 25% of AAS (26.58, 24.80, 22.07, and 26.58%, respectively). On the other hand, BB was revealed to contain notable amounts of proline and glutamic and aspartic acids (41.32, 39.32, and 14.74 mg/g, respectively). Egyptian BB had higher contents of amino acids than Malaysian BB according to data reported by [21]. RJ was found to contain good amounts of threonine, phenylalanine + tyrosine, lysine, and histidine, covering 23.54, 18.13, 18.05, and 17.07% of AAS, respectively. RJ contained limited amounts of non-essential amino acids, as aspartic acid had the highest content (11.56 mg/g). The obtained data on the amino acid composition of RJ are in agreement with [31]. Notably, both BP and RJ showed a deficiency of sulfur-containing amino acids, namely, methionine + cysteine, as they were shown to be their limiting amino acids (2.63 and 1.13 mg/g), while they are adequate in BB, covering 26.85% of AAS. Consequently, mixing protein sources with complementary essential amino acids within the same meal may achieve the limit of complete protein with high quality in human diets, ensuring long-term adequacy [32].
2.3. LC–MS–MS Analysis and International Molecular Networking GNPS Database of Bee Product Metabolites
Royal jelly, BB, and BB are examples of bee products that are rich in active compounds, such as proteins, amino acids, enzymes, fatty acids, esters, peptides, phenolic compounds, minerals, and vitamins [8,33,34]. Many factors, including climatic, botanical, geographic, and storage conditions, have an impact on the nutritional and chemical compositions of bee products [35,36].
The bee products’ metabolomic mass profiles were determined using Global Natural Products Social Molecular Networking (GNPS) and MS-MS data in positive ionization mode, as shown in Figure 1 and Table 3. Each metabolite is represented by a node in the GNPS network, where metabolites of similar classes are grouped together to form a single cluster. A total of 222 nodes of bee products (BP, BB, and RJ) were identified. BP metabolites are demonstrated as pink-colored nodes, BB is blue, and orange nodes are RJ, as shown in Figure 1A.
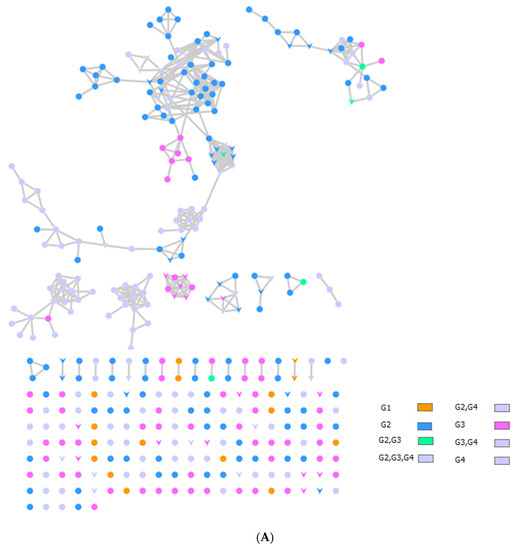
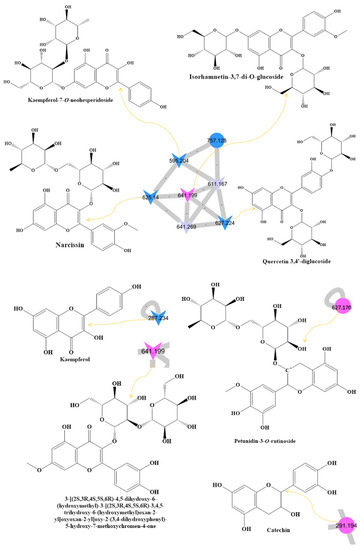
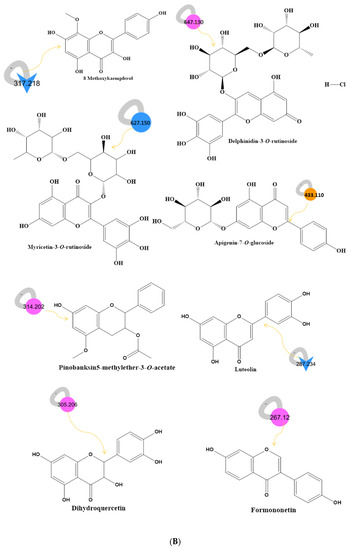
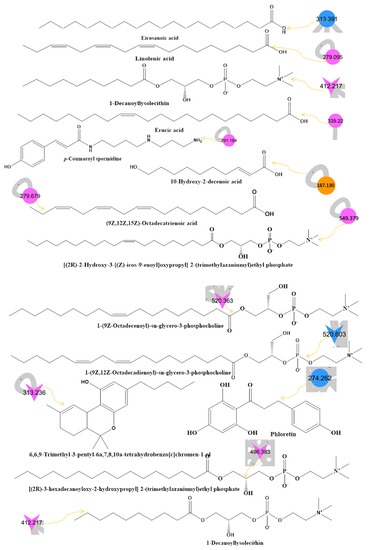
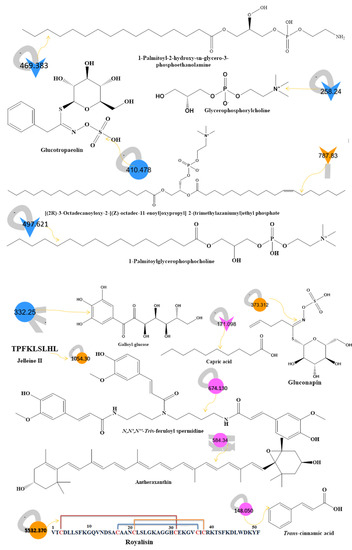
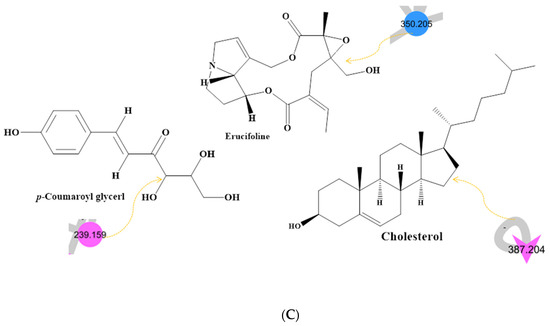
Figure 1.
(A) Metabolite molecular network of the bee product (royal jelly, bee bread, and bee pollen) extracts and the blank injected before the extracts. The nodes refer to parent masses of the extract metabolites. The circular nodes refer to whole-parent masses that have unique detected peaks in the molecular network. The triangle nodes represent parent ions that have been identified in the GNPS molecular network. G1: royal jelly nodes are orange; G2: bee pollen is colored pink; G3: bee bread is blue; and G4: blank solvent. (B) Flavonoids found in bee product extracts, including royal jelly, bee bread, and bee pollen. (C) Fatty acid, glycerophospholipid, styrene, and phenol metabolites from bee product (royal jelly, bee bread, and bee pollen) extracts.

Table 3.
LC-MS/MS data of the annotated metabolites in bee product extracts.
Through LC-MS-MS analysis and molecular networking analysis, a total of 46 compounds were identified. These compounds included flavonoids, fatty acids, alkylglucosinolates, peptides, pyrrolizidine alkaloid, tannins, glycerophospholipids, steroids, epoxycarotenol, styrenes, phenols, glycerophospholipids, dihydrochalcone, and benzopyrans (Table 3). Sixteen parent ions matched known standards in the GNP library. The two main metabolites found in bee products, as listed in Table 3, are flavonoids and fatty acids. Flavonoids were found in all samples, and they were the most common class of natural products found (Figure 1B). Flavonoids were potent in BB and BB, whereas very few flavonoids were identified in the samples of RJ. Flavonoids appeared in the aglycone or flavonoid glycoside form as flavonol, flavone, and isoflavone. Since flavonoids possess anti-inflammatory, antibacterial, antifungal, antiviral, and anticancer activities, they are beneficial to human health [17,37,38]. RJ is distinguished from other bee products by the presence of an unsaturated fatty acid termed 10-hydroxy-2-decenoic acid (Table 3 and Figure 1C). This compound has anti-inflammatory and bactericidal effects on human colon cancer cells [39] and could be a potential medicine for rheumatoid arthritis [40]. The antibacterial compounds royalisin and jelleine II were found in RJ [41,42].
2.4. Antioxidant Potentials
Figure 2A–C present the antioxidant potentials of BP, BB, RJ, and their mixes, which are in accordance with the chemical profiling results. BP had the highest total phenolic content (TPC) (105.68 μg/g), followed by RJ and bee bread (66.35 and 56.93 μg/g) (Figure 2A). Just as the chemical profiling showed the potent contents of flavonoids in BP and BB, the total flavonoid content (TFC) assessment also revealed that BP had the highest contents of these compounds, followed by BB and then RJ (43.91, 20.49, and 15.29 μg/g, respectively) (Figure 2B).
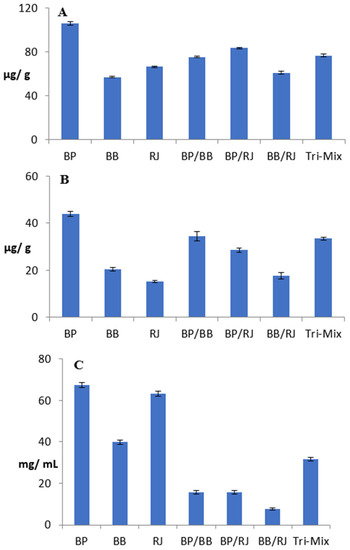
Figure 2.
Antioxidant potentials of bee pollen, bee bread, royal jelly, and their mixes. (A): Total phenolic content (μg/g); (B): total flavonoid content (μg/g); (C): antioxidant potentials represented as IC50 (mg/mL), the inhibitory concentration at which 50% of DPPH radicals are scavenged. BP: bee pollen; BB: bee bread; RJ: royal jelly. Data represented are means of duplicates ± SD.
The bi-mix (BP/RJ, BP/BB, and BB/royal jelly) and tri-mix results highlighted the role of BP in increasing TPC and TFC, which are correlated with the mixing ratios (1:1 or 1:1:1). On the other hand, while scavenging potentials, represented as IC50 (Figure 2C), of BP, bee bread, and RJ were recorded at 67.37, 39.84, and 63.10 mg/mL, the bi-mixes and tri-mix were found to have significantly higher antioxidant powers with lower IC50 values than those of the individual components. The IC50 values of the two BP bi-mixes were comparable (15.67 and 15.71 mg/mL), while the Tri-Mix showed less of an antioxidant effect with a higher IC50 of 31.43 mg/mL. Özkök & Silici (2017) reported similar results for BP, RJ, and their mixtures. The BB/RJ bi-mix showed remarkable results, as its IC50 had the lowest value (7.54 mg/mL); this significant result drew attention to another source of scavenging potentials besides phenolic and flavonoid contents, specifically enzymatic activity. Enzymatic hydrolysis was reported to improve the functional properties and biological activities of protein by-products, in addition to the DPPH radical-scavenging potentials of proteases that were previously reported by [12,69]. In order to support this hypothesis, qualitative, quantitative, and SDS-PAGE analyses were performed to assess the proteolytic activities of honeybee products and their mixes.
2.5. Protease Activity of Honeybee Products
Honeybee products’ crude extracts and their mixes were evaluated qualitatively and quantitatively for their potential proteolytic activities, and the results are illustrated in Table 4. In qualitative screening, royal jelly, BP/RJ, BB/RJ, and the tri-mix (BP/BB/RJ) exhibited positive proteolytic activity on casein agar (pH 7), with diameters of 1, 0.5, 0.5, and 0.5 mm, respectively (Table 4 and Figure 3). When comparing the screening results of the quantitative analysis at pH 7, RJ (5.22 U) showed approx. two times the proteolytic activity of BP and bee bread (2.20, 2.73 U), as the latter two bee products’ activities could not be revealed via screening by the qualitative method.

Table 4.
Qualitative and quantitative assessment of protease activities for bee products and their mixes.
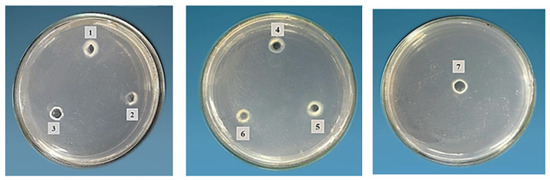
Figure 3.
Proteolytic activity on casein agar of bee products: 1, bee pollen; 2, bee bread; 3, royal jelly; 4, bee pollen/bee bread; 5, bee pollen/royal jelly; 6, bee bread/royal jelly; 7, tri-mix.
On the other hand, mixing RJ with BP significantly decreased its activity to 4.23 U. On the contrary, royal jelly mixed with bee bread significantly enhanced its activity to 6.52 U, which may be attributable to the composition of BB, which harbors bee salivary enzymes that showed a symbiotic effect with royal jelly. The tri-mix of the three tested bee products was equal to RJ alone (5.21 U) due to the two above-mentioned reverse interactions. The results reveal that pH is a crucial factor in protease activity. Due to the variations in optimal enzymatic protease activities, BB/RJ and the tri-mix showed the highest enzymatic activities at different pH values [70]. The highest proteolytic activity at pH 5.0 was exhibited by the BB/RJ extract (5.72 ± 0.07U). The tri-mix and BP/RJ showed activities of 4.35 ± 0.13 U and 3.23 ± 0.15 U, respectively. These values differed statistically based on a Duncan test at 0.05 (Table 5). Proteases with enzymatic activity optima at pH 5.0 could be used to coagulate milk proteins in the dairy industry, as debittering agents in cheese, and in peptide synthesis [71]. The evaluated samples that showed their highest proteolytic activities at pH 9 were BB/RJ (8.4 ± 0.17 U), RJ (7.28 ± 0.17 U), and the tri-mix (7.11 ± 0.16 U) (Table 5). The presence of proteases in RJ has been previously hypothesized and discussed, but only a trypsin-like protease and two serine-proteinases in RJ derived from thoracic glands were identified [15]. Matsuoka et al. (2012) indicated that RJ proteins consumed by honeybee queen larvae are hydrolyzed by enzymes in the larvae that are alkaline in nature, as these activities were stronger in the alkaline range at pH 9, at which most bands were hydrolyzed. This could explain the obtained results at pH 9.0 in the current research, which showed significant increases in proteolytic activities in bee products and their mixes, which indicates the alkaline nature of these proteases. These findings may provide us with novel knowledge that could be used in food technology as ingredients in functional foods and nutraceuticals [13].

Table 5.
Physicochemical characteristics of fortified fermented milk products.
2.6. SDS–PAGE of Honeybee Products
The electrophoresis patterns of honeybee products’ and their mixes’ extracted proteins in aqueous extracts are shown in Figure 4. SDS-PAGE showed bands with molecular weights ranging from ~10 to 118 kDa. Based on the band width and intensity, the major polypeptides present were in the range of 20–85 kDa. Proteins with MWs of 20, 26, 36, 47, and 77 KDa were found in all samples, while the protein with an MW of 23.6 KDa in BB and RJ corresponds to papain [72,73]. One protein band less than 20 KDa, corresponding to albumin components according to [74], was found in all samples with very high expression. It is noteworthy that the tri-mix (seventh lane) showed all band widths with high intensities, which agrees with the concept of mixing complementary protein sources to achieve a better balance of amino acids.
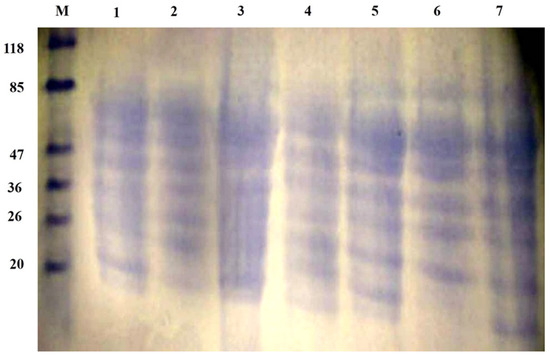
Figure 4.
SDS-PAGE of honeybee products and their mixes. M, protein marker; 1, bee pollen; 2, bee bread; 3, royal jelly; 4, bee pollen/bee bread; 5, bee pollen/royal jelly; 6, bee bread/royal jelly; 7, tri-mix.
2.7. Physicochemical Characteristics of Developed Functional Fermented Dairy Products
Fermented dairy products fortified with PB, BB, RJ, and the tri-mix and the control are exhibited in Figure 5, and Table 5 illustrates the physicochemical characteristics of fortified fermented dairy products. The results revealed that compared to plain control yogurt (pH = 4.93), fortification with bee products and their mixture significantly decreased the pH to 4.83, 4.62, and 4.62 in TBB, TRJ, and TMix, respectively, and increased the titratable acidity from 0.350% in plain fermented milk to 0.399% in fortified fermented dairy. This decrease may be due to either bee products’ synergistic effect on the microbial fermentation of starter cultures or enzymatic activity producing amino and fatty acids that could affect acidity. These results disagree with the results of [75], who reported no significant changes in the acidity of fresh bio-yogurt fortified with RJ. The addition of BP, BB, RJ, and their mixture significantly improved the product texture compared to the control, as the viscosity significantly increased from 10.55 cP in control yogurt to 11.55, 12.15, 12.50, and 12.65 cP, respectively (p < 0.05). These results agree with [76], who reported that the fortification of yogurt with BP and RJ enhanced the textural properties. Additionally, Karabagias et al. reported that ground BP may act as a surface or interface enhancer in material or food science based on its total protein content. The obtained results emphasized that the fortification of fermented milk with honeybee products improved the physical properties of the products [77].

Figure 5.
Fortified fermented milk products with different treatments. (A): Plain fermented milk (control); (B): fermented milk fortified with bee pollen (TBP), (C): fermented milk fortified with bee bread (TBB); (D): fermented milk fortified with RJ (TRJ); (E) fermented milk fortified with tri-mix (TMix).
2.8. Effects of Fortification on LAB Starter Culture
The effects of fortification on starter cultures in fermented dairy products are illustrated in Figure 6. The main aim of microbiological analyses of fortified fermented dairy products was to ensure that these fortifications do not represent obstructions to the viability of lactic acid bacteria starter cultures, represented by St. thermophiles and Lb. delbrueckii subsp. bulgaricus, in addition to the assessment of the best-before date. Figure 6A describes the total counts in fermented dairy products over 15 days of cold storage. The counts were recorded at 7.7 × 10, 7.3 × 10, 1.42 × 102, 1.63 × 102, and 1.08 × 102 CFU/g in the control, TBP, TBB, TRJ, and TMix, respectively, at zero time. The results agree with Nowak’s observations that the Lactobacillus genus showed higher counts in royal jelly samples and Hassan et al.’s finding that the viable counts of probiotics were boosted by fortification with RJ [78,79].
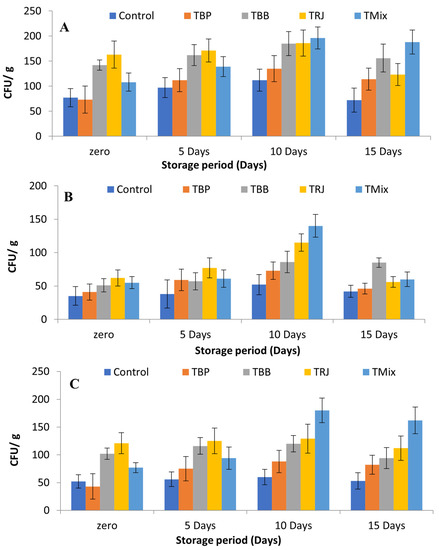
Figure 6.
Effects of fortification on starter cultures in fermented milk products during cold storage. (A): Total counts on NA; (B): lactic acid bacteria counts on M17 for Streptococcus thermophilus; (C): lactic acid bacteria counts on MRS for Lactobacillus delbrueckii subsp. Bulgaricus. Data presented as means of duplicates ± SD. Control, plain fermented milk; TP, fermented milk fortified with bee pollen; TBB, fermented milk fortified with bee bread; TRJ, fermented milk fortified with royal jelly; TMix, fermented milk fortified with tri-mix.
These results reflect the pH results (Table 5), which showed significantly lower pH in the fortified products TBB, TRJ, and TMix. The total counts then gradually increased until the 10th day of storage to 1.12 × 102, 1.35 × 102, 1.85 × 102, 1.86 × 102, and 1.96 × 102 CFU/g, respectively, before starting to decrease on the 15th day. The same pattern was observed for the enumeration of Streptococcus thermophilus (Figure 6B) and counts of Lactobacillus delbrueckii subsp. bulgaricus (Figure 6C). The obtained results of the four forms of bee product fortification did not affect the total viable counts of the Streptococci or Lactobacilli group compared to control unfortified fermented milk during the storage period until the 10th day. Additionally, yeast and molds were not detected in any treatments up to the 15th storage day, which indicates good hygienic conditions of processing. The obtained results disagree with those of Atallah, who showed decreased counts during cold storage [76]. However, the obtained results indicate that these fermented dairy products are best consumed before 15 days of cold storage.
2.9. Sensory Evaluation
The sensory evaluations of fresh fortified fermented dairy products are illustrated in Figure 7. Sensory evaluations showed differences among treatments in the color, odor, taste, texture, appearance, and overall acceptance of fresh products (zero time). Fermented dairy fortified with RJ (TRJ) and the tri-mix (TMix) scored the highest total scores, which exceeded that of the plain control product, which may be related to pH and viscosity characteristics (Table 5) that served to increase consumer acceptability by enhancing the taste and texture. On the other hand, higher levels of probiotic starter culture counts in TRJ (Figure 6) may enhance the sensory properties due to the produced flavor compounds. On the contrary, fermented products fortified with BP (TBP) and BB (TBB) scored the lowest total scores. This could be related to the distribution of grains in the products that negatively affected taste, texture, and appearance scores (6.9, 7.0, and 7.8 for TBP and 7.7, 7.4, and 7.1 for TBB, respectively). The obtained findings are in agreement with [75,76], who reported the improved sensory quality of RJ-fortified bio-yogurt without adverse effects on lactic acid bacteria counts and organoleptic properties.
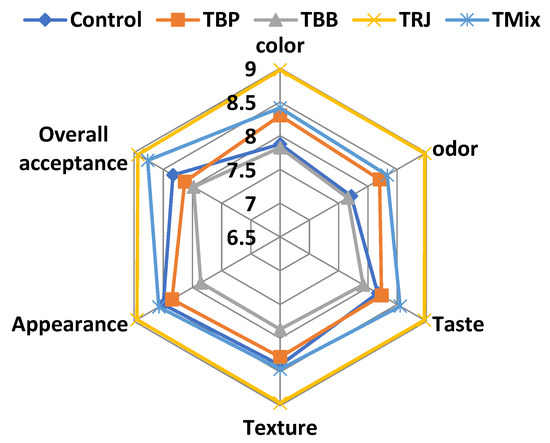
Figure 7.
Sensory evaluation of fresh fortified fermented milk products. Control, plain fermented milk; TBP, fermented milk fortified with bee pollen; TBB, fermented milk fortified with bee bread; TRJ, fermented milk fortified with royal jelly; TMix, fermented milk fortified with tri-mi.
3. Materials and Methods
3.1. Sampling and Extracting
Samples of BP from different botanical origins, BB, and RJ were collected between May and July of 2019 from a honeybee apiary located in the Experimental Farm, City of Scientific Research and Technological Applications, Alexandria, Egypt. Beehives were equipped with bottom-fitted BP traps (Figure 8A) to collect BP (pollen grains) (Figure 8B), while BB (Figure 8D) was collected manually from honeycombs (Figure 8C). For royal jelly (RJ) production, Laidlaw and Page’s grafting method was applied. Two colonies were selected. One was a breeding colony headed by a young open-mated queen to produce a sufficient number of eggs that provided young larvae for grafting at 24–36 h old (Figure 8E) [80].
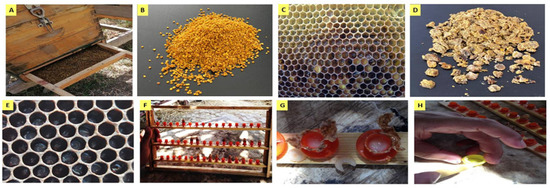
Figure 8.
Bee products’ raw materials and grafting method for royal jelly production. (A) Bee pollen trap; (B) collected bee pollen; (C) bees’ honeycomb showing bee bread stored inside hexagonal bee cells; (D) collected bee bread; (E) young larvae less than 36 h old; (F) rearing queen cells; (G) moving larvae from grafted cups; (H) royal jelly harvesting.
The second colony was the rearing colony (Figure 8F); this colony had a large population of nursing worker bees and was dequeened 48 h before grafting young larvae. With a grafting tool, young larvae were grafted and moved from breeding colony combs to queen cups in the rearing colony. After 48 h, all grafted cups were collected, the larvae were moved (Figure 8G), and then fresh RJ was harvested (Figure 8H). In the field, collected RJ was kept in an ice box, and it was directly moved to the laboratory and kept at −20 °C until being tested and used in experiments. Similarly, BP and BB samples were stored in a deep freezer (−20 °C), while mixes of honeybee products were freshly prepared before analyses.
For extraction, bee pollen and BB were crushed in a mortar, and then samples were extracted individually in Milli-Q (Ultra Clear Water Purification Systems, Series 2000, Siemens, Washington, DC, USA) with a ratio of 1:10 w/v, blended using a vortex mixer for 10 min, and then centrifuged at 4000 rpm/15 min, while RJ was dissolved with the same ratio. Bi-mixes were prepared with equal portions (1:1) w/w, as was the tri-mix (1:1:1) w/w/w.
3.2. Chemical Compositions of Honeybee Products
Moisture content was determined according to AOAC 925.10 [81], and ash content was determined as described by AOAC 923.03 [81]. Total protein content was determined by the binding of Coomassie Brilliant Blue G-250 to protein, using bovine serum albumin as a standard [82]. The phenol-sulfuric acid colorimetric method, using a T80 UV/VIS spectrophotometer (PG Instrument Ltd., Lutterworth, UK) at 490 nm, was employed to quantify the total soluble-sugar concentration in honeybee products, as described by [83].
3.3. Mineral Contents of Honeybee Products
Samples were prepared by dry ashing before dissolving in dilute aqua regia (10 mL concentrated HNO3: HCl, 1:3); the solution was diluted with Milli-Q water up to 50 mL in a volumetric flask, and then mineral concentrations were determined using Atomic Absorption Spectrometry (AAS) according to the method of [84], except for phosphorus, which was determined colorimetrically using the method of [85].
3.4. Daily Values (DVs%)
Daily values (%) for nutrition labeling were calculated based on a daily intake of 2000 calories, which has been established as the reference for adults and children 4 or more years of age according to [86].
3.5. Amino Acid Compositions of Honeybee Products
Amino acid analysis was carried out using an Automatic Amino Acid Analyzer (AAA 400 INGOS Ltd.) using the performic acid oxidation method according to [87,88]. The required pattern values were in accordance with previously established values (FAO/WHO/UNU, 1985; FAO/WHO, 1973; Recommended Dietary Allowances—Food and Nutrition/ Board Commission on Life/Sciences National Research Council, 1989), and the Amino Acid Score (AAS%) was calculated as “Percentage of adequacy” as follows:
3.6. Liquid Chromatography–Mass Spectrometry (LC-MS-MS) Analysis
Extracts of bee products were analyzed using LC–MS–MS. A Shimadzu LC-10 HPLC instrument with a Grace Vydac Everest Narrowbore C18 column (100 mm × 2.1 mm i.d., 5 µm, 300 Å). LC-MS connected to an LCQ electrospray ion trap MS (Thermo Finnigan, San Jose, CA, USA) was utilized with a mass range of 200–5000 m/z. A 2 µL sample was injected using an autosampler. The solvents used were 95% H2O in formic acid (0.1%) (A) and 95% ACN in formic acid (0.1%) (B). Gradient elution ranged from 5% to 95% (B), followed by column conditioning to 5% (B) at a 300 µL/min flow rate. The elution time was 60 min.
Foundation 3.1 Xcalibur 3.1.6610 was used to analyze the data. Additionally, MS Convert from the ProteoWizard suite (https://proteowizard.sourceforge.io/download.html; accessed on 8 November 2022) was used to convert the raw data files to mzXML format. The Global Natural Products Social Molecular Networking (GNPS) online workflow was used to generate the molecular network [3,4]. The network’s spectra were then validated against the spectral libraries and literature data of GNPS.
Cytoscape software was used to analyze and edit the molecular networks. The parent mass of each node served as a label. A pie slice proportional to the number of MS/MS spectra for each parent mass was established, with colors designating the sources of the samples.
3.7. Antioxidant Potentials of Honeybee Products
3.7.1. Total Phenolic Content
The total phenolic content was assessed using the Folin–Ciocalteu reagent according to [89]. The absorbance was measured at 760 nm using a T80 UV/VIS spectrophotometer (PG Instrument Ltd., UK) and was expressed as gallic acid equivalents (GAE) in milligrams per gram sample. Measurements were performed in duplicate.
3.7.2. Total Flavonoid Content
The total flavonoid content of aqueous extracts was assessed using Sakanaka et al.’s [90] colorimetric method [90], reading the absorbance at 510 nm using a T80 UV/VIS spectrophotometer (PG Instrument Ltd., UK). The results were expressed as μg/g of sample.
3.7.3. DPPH Scavenging Activity
The DPPH (2,2-diphenyl-1-picrylhydrazyl) assay was used as described by [91], reading the absorbance at 517 nm using a T80 UV/VIS spectrophotometer (PG Instrument Ltd., UK).
3.8. Screening and Quantitative Determination of Proteolytic Activity
The qualitative proteolytic activity was detected by casein hydrolysis on agar plates containing YNB medium supplemented with 0.5% casein, 0.5% glucose, and 2% agar (w/v-1), pH 7.0 [92]. The plates were incubated at 28 °C for 3 days. Enzyme activity was indicated by the formation of a clear zone around the wells after precipitation with 1 M HCl solution. A commercial protease solution at 0.001% (w/v-) was used as the positive control.
A proteolytic qualitative activity assay, using casein as the substrate, was performed according to the method described by [93], with some modifications. Enzyme activity was determined by incubating 250 µL of the culture supernatant with 500 µL of 1% (w/v) casein sodium salt in 50 mM buffer (pH 5.0, 7.0, and 9.0) for 2h/30 °C. The reaction was stopped by the addition of 375 µL of 20% (w/v-1) trichloroacetic acid (TCA). The tubes were placed in an ice bath for 30 min and then centrifuged at 5000× g for 15 min at 4 °C. Proteolytic activity was determined from the absorbance reading of the supernatant at 280 nm versus an appropriate blank. One unit (U) of enzyme activity is defined as the amount of enzyme that, under the assay conditions described, gives rise to an increase of 0.1 unit of absorbance (280 nm) in 60 min at 30 °C (T80UV/Vis spectrometer PG Instruments LDT, United Kingdom).
The qualitative protease activity was assayed quantitatively using different pH values of the substrate solution (5.0, 7.0, and 9.0). The substrate was prepared in three different 50 mM buffers: sodium citrate (pH 5.0), sodium phosphate (pH 7.0), and Tris-HCl (pH 9.0) [94].
3.9. SDS-PAGE
The polypeptide patterns of honeybee products (bee pollen, BB, and RJ) were analyzed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) according to [95]. Honeybee products were diluted to 1 mg/mL with 50 mM Tris buffer (pH 7.4) containing 1 mM EDTA (Merck, Darmstadt, Germany) and further mixed 1:1 (v:v) with Laemmli buffer containing 10% dithiothreitol (DTT) (Sigma-Aldrich, Steinheim, Germany). Subsequently, the mixture was boiled for 3 min and centrifuged for 3 min at 6000 g. Samples and a See-Blue Standard (10 xL) were loaded onto a 12% SDS-PAGE gel and run with running buffer at 200 V for approximately 50 min. Finally, the gels were stained with Coomassie Brilliant Blue G-250 overnight and washed with a distaining solution (15% ethanol and 5% acetic acid) until protein bands became clearly visible in the colorless gel matrix.
3.10. Application in Fermented Dairy Products
3.10.1. Preparation of Honeybee-Product-Fortified Fermented Dairy Products
Pasteurized cow milk (3% fat) was standardized using skimmed milk powder (SMP) to raise the solid non-fat (SNF) from 8.5 to 13%. A sugar solution containing 65% sugar (w/v) was prepared and pasteurized at 65 °C/30 min [96]. Milk was warmed to 42 ± 2 °C and divided into five equal portions: C; control plain fermented milk; TBP; fermented milk fortified with bee pollen grains; TBB; fermented milk fortified with BB; TRJ; fermented milk fortified with RJ; and TMix; fermented milk fortified with tri-mix (BP, BB, RJ (1:1:1)). Honeybee products were added for a fortification percent of 1% (w/v), and the sugar solution was added at 7% (v/v). The fortified milk was then inoculated with the commercial starter culture YO-MIX® (Danisco, France) containing Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus, with an inoculation level of 10 Direct Culture Units (DCU) for 100 L milk (the lowest recommended inoculation dose), was poured into 100 mL bottles and incubated at 42 ± 2 °C until coagulation at pH ~4.6 (about 4 h) and then cooled and stored at 4 °C [97].
3.10.2. Physicochemical Characterization of Fortified Fermented Dairy Products
pH was measured using a pH meter (Adwa AD1030, Hungary), and the titratable acidity of fortified fermented dairy products was expressed as an equivalent percentage of lactic acid according to [98].
The viscosity of fermented dairy products was determined using a J.P. Selecta Wide Range Rotary Viscometer (Model STS-2011, Valencia, Spain), with spindle number L3 at 100 rpm/20 °C. Fortified fermented dairy samples were left for 10 min at room temperature and stirred for 40 sec before analysis, and results were recorded in centipoises (cP) after 50 s of shearing [99].
3.10.3. Microbiological Analysis
The conventional dilution pour-plate technique was used to enumerate microbes in the products. For the total viable microbial count, NA (Biolife, Milano, Italy) was used; members of Lactobacilli sp. were grown on MRS agar (Biolife, Milano, Italy), members of Lactococci sp. were grown on M17 agar (HiMedia, Mumbai, India), and the enumeration of yeast and mold was performed on potato dextrose agar (Biolife, Milano, Italy), as described by Standard Methods for the Examination of Dairy Products [100]. The results are represented as colony-forming units (CFU/g).
3.10.4. Sensory Evaluation
Ten panelists (six men and four women, aged between 27 and 50 years) conducted sensory evaluations of fresh honeybee-fortified fermented dairy products (control plain fermented dairy, fermented dairy fortified with bee pollen grains, fermented dairy fortified with BB, fermented dairy fortified with RJ, and fermented dairy fortified with the tri-mix) at Food Technology Dept., Arid Lands Cultivation Research Institute, SRTA-City, Alexandria, Egypt, as described by [101,102] with some modifications. The criteria for selection depended on their experience and background related to fermented dairy products. The samples, which were stored at 4 °C, were allowed to rest at room temperature (20 °C ± 2) for 10 min before the evaluation. The samples were evaluated using a 9-point Hedonic scale according to ISO 22935-3 | IDF, 2009 [103]. This scale consisted of the test parameters of color, odor, taste, texture, appearance, and overall acceptability, accompanied by a scale of nine categories: 1 = dislike extremely; 2 = dislike very much; 3 = dislike moderately; 4 = dislike slightly, 5 = neither dislike nor like, 6 = like slightly; 7 = like moderately; 8 = like very much; 9 = like extremely. The data presented are averages of n = 10 ± SD.
3.11. Statistical Analysis
Data are expressed as means of duplicates ± standard deviation (SD). Data were analyzed using one-way analysis of variance (ANOVA) for multiple comparisons using the Duncan test in the IBM SPSS Statistics 23 software program, where a probability of p < 0.05 was considered statistically significant [104].
4. Conclusions
A molecular networking analysis identified a total of 46 compounds in three bee products that could be potential medicines, including royalisin and jelleine II, with anti-inflammatory activity, anti-colon-cancer effects, and antioxidant potential. Functional fermented dairy products can be successfully employed as vehicles to ease and broaden the delivery of honeybee products. Bee pollen, bee bread, and RJ had no adverse effects on starter cultures or organoleptic properties. Mixing protein sources with complementary essential amino acids and functional properties may help to achieve products with high nutritional quality for healthier diets. Consuming honeybee-product-fortified fermented dairy on a daily basis may add valuable nutrients due to their high nutritional value, high protein quality, in vitro antioxidant capacities, and proteolytic activities in parallel with preferable sensorial aspects. In conclusion, the complexity of honeybee products in terms of their nutrient compositions and chemical, functional, and proteolytic properties can open future research opportunities of great importance for the development of functional dairy products on the industrial scale.
Author Contributions
Conceptualization, A.M.G.D.; formal analysis, A.M.G.D., S.A.E.-S., H.R.E.-S., H.M.M., and M.G.S.; funding acquisition, H.R.E.-S.; methodology, A.M.G.D., M.G.S., S.A.E.-S., A.A.A.E.-W., S.H.D.M., and H.R.E.-S.; resources, S.H.D.M.; supervision, H.R.E.-S., A.M.G.D., and S.A.E.-S.; writing—original draft preparation, A.M.G.D., M.G.S., H.R.E.-S., S.A.E.-S., A.A.A.E.-W., and S.A.M.K.; writing—review and editing, A.M.G.D., S.A.E.-S., M.G.S., H.M.M., S.H.D.M., A.A.A.E.-W., S.A.M.K., and H.R.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
H.R.E.-S. highly appreciated and thankful to the support of: Plan of High end Foreign Experts of the Ministry of Science and Technology (G2022016009L), China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Valdés, A.; Álvarez-Rivera, G.; Socas-Rodríguez, B.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Foodomics: Analytical opportunities and challenges. Anal. Chem. 2022, 94, 366–381. [Google Scholar] [CrossRef] [PubMed]
- West, K.A.; Schmid, R.; Gauglitz, J.M.; Wang, M.; Dorrestein, P.C. FoodMASST a mass spectrometry search tool for foods and beverages. NPJ Sci. Food 2022, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- El-Garawani, I.; Hassab El-Nabi, S.; El Kattan, A.; Sallam, A.; Elballat, S.; Abou-Ghanima, S.; El Azab, I.H.; El-Seedi, H.R.; Khalifa, S.A.M.; El-Shamy, S. The ameliorative role of Acacia senegal gum against the oxidative stress and genotoxicity induced by the radiographic contrast medium (Ioxitalamate) in albino rats. Antioxidants 2021, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- El-Din, M.I.G.; Fahmy, N.M.; Wu, F.; Salem, M.M.; Khattab, O.M.; El-Seedi, H.R.; Korinek, M.; Hwang, T.L.; Osman, A.K.; El-Shazly, M.; et al. Comparative LC–LTQ–MS–MS analysis of the leaf extracts of Lantana camara and Lantana montevidensis growing in Egypt with insights into their anti-Inflammatory, and cytotoxic activities. Plants 2022, 11, 1699. [Google Scholar] [CrossRef]
- Özkök, D.; Silici, S. Antioxidant activities of honeybee products and their mixtures. Food Sci. Biotechnol. 2017, 26, 201–206. [Google Scholar] [CrossRef]
- Bakour, M.; Campos, M.d.G.; Imtara, H.; Lyoussi, B. Antioxidant content and identification of phenolic/flavonoid compounds in the pollen of fourteen plants using HPLC-DAD. J. Apic. Res. 2020, 59, 35–41. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Ka, J.; Mencner, L.; Olczyk, K. Bee pollen: Chemical composition and therapeutic application. Evid.-Based Complement. Altern. Med. 2015, 2015, 297425. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elashal, M.; Kieliszek, M.; Ghazala, N.E.; Farag, M.A.; Saeed, A.; Sabir, J.S.M.; Battino, M.; Xiao, J.; Zou, X.; et al. Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci. Technol. 2020, 97, 300–316. [Google Scholar] [CrossRef]
- Salama, S.; Shou, Q.; Abd El-Wahed, A.A.; Elias, N.; Xiao, J.; Swillam, A.; Umair, M.; Guo, Z.; Daglia, M.; Wang, K.; et al. Royal jelly: Beneficial properties and synergistic effects with chemotherapeutic drugs with particular emphasis in anticancer strategies. Nutrients 2022, 14, 4166. [Google Scholar] [CrossRef]
- Sabatini, A.G.; Marcazzan, G.L.; Caboni, M.F.; Bogdanov, S.; de Almeida-Muradian, L.B. Quality and standardisation of royal jelly. J. ApiProduct ApiMedical Sci. 2009, 1, e78298. [Google Scholar] [CrossRef]
- Brix, K.; Stocker, W. Proteases: Structure and Function; Springer: Wien, Austria, 2013; Volume 9783709108, ISBN 9783709108857. [Google Scholar]
- Pokora, M.; Eckert, E.; Zambrowicz, A.; Bobak, Ł.; Szołtysik, M.; Dąbrowska, A.; Chrzanowska, J.; Polanowski, A.; Trziszka, T. Biological and functional properties of proteolytic enzyme-modified egg protein by-products. Food Sci. Nutr. 2013, 1, 184–195. [Google Scholar] [CrossRef]
- Matsuoka, T.; Kawashima, T.; Nakamura, T.; Kanamaru, Y.; Yabe, T. Isolation and characterization of proteases that hydrolyze royal jelly proteins from queen bee larvae of the honeybee, Apis mellifera. Apidologie 2012, 43, 685–697. [Google Scholar] [CrossRef]
- Ismail, B.; Mohammed, H.; Nair, A.J. Influence of Proteases on Functional Properties of Food. In Green Bio-Processes, Energy, Environment, and Sustainability; Parameswaran, B., Varjani, S., Raveendran, S., Eds.; Springer: Singapore, 2019; pp. 31–53. ISBN 9789811332630. [Google Scholar]
- Sagona, S.; Coppola, F.; Giannaccini, G.; Betti, L.; Palego, L.; Tafi, E.; Casini, L.; Piana, L.; Dall’Olio, R.; Felicioli, A. Impact of Different Storage Temperature on the Enzymatic Activity of Apis mellifera Royal Jelly. Foods 2022, 11, 3165. [Google Scholar] [CrossRef]
- Algethami, J.S.; El-Wahed, A.A.A.; Elashal, M.H.; Ahmed, H.R.; Elshafiey, E.H.; Omar, E.M.; Al Naggar, Y.; Algethami, A.F.; Shou, Q.; Alsharif, S.M. Bee pollen: Clinical trials and patent applications. Nutrients 2022, 14, 2858. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; El-Wahed, A.A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-viral and immunomodulatory properties of propolis: Chemical diversity, pharmacological properties, preclinical and clinical applications, and in silico potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Aufschnaiter, A.; Kohler, V.; Khalifa, S.; El-Wahed, A.; Du, M.; El-Seedi, H.; Büttner, S. Apitoxin and its components against cancer, neurodegeneration and rheumatoid arthritis: Limitations and possibilities. Toxins 2020, 12, 66. [Google Scholar] [CrossRef]
- Spence, S.; Delve, J.; Stamp, E.; Matthews, J.N.S.; White, M.; Adamson, A.J. The impact of food and nutrient-based standards on primary school children’s lunch and total dietary intake: A natural experimental evaluation of government policy in England. PLoS ONE 2013, 8, e78298. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.F.; Wu, L.M.; Hu, F.L. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Mohammad, S.M.; Mahmud-Ab-Rashid, N.K.; Zawawi, N. Botanical origin and nutritional values of bee bread of stingless bee (Heterotrigona itama) from Malaysia. J. Food Qual. 2020, 2020, 15–17. [Google Scholar] [CrossRef]
- Mayda, N.; Özkök, A.; Ecem Bayram, N.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Kaplan, M.; Karaoglu, Ö.; Eroglu, N.; Silici, S. Fatty acid and proximate composition of bee bread. Food Technol. Biotechnol. 2016, 54, 497–504. [Google Scholar] [CrossRef]
- Bogdanov, S. Royal Jelly, Bee Brood: Composition, Health, Medicine: A Review. Lipids 2011, 38, 19. [Google Scholar]
- Nehete, J.; Bhambar, R.; Narkhede, M.; Gawali, S. Natural proteins: Sources, isolation, characterization and applications. Pharmacogn. Rev. 2013, 7, 107–116. [Google Scholar] [CrossRef]
- FAO/WHO/UNU. Energy and Protein Requirements—FAO/WHO/UNU Expert Consultation; Food and Agriculture Organization of the United States/World Health Organization/United Nations University, Energy and Protein requirements. Report of a joint FAO/WHO/UNU meeting; World Health Organization: Geneva, Switzerland, 1985. [Google Scholar]
- FAO/WHO. Energy and Protein Requirements; World Health Organization: Geneva, Switzerland, 1973; p. 118. [Google Scholar]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. Recommended Dietary Allowances—Food and Nutrition/ Board Commission on Life/Sciences National Research Council, 10th ed.; National Academy Press: Washington, DC, USA, 1989; ISBN 0309536065. [Google Scholar]
- Watford, M. Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J. Nutr. 2008, 138, 2003S–2007S. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Ahmed, O.M.; Hozayen, W.G.; Ahmed, M.A. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-Induced diabetic wistar rats. Biomed. Pharmacother. 2018, 97, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Bărnuţiu, L.I.; Mărghitaş, L.A.; Dezmirean, D.S.; Mihai, C.M.; Bobiş, O. Chemical composition and antimicrobial activity of royal jelly. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 67–72. [Google Scholar]
- Mariotti, F.; Gardner, C.D. Dietary protein and amino acids in vegetarian diets—A review. Nutrients 2019, 11, 2661. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee pollen: Current status and therapeutic potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Bagameri, L.; Baci, G.-M.; Dezmirean, D.S. Royal jelly as a nutraceutical natural product with a focus on its antibacterial activity. Pharmaceutics 2022, 14, 1142. [Google Scholar] [CrossRef]
- Becerril-s, A.L.; Quintero-Salazar, B.; Dubl, O.; Escalona-Buend, B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Nogueira, C.; Iglesias, A.; Feás, X.; Estevinho, L.M. Commercial bee pollen with different geographical origins: A comprehensive approach. Int. J. Mol. Sci. 2012, 13, 11173–11187. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The profile of polyphenolic compounds, contents of total phenolics and flavonoids, and antioxidant and antimicrobial properties of bee products. Molecules 2022, 27, 1301. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical prospects of bee products: Special focus on anticancer, antibacterial, antiviral, and antiparasitic properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Lin, I.P.; Peng, C.C. 10-Hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Yang, D.S.; Zhang, W.; Wang, J.M.; Li, C.Y.; Ye, H.; Lei, K.F.; Chen, X.F.; Shen, N.H.; Jin, L.Q.; et al. 10-Hydroxy-2-decenoic acid from royal jelly: A potential medicine for RA. J. Ethnopharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.; Mendes, M.A.; de Souza, B.M.; Konno, K.; César, L.M.M.; Malaspina, O.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; Aguilera-Luiz, M.d.M.; Romero-González, R.; Vidal, J.L.M.; Garrido Frenich, A. Fast analysis of polyphenols in royal jelly products using automated TurboFlowTM-liquid chromatography-Orbitrap high resolution mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 973, 17–28. [Google Scholar] [CrossRef]
- Fuenmayor, B.C.; Zuluaga, D.C.; Díaz, M.C.; de Quicazán, C.M.; Cosio, M.; Mannino, S. Evaluation of the physicochemical and functional properties of Colombian bee pollen. Rev. MVZ Córdoba 2014, 19, 4003–4014. [Google Scholar] [CrossRef]
- Li, F.; Guo, S.; Zhang, S.; Peng, S.; Cao, W.; Ho, C.T.; Bai, N. Bioactive constituents of f. esculentum bee pollen and quantitative analysis of samples collected from seven areas by HPLC. Molecules 2019, 24, 2705. [Google Scholar] [CrossRef]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Development and validation of a liquid chromatography-tandem mass spectrometry method to determine intact glucosinolates in bee pollen. J. Chromatogr. B 2015, 1000, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Boppré, M.; Colegate, S.M.; Edgar, J.A.; Fischer, O.W. Hepatotoxic pyrrolizidine alkaloids in pollen and drying-related implications for commercial processing of bee pollen. J. Agric. Food Chem. 2008, 56, 5662–5672. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Román, D.; Zurek, G.; Bäßmann, C.; Almaraz-Abarca, N.; Quirantes, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of phenolic compounds from pollen extracts using capillary electrophoresis-electrospray time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2007, 389, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Serra Bonvehi, J.; Soliva Torrentó, M.; Centelles Lorente, E. Evaluation of polyphenolic and flavonoid compounds in honeybee-collected pollen produced in Spain. J. Agric. Food Chem. 2001, 49, 1848–1853. [Google Scholar] [CrossRef]
- Aylanc, V.; Falcão, S.I.; Ertosun, S.; Vilas-Boas, M. From the hive to the table: Nutrition value, digestibility and bioavailability of the dietary phytochemicals present in the bee pollen and bee bread. Trends Food Sci. Technol. 2021, 109, 464–481. [Google Scholar] [CrossRef]
- Ismail, N.I.; Kadir, M.R.A.; Zulkifli, R.M. Isolation and identification of potential antineoplastic bioactive phenolic compounds in malaysian honeys. J. Appl. Pharm. Sci. 2015, 5, 59–66. [Google Scholar] [CrossRef]
- Šarić, A.; Balog, T.; Sobočanec, S.; Kušić, B.; Šverko, V.; Rusak, G.; Likić, S.; Bubalo, D.; Pinto, B.; Reali, D.; et al. Antioxidant effects of flavonoid from Croatian Cystus incanus L. rich bee pollen. Food Chem. Toxicol. 2009, 47, 547–554. [Google Scholar] [CrossRef]
- El Ghouizi, A.; El Menyiy, N.; Falcão, S.I.; Vilas-Boas, M.; Lyoussi, B. Chemical composition, antioxidant activity, and diuretic effect of Moroccan fresh bee pollen in rats. Vet. World 2020, 13, 1251–1261. [Google Scholar] [CrossRef]
- Wei, X.E.; Korth, J.; Brown, S.H.J.; Mitchell, T.W.; Truscott, R.J.W.; Blanksby, S.J.; Willcox, M.D.P.; Zhao, Z. Rapid quantification of free cholesterol in tears using direct insertion/electron ionization-Mass spectrometry. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8027–8035. [Google Scholar] [CrossRef]
- Bárbara, M.S.; Machado, C.S.; Sodré, G.D.S.; Dias, L.G.; Estevinho, L.M.; De Carvalho, C.A.L. Microbiological assessment, nutritional characterization and phenolic compounds of bee pollen from Mellipona mandacaia Smith, 1983. Molecules 2015, 20, 12525–12544. [Google Scholar] [CrossRef]
- Mohammad, S.M.; Mahmud-Ab-Rashid, N.K.; Zawawi, N. Stingless bee-collected pollen (Bee Bread): Chemical and microbiology properties and health Benefits. Molecules 2021, 26, 957. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.S.; Camara, C.A.; Lins, A.C.S.; Agra, M.d.F.; Silva, E.M.S.; Reis, I.T.; Freitas, B.M. Chemical composition, botanical evaluation and screening of radical scavenging activity of collected pollen by the stingless bees Melipona rufiventris (Uruçu-amarela). An. Acad. Bras. Cienc. 2009, 81, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Carreiras, J.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Matos, A.R.; Caçador, I.; Duarte, B. Heavy metal pre-conditioning history modulates Spartina patens physiological tolerance along a salinity gradient. Plants 2021, 10, 2072. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Suarez, J.M. Bee Products—Chemical and Biological Properties; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319596891. [Google Scholar]
- Chang, C.L.; Wu, R.T. Quantification of (+)-catechin and (−)-epicatechin in coconut water by LC-MS. Food Chem. 2011, 126, 710–717. [Google Scholar] [CrossRef]
- Di Paola-Naranjo, R.D.; Sánchez-Sánchez, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C. Liquid chromatographic-mass spectrometric analysis of anthocyanin composition of dark blue bee pollen from Echium plantagineum. J. Chromatogr. A 2004, 1054, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-decenoic acid, the major lipid component of royal jelly, extends the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, R.; Lu, Q. Separation and characterization of phenolamines and flavonoids from rape bee pollen, and comparison of their antioxidant activities and protective effects against oxidative stress. Molecules 2020, 25, 1264. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Rocchetti, G.; Castiglioni, S.; Maldarizzi, G.; Carloni, P. Original article UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin. Int. J. Food Sci. Technol. 2019, 54, 335–346. [Google Scholar] [CrossRef]
- De-Melo, A.A.M.; Estevinho, L.M.; Moreira, M.M.; Delerue-Matos, C.; de Freitas, A.d.S.; Barth, O.M.; de Almeida-Muradian, L.B. A multivariate approach based on physicochemical parameters and biological potential for the botanical and geographical discrimination of Brazilian bee pollen. Food Biosci. 2018, 25, 91–110. [Google Scholar] [CrossRef]
- Kostić, A.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT-Food Sci. Technol. 2019, 112, 108244–108250. [Google Scholar] [CrossRef]
- Čeksterytė, V.; Jansen, E.H.J.M. Composition and content of fatty acids in beebread of various floral origin, collected in Lithuania and prepared for storage in different ways. Chemine Technol. 2012, 2, 57–61. [Google Scholar] [CrossRef]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Production, characterization and antioxidant potential of protease from Streptomyces sp. MAB18 using poultry wastes. Biomed Res. Int. 2013, 2013, 496586. [Google Scholar] [CrossRef] [PubMed]
- Koka, R.; Weimer, B.C. Isolation and characterization of a protease from Pseudomonas fluorescens RO98. J. Appl. Microbiol. 2000, 89, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Sumantha, A.; Larroche, C.; Pandey, A. Microbiology and industrial biotechnology of food-grade proteases: A perspective. Food Technol. Biotechnol. 2006, 44, 211–220. [Google Scholar]
- Azarkan, M.; El Moussaoui, A.; Van Wuytswinkel, D.; Dehon, G.; Looze, Y. Fractionation and purification of the enzymes stored in the latex of Carica papaya. J. Chromatogr. B 2003, 790, 229–238. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, B.P.; Hemmings, A.M.; Buttle, D.J.; Pearl, L.H. Crystal Structure of Glycyl Endopeptidase from Carica papaya: A Cysteine Endopeptidase of Unusual Substrate Specificity. Biochemistry 1995, 34, 13190–13195. [Google Scholar] [CrossRef]
- Brinegar, C.; Sine, B.; Nwokocha, L. High-cysteine 2S seed storage proteins from quinoa (Chenopodium quinoa). J. Agric. Food Chem. 1996, 44, 1621–1623. [Google Scholar] [CrossRef]
- Metry, W.A.; Owayss, A.A. Influence of incorporating honey and royal jelly on the quality of yoghurt during storage. Egypt. J. Food Sci. 2009, 37, 115–131. [Google Scholar]
- Atallah, A.A. The production of bio-yoghurt with probiotic bacteria, royal jelly and bee pollen grains. J. Nutr. Food Sci. 2016, 6, 1000510–1000516. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabagias, V.K.; Gatzias, I.; Riganakos, K.A. Bio-functional properties of bee pollen: The case of “bee pollen yoghurt”. Coatings 2018, 8, 423. [Google Scholar] [CrossRef]
- Nowak, A.; Szczuka, D.; Górczyńska, A.; Motyl, I.; Kręgiel, D. Characterization of Apis mellifera Gastrointestinal Microbiota and Lactic Acid Bacteria for Honeybee Protection—A Review. Cells 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.M.; Elenany, Y.E.; Nassrallah, A.; Cheng, W.; Abd El-Maksoud, A.A. Royal jelly improves the physicochemical properties and biological activities of fermented milk with enhanced probiotic viability. LWT 2022, 155, 112912. [Google Scholar] [CrossRef]
- Laidlaw, H.H.; Page, R.E. Queen Rearing and Bee Breeding; Wicwas Press: New York, NY, USA, 1997. [Google Scholar]
- AOAC. AOAC: Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S. Food Analysis Laboratory Manual, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-1-4419-1462-0. [Google Scholar]
- Beaty, R.D.; Kerber, J.D. Concepts, Instrumentation and Techniques in Atomic Absorption Spectrophotometry, 2nd ed.; The Perkin-Elmer Corporation: Norwalk, CT, USA, 1993. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2.2, 2nd ed.; Page, A.L., Ed.; Agronomy Monographs; ASA, CSSA, SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- FDA. 14. Appendix F: Calculate the Percent Daily Value for the Appropriate Nutrients. In Guidance for Industry: A Food Labeling Guide; FDA: Silver Spring, MD, USA, 2004. [Google Scholar]
- INGOS. Automatic Amino Acid Analyser AAA 400 User Manual; INGOS: Strasbourg, France, 2006. [Google Scholar]
- Smith, A.J. Post column amino acid analysis. In Methods in Molecular Biology (Clifton, N.J.); Smith, A.J., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2003; Volume 211, pp. 133–141. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin–Ciocalteau Reagent. Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 9, 569–575. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Larsen, M.D.; Kristiansen, K.R.; Hansen, T.K. Characterization of the proteolytic activity of starter cultures of Penicillium roqueforti for production of blue veined cheeses. Int. J. Food Microbiol. 1998, 43, 215–221. [Google Scholar] [CrossRef]
- Ramakrishna, T.; Pandit, M.W. Self-association of α-chymotrypsin: Effect of amino acids. J. Biosci. 1988, 13, 215–222. [Google Scholar] [CrossRef]
- Rodarte, M.P.; Dias, D.R.; Vilela, D.M.; Schwan, R.F. Proteolytic activities of bacteria, yeasts and filamentous fungi isolated from coffee fruit (Coffea arabica L.). Acta Sci.-Agron. 2011, 33, 457–464. [Google Scholar] [CrossRef]
- Laemmli, U.K.; Beguin, F.; Gujer-Kellenberger, G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J. Mol. Biol. 1970, 47, 69–85. [Google Scholar] [CrossRef]
- Sutikno, B.T.; Rizal, S.; Marniza. Effects of sugar type and concentration on the characteristics of fermented turi (Sesbania grandiflora (L.) poir) milk. Emirates J. Food Agric. 2013, 25, 576–584. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robenson, R.K. Tamime and Robenson’s Yoghurt Science and Technology, 3rd ed.; Woodhead Publishing Ltd. and CRC Press LLC: Cambridge, UK, 2007; ISBN 9781855735842. [Google Scholar]
- Ling, E.R. A Textbook of Dairy Chemistry, 2nd ed.; Chapman and Hall LTD: London, UK, 1945. [Google Scholar]
- Akalın, A.S.; Unal, G.; Dinkci, N.; Hayaloglu, A.A. Microstructural, textural, and sensory characteristics of probiotic yogurts fortified with sodium calcium caseinate or whey protein concentrate. J. Dairy Sci. 2012, 95, 3617–3628. [Google Scholar] [CrossRef] [PubMed]
- Marth, E.H. Standard Methods for the Examination of Dairy Products, 14th ed.; American Public Health Association: Washington, DC, USA, 1978; p. 20036. [Google Scholar]
- Darwish, A.M.G.; Khalifa, R.E.; El Sohaimy, S.A. Functional Properties of Chia Seed Mucilage Supplemented in Low Fat Yoghurt. Alexandria Sci. Exch. J. An Int. Q. J. Sci. Agric. Environ. 2018, 39, 450–459. [Google Scholar] [CrossRef]
- Senaka Ranadheera, C.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- 103. ISO 22935-3|IDF 099-3: 2009; Milk and Milk Products—Sensory Analysis—Part 3: Guidance on a Method for Evaluation of Compliance with Product Specifications for Sensory Properties by Scoring, First Edition. ISO and IDF: Geneva, Switzerland, 2009; ISBN 2831886376.
- IBM Corp. IBM SPSS Statistics for Windows, Version 23.0; IBM Corp: Armonk, NY, USA, 2015.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).