Characterisation of Bee Pollen from the Marche Region (Italy) According to the Botanical and Geographical Origin with Analysis of Antioxidant Activity and Colour, Using a Chemometric Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Palynological Analysis
2.2. Total Phenolic Content and In Vitro Antioxidant Capacity
2.2.1. Total Phenolic Content (TPC Assay)
2.2.2. ABTS Assay
2.2.3. DPPH Assay
2.2.4. ORAC Assay
2.3. Instrumental Colour Measurement
2.4. Pollen Load Size Distribution
2.5. Protein and Moisture Content
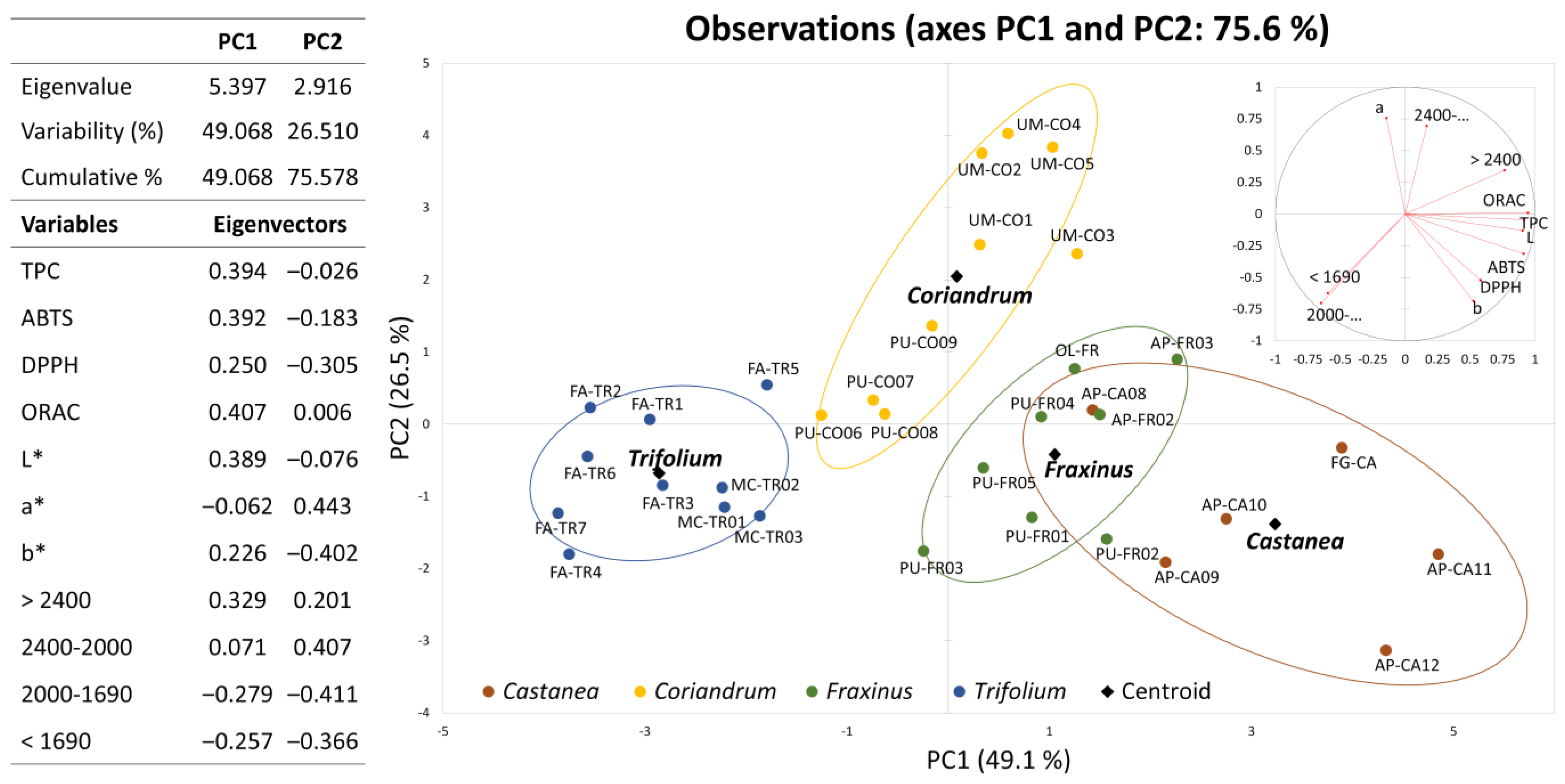
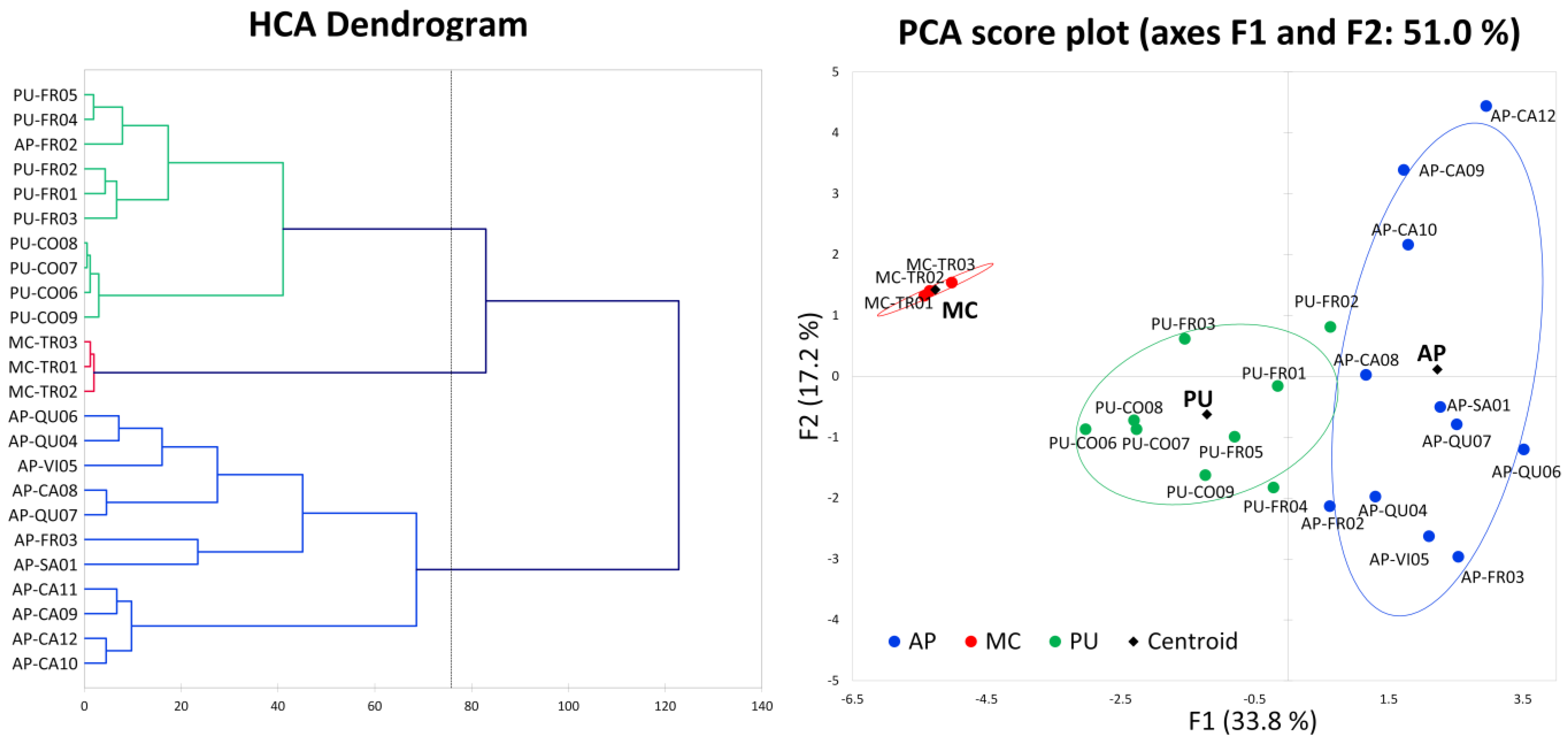
2.6. General Consideration and Multivariate Analysis
3. Materials and Methods
3.1. Chemicals and Equipment
3.2. Pollen Samples
3.3. Palynological Analysis
3.4. Preparation of Pollen Extracts
3.5. Determination of Total Phenolic Content (TPC)
3.6. Determination of In Vitro Antioxidant Capacity (ABTS, DPPH, ORAC)
3.7. Instrumental Colour Measurement
3.8. Pollen Load Size Distribution
3.9. Total Protein Content (%) and Moisture Determination
3.10. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, M.; Nanda, V. Composition and functionality of bee pollen: A review. Trends Food Sci. Technol. 2020, 98, 82–106. [Google Scholar] [CrossRef]
- Dimou, M.; Tananaki, C.; Liolios, V.; Thrasyvoulou, A. Pollen Foraging by Honey Bees (Apis mellifera L.) in Greece: Botanical and Geographical Origin. J. Apic. Sci. 2014, 58, 11–23. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Plutino, M.; Lucini, L.; Aromolo, R.; Martinelli, E.; Souto, E.B.; Santini, A.; Pignatti, G. Bee Products: A Representation of Biodiversity, Sustainability, and Health. Life 2021, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X.; et al. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Kieliszek, M.; Piwowarek, K.; Kot, A.M.; Błażejak, S.; Chlebowska-Śmigiel, A.; Wolska, I. Pollen and bee bread as new health-oriented products: A review. Trends Food Sci. Technol. 2018, 71, 170–180. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Morais, M.; Moreira, L.L.; Feás, X.; Estevinho, L.M. Honeybee-collected pollen from five Portuguese Natural Parks: Palynological origin, phenolic content, antioxidant properties and antimicrobial activity. Food Chem. Toxicol. 2011, 49, 1096–1101. [Google Scholar] [CrossRef]
- Rocchetti, G.; Castiglioni, S.; Maldarizzi, G.; Carloni, P.; Lucini, L. UHPLC-ESI-QTOF-MS phenolic profiling and antioxidant capacity of bee pollen from different botanical origin. Int. J. Food Sci. Technol. 2019, 54, 335–346. [Google Scholar] [CrossRef]
- Alimoglu, G.; Guzelmeric, E.; Yuksel, P.I.; Celik, C.; Deniz, I.; Yesilada, E. Monofloral and polyfloral bee pollens: Comparative evaluation of their phenolics and bioactivity profiles. LWT 2021, 142, 110973. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of Pollen Nutrition on Honey Bee Health: Do Pollen Quality and Diversity Matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef]
- Castiglioni, S.; Stefano, M.; Pisani, M.; Carloni, P. Geographical characterisation of multifloral honeys from the Marche region (Italy) according to their antioxidant activity and colour using a chemometric approach. Int. J. Food Sci. Technol. 2017, 53, 571–581. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Ilie, C.-I.; Oprea, E.; Geana, E.-I.; Spoiala, A.; Buleandra, M.; Gradisteanu Pircalabioru, G.; Badea, I.A.; Ficai, D.; Andronescu, E.; Ficai, A.; et al. Bee Pollen Extracts: Chemical Composition, Antioxidant Properties, and Effect on the Growth of Selected Probiotic and Pathogenic Bacteria. Antioxidants 2022, 11, 959. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Parri, E.; Felicioli, A.; Sagona, S.; Pozzo, L.; Biondi, C.; Domenici, V.; Pucci, L. Phytochemical composition and antioxidant activity of Tuscan bee pollen of different botanic origins. Ital. J. Food Sci. 2015, 27, 248–259. [Google Scholar] [CrossRef]
- Salonen, A.; Lavola, A.; Virjamo, V.; Julkunen-Tiitto, R. Protein and phenolic content and antioxidant capacity of honey bee-collected unifloral pollen pellets from Finland. J. Apic. Res. 2021, 60, 744–750. [Google Scholar] [CrossRef]
- de Arruda, V.A.S.; Pereira, A.A.S.; de Freitas, A.S.; Barth, O.M.; de Almeida-Muradian, L.B. Dried bee pollen: B complex vitamins, physicochemical and botanical composition. J. Food Compos. Anal. 2013, 29, 100–105. [Google Scholar] [CrossRef]
- Salazar-González, C.Y.; Rodríguez-Pulido, F.J.; Terrab, A.; Díaz-Moreno, C.; Fuenmayor, C.A.; Heredia, F.J. Analysis of Multifloral Bee Pollen Pellets by Advanced Digital Imaging Applied to Functional Food Ingredients. Plant Foods Hum. Nutr. 2018, 73, 328–335. [Google Scholar] [CrossRef]
- Castiglioni, S.; Astolfi, P.; Conti, C.; Monaci, E.; Stefano, M.; Carloni, P. Morphological, Physicochemical and FTIR Spectroscopic Properties of Bee Pollen Loads from Different Botanical Origin. Molecules 2019, 24, 3974. [Google Scholar] [CrossRef]
- Liolios, V.; Tananaki, C.; Papaioannou, A.; Kanelis, D.; Rodopoulou, M.-A.; Argena, N. Mineral content in monofloral bee pollen: Investigation of the effect of the botanical and geographical origin. J. Food Meas. Charact. 2019, 1674–1682. [Google Scholar] [CrossRef]
- Liolios, V.; Tananaki, C.; Dimou, M.; Kanelis, D.; Goras, G.; Karazafiris, E.; Thrasyvoulou, A. Ranking pollen from bee plants according to their protein contribution to honey bees. J. Apic. Res. 2015, 54, 582–592. [Google Scholar] [CrossRef]
- Ganser, D.; Albrecht, M.; Knop, E. Wildflower strips enhance wild bee reproductive success. J. Appl. Ecol. 2021, 58, 486–495. [Google Scholar] [CrossRef]
- Joseph, J.; Santibáñez, F.; Laguna, M.F.; Abramson, G.; Kuperman, M.N.; Garibaldi, L.A. A spatially extended model to assess the role of landscape structure on the pollination service of Apis mellifera. Ecol. Model. 2020, 431, 109201. [Google Scholar] [CrossRef]
- Barth, O.M.; Freitas, A.S.; Oliveira, É.S.; Silva, R.A.; Maester, F.M.; Andrella, R.R.S.; Cardozo, G.M.B.Q. Evaluation of the botanical origin of commercial dry bee pollen load batches using pollen analysis: A proposal for technical standardization. An. Acad. Bras. Cienc. 2010, 82, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Packer, L., Ed.; Academic Press: New York, NY, USA, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Gillespie, K.M.; Chae, J.M.; Ainsworth, E.A. Rapid measurement of total antioxidant capacity in plants. Nat. Protoc. 2007, 2, 867–870. [Google Scholar] [CrossRef]
- Owusu-Apenten, R. Kjeldahl method, quantitative amino acid analysis and combustion analysis. In Food Protein Analysis: Quantitative Effects on Processing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–46. [Google Scholar] [CrossRef]
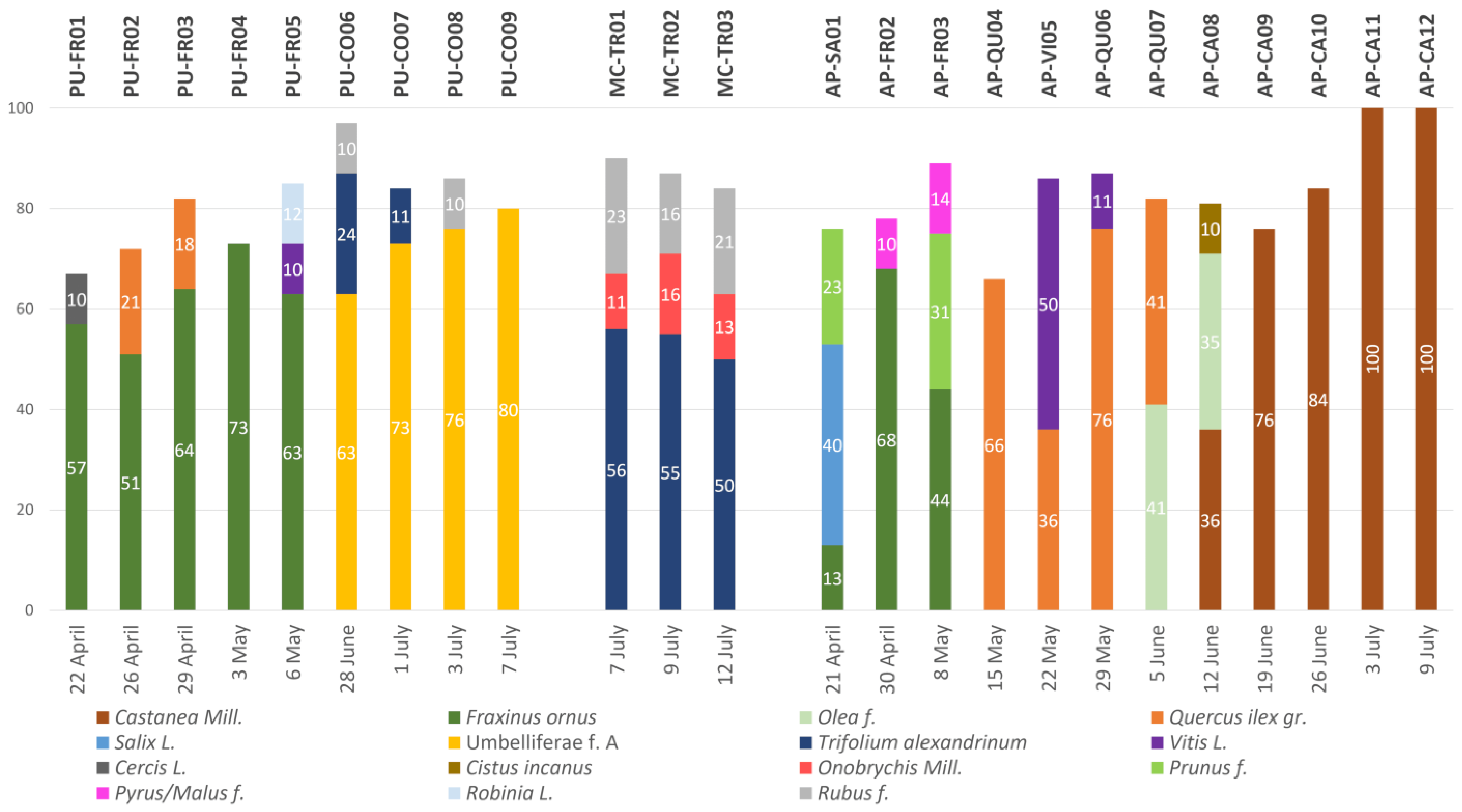
| Code | Period | Zone | Province | Protein | Moisture | Prevalent Pollen | |
|---|---|---|---|---|---|---|---|
| % | % | Type | % | ||||
| PU-FR01 | 22 April | Isola del Piano | Pesaro-Urbino (PU) | 22.49 | 16 | Fraxinus ornus | 57 |
| PU-FR02 | 26 April | Isola del Piano | Pesaro-Urbino (PU) | 20.64 | 21.7 | Fraxinus ornus | 51 |
| PU-FR03 | 29 April | Isola del Piano | Pesaro-Urbino (PU) | 18.66 | 22.2 | Fraxinus ornus | 64 |
| PU-FR04 | 3 May | Isola del Piano | Pesaro-Urbino (PU) | 19.97 | 14.8 | Fraxinus ornus | 73 |
| PU-FR05 | 6 May | Isola del Piano | Pesaro-Urbino (PU) | 20.58 | 17.8 | Fraxinus ornus | 63 |
| PU-CO06 | 28 June | Isola del Piano | Pesaro-Urbino (PU) | 20.8 | 14 | Coriandrum | 63 |
| PU-CO07 | 1 July | Isola del Piano | Pesaro-Urbino (PU) | 20.52 | 13.5 | Coriandrum | 73 |
| PU-CO08 | 3 July | Isola del Piano | Pesaro-Urbino (PU) | 20.1 | 12.8 | Coriandrum | 76 |
| PU-CO09 | 7 July | Isola del Piano | Pesaro-Urbino (PU) | 19.88 | 15 | Coriandrum | 80 |
| AP-SA01 | 21 April | Cavaceppo | Ascoli Piceno (AP) | 21.09 | 15.8 | Salix | 40 |
| AP-FR02 | 30 April | Cavaceppo | Ascoli Piceno (AP) | 21.42 | 13.9 | Fraxinus ornus | 68 |
| AP-FR03 | 8 May | Cavaceppo | Ascoli Piceno (AP) | 24.52 | 11.9 | Fraxinus ornus | 44 |
| AP-QU04 | 15 May | Cavaceppo | Ascoli Piceno (AP) | 19.88 | 13.8 | Quercus ilex | 66 |
| AP-VI05 | 22 May | Cavaceppo | Ascoli Piceno (AP) | 20.84 | 13.1 | Vitis | 50 |
| AP-QU06 | 29 May | Cavaceppo | Ascoli Piceno (AP) | 18.04 | 14.9 | Quercus ilex | 76 |
| AP-QU07 | 5 June | Cavaceppo | Ascoli Piceno (AP) | 17.78 | 11.3 | Quercus ilex | 41 |
| AP-CA08 | 12 June | Cavaceppo | Ascoli Piceno (AP) | 16.02 | 16.9 | Castanea | 36 |
| AP-CA09 | 19 June | Cavaceppo | Ascoli Piceno (AP) | 18.81 | 12.9 | Castanea | 76 |
| AP-CA10 | 26 June | Cavaceppo | Ascoli Piceno (AP) | 20.75 | 10.5 | Castanea | 84 |
| AP-CA11 | 3 July | Cavaceppo | Ascoli Piceno (AP) | 22.1 | 10.8 | Castanea | 100 |
| AP-CA12 | 9 July | Cavaceppo | Ascoli Piceno (AP) | 21.97 | 13.2 | Castanea | 100 |
| MC-TR01 | 7 July | Matelica | Macerata (MC) | 21.93 | 14.9 | T. alexandrinum | 46 |
| MC-TR02 | 9 July | Matelica | Macerata (MC) | 21.54 | 16.5 | T. alexandrinum | 55 |
| MC-TR03 | 12 July | Matelica | Macerata (MC) | 22.24 | 14.7 | T. alexandrinum | 50 |
| TPC | ABTS | DPPH | ORAC | |
|---|---|---|---|---|
| mg GA/g DW * | μmol TE/g DW ** | μmol TE/g DW ** | μmol TE/g DW ** | |
| PU-FR01 | 18.3 ± 1.7 E | 180.7 ± 14.9 C | 50.7 ± 9.8 HIJK | 553.4 ± 79.8 EF |
| PU-FR02 | 23 ± 2.2 D | 211.8 ± 20.7 BC | 69.9 ± 8.1 EF | 669.9 ± 79.2 DE |
| PU-FR03 | 19.1 ± 1.2 EF | 160.7 ± 18.3 DE | 52.1 ± 11.2 HIJKL | 491 ± 68.9 G |
| PU-FR04 | 16.1 ± 1.7 FG | 151.9 ± 12.8 D | 48.9 ± 8 HIJKL | 464.4 ± 66.3 FG |
| PU-FR05 | 15.2 ± 1.3 GH | 148.5 ± 16.7 DEF | 52.7 ± 8.2 HIJK | 452.2 ± 40.7 GH |
| PU-CO06 | 13.9 ± 1.1 HIJ | 123.9 ± 9.6 FG | 43.8 ± 5.2 JKL | 359.9 ± 42.5 HIJ |
| PU-CO07 | 16.6 ± 1 EF | 139.9 ± 14.7 DEF | 40.9 ± 7.5 KL | 442.9 ± 59.7 G |
| PU-CO08 | 16.2 ± 1.5 EF | 145.8 ± 18 DE | 40.7 ± 6.1 KL | 453.4 ± 40.1 FG |
| PU-CO09 | 17 ± 2 EF | 143.4 ± 11.3 DEF | 43.8 ± 9.3 KL | 404.9 ± 35.1 GHI |
| PU samples | 17.3 ± 1.5 ab | 156.3 ± 15.2 b | 49.3 ± 1.22 a | 476.9 ± 56.9 b |
| AP-SA01 | 20.8 ± 1.6 D | 195.2 ± 20.1 C | 54.2 ± 9.4 GHI | 759.7 ± 86.2 ABC |
| AP-FR02 | 17 ± 1.4 EF | 177.5 ± 17.4 C | 39.4 ± 7.7 LM | 617.6 ± 62.3 DE |
| AP-FR03 | 16.8 ± 1.5 EF | 187.5 ± 16.9 C | 30.3 ± 7.5 M | 701.9 ± 62.5 BC |
| AP-QU04 | 17.5 ± 1.8 E | 182.8 ± 19.7 C | 47.3 ± 7 HIJKL | 559.8 ± 59.3 E |
| AP-VI05 | 17.3 ± 1 E | 180.8 ± 18.5 C | 61 ± 5.9 EFG | 704.2 ± 77.9 BC |
| AP-QU06 | 21.3 ± 1.9 D | 192.8 ± 19.2 C | 55.7 ± 10 FGH | 783.9 ± 47.1 AB |
| AP-QU07 | 20.2 ± 2.7 D | 188.9 ± 17 BC | 79.1 ± 10.5 D | 653 ± 84.7 CD |
| AP-CA08 | 17.2 ± 1.3 EF | 152.1 ± 14.1 DE | 71.9 ± 11.6 E | 475.6 ± 56.5 FG |
| AP-CA09 | 23.9 ± 1.8 C | 192.6 ± 16.3 BC | 101.7 ± 8.5 C | 607.8 ± 54.8 DE |
| AP-CA10 | 24.4 ± 1.7 C | 205 ± 14.3 B | 110.5 ± 6.7 B | 716.6 ± 61.8 ABC |
| AP-CA11 | 28.5 ± 1.9 B | 233.8 ± 16.4 A | 143.2 ± 8 A | 772.5 ± 93.6 A |
| AP-CA12 | 31 ± 2 A | 257.6 ± 15.6 A | 147.4 ± 9.9 A | 801.6 ± 117.5 A |
| AP samples | 21.3 ± 1.7 a | 195.5 ± 17.1 a | 78.4 ± 8.6 a | 679.5 ± 72.0 a |
| MC-TR01 | 12.8 ± 0.9 IJ | 118.6 ± 18.2 G | 49.2 ± 8.9 HIJKL | 328.2 ± 35 IJ |
| MC-TR02 | 12.8 ± 1.2 J | 112.1 ± 20.2 G | 45.7 ± 10 IJKL | 300.1 ± 44.3 J |
| MC-TR03 | 14.1 ± 1.4 HI | 128.3 ± 18.5 EFG | 53.5 ± 9.1 GHIJ | 338.7 ± 62.6 IJ |
| MC samples | 13.2 ± 1.2 b | 119.7 ± 18.9 b | 49.5 ± 9.3 a | 322.3 ± 47.3 c |
| Total samples | 18.8 ± 1.6 | 171.3 ± 16.6 | 63.9 ± 8.5 | 558.9 ± 63.3 |
| Code | L* (I) | a* (I) | b* (I) | L* (M) | a* (M) | b* (M) | >2400 | 2400–2000 | 2000–1690 | <1690 |
|---|---|---|---|---|---|---|---|---|---|---|
| PU-FR01 | 60.4 | 4.4 | 40.5 | 65.6 | 6.8 | 50.1 | 16% | 46% | 28% | 11% |
| PU-FR02 | 58.2 | 7.6 | 41.3 | 63.4 | 8.4 | 48.1 | 17% | 40% | 29% | 14% |
| PU-FR03 | 58.1 | 8.5 | 46.2 | 64.4 | 9.0 | 53.1 | 6% | 43% | 30% | 21% |
| PU-FR04 | 60.7 | 6.2 | 46.9 | 64.3 | 9.2 | 53.6 | 18% | 59% | 16% | 7% |
| PU-FR05 | 61.3 | 7.7 | 46.7 | 64.2 | 8.2 | 54.3 | 16% | 49% | 22% | 12% |
| PU-CO06 | 50.5 | 8.0 | 33.0 | 57.7 | 11.2 | 44.8 | 7% | 55% | 27% | 11% |
| PU-CO07 | 50.8 | 9.3 | 32.5 | 56.9 | 12.3 | 48.0 | 9% | 55% | 22% | 14% |
| PU-CO08 | 51.8 | 9.2 | 35.1 | 57.8 | 11.3 | 47.8 | 8% | 54% | 27% | 11% |
| PU-CO09 | 50.6 | 11.1 | 33.1 | 55.5 | 11.5 | 46.5 | 16% | 59% | 18% | 7% |
| AP-SA01 | 57.4 | 6.9 | 37.6 | 62.5 | 7.5 | 51.8 | 34% | 43% | 13% | 11% |
| AP-FR02 | 61.5 | 5.8 | 43.3 | 64.3 | 7.2 | 48.2 | 20% | 56% | 19% | 4% |
| AP-FR03 | 57.7 | 4.8 | 35.0 | 62.3 | 5.1 | 41.6 | 41% | 48% | 8% | 3% |
| AP-QU04 | 59.6 | 6.0 | 40.2 | 64.8 | 7.0 | 52.5 | 27% | 58% | 11% | 5% |
| AP-VI05 | 60.5 | 6.0 | 47.3 | 62.7 | 7.4 | 53.9 | 27% | 61% | 11% | 1% |
| AP-QU06 | 62.0 | 6.4 | 49.6 | 61.6 | 7.8 | 52.5 | 46% | 45% | 7% | 2% |
| AP-QU07 | 60.4 | 8.2 | 51.9 | 61.0 | 11.0 | 61.9 | 36% | 49% | 10% | 4% |
| AP-CA08 | 57.3 | 9.3 | 43.7 | 60.5 | 14.8 | 68.1 | 37% | 42% | 15% | 6% |
| AP-CA09 | 60.8 | 6.9 | 41.8 | 64.0 | 10.2 | 61.0 | 29% | 32% | 26% | 12% |
| AP-CA10 | 61.8 | 6.4 | 43.5 | 66.6 | 6.9 | 53.3 | 18% | 50% | 24% | 8% |
| AP-CA11 | 65.1 | 6.0 | 52.6 | 68.3 | 7.1 | 58.8 | 39% | 40% | 18% | 4% |
| AP-CA12 | 66.8 | 5.0 | 52.1 | 69.2 | 6.9 | 58.4 | 19% | 41% | 32% | 8% |
| MC-TR01 | 47.4 | 8.4 | 31.0 | 53.2 | 11.1 | 47.6 | 8% | 39% | 31% | 22% |
| MC-TR02 | 46.7 | 9.9 | 34.4 | 52.4 | 11.1 | 50.6 | 10% | 38% | 34% | 18% |
| MC-TR03 | 49.7 | 8.3 | 31.4 | 53.7 | 12.5 | 51.3 | 8% | 39% | 31% | 22% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castiglioni, S.; Stefano, M.; Astolfi, P.; Pisani, M.; Carloni, P. Characterisation of Bee Pollen from the Marche Region (Italy) According to the Botanical and Geographical Origin with Analysis of Antioxidant Activity and Colour, Using a Chemometric Approach. Molecules 2022, 27, 7996. https://doi.org/10.3390/molecules27227996
Castiglioni S, Stefano M, Astolfi P, Pisani M, Carloni P. Characterisation of Bee Pollen from the Marche Region (Italy) According to the Botanical and Geographical Origin with Analysis of Antioxidant Activity and Colour, Using a Chemometric Approach. Molecules. 2022; 27(22):7996. https://doi.org/10.3390/molecules27227996
Chicago/Turabian StyleCastiglioni, Sara, Mariassunta Stefano, Paola Astolfi, Michela Pisani, and Patricia Carloni. 2022. "Characterisation of Bee Pollen from the Marche Region (Italy) According to the Botanical and Geographical Origin with Analysis of Antioxidant Activity and Colour, Using a Chemometric Approach" Molecules 27, no. 22: 7996. https://doi.org/10.3390/molecules27227996
APA StyleCastiglioni, S., Stefano, M., Astolfi, P., Pisani, M., & Carloni, P. (2022). Characterisation of Bee Pollen from the Marche Region (Italy) According to the Botanical and Geographical Origin with Analysis of Antioxidant Activity and Colour, Using a Chemometric Approach. Molecules, 27(22), 7996. https://doi.org/10.3390/molecules27227996











