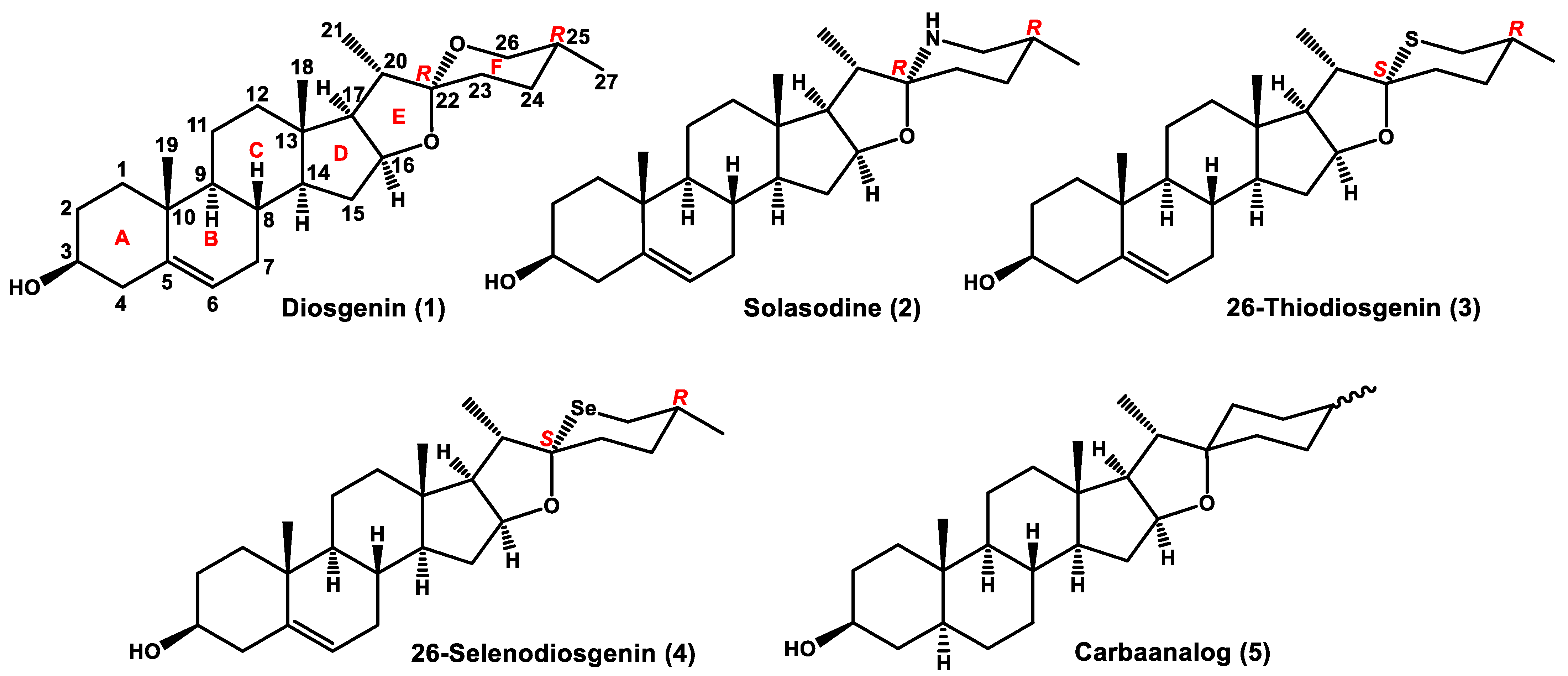
3.1. Chemistry
3.1.1. General
The reagents were purchased from Merck, Alfa Aesar, or Acros. All solvents were freshly distilled prior to use. The dry solvents were prepared by distillation over the following drying agents: DMF (4 Å molecular sieves), THF (Na/benzophenone), CH2Cl2 (CaH2).
The reactions were monitored by TLC on silica gel plates 60 F254 (Merck, Darmstadt, Germany) and spots were visualized either by UV Hand Lamp (Type: NU-4; 254 nm/365 nm, 2x4W, Herolab GmbH Laborgeräte, Wiesloch, Germany) or by charring with molybdophosphoric acid/cerium(IV) sulfate in H2SO4. The reaction products were isolated by chromatographic methods using JT Baker silica gel (J.T. Baker, Phillipsburg, NJ, USA), pore size 40 Å (70–230 mesh) (column chromatography) or 60 Å (230–400 mesh) (gravity flow column chromatography and dry flash chromatography).
1H and
13C NMR (400 and 100 MHz, respectively) spectra of all compounds were recorded using Bruker Avance II spectrometer (Bruker, Fällanden, Switzerland) in CDCl
3 and referenced to TMS (0.0 ppm) and CDCl
3 (77.0 ppm), respectively. Only selected signals in the
1H NMR spectra are reported. The original
1H and
13C NMR spectra are contained in the
Supplementary Materials. Infrared spectra were recorded using Attenuated Total Reflectance (ATR) as solid samples with Nicolet 6700 FT-IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Mass spectra were obtained at Accurate-Mass Q-TOF LC/MS 6530 spectrometer (Agilent, Santa Clara, NJ, USA) with electrospray ionization (ESI). Melting points were determined on a Kofler bench (Boetius type, Nagema, VEB Wägetechnik Rapido Radebeul, Dresden, Germany) melting point apparatus.
26-Thiodiosgenin (
3) [
10] and carbaanalog (
5) [
15] were prepared according to literature procedures.
3.1.2. Synthesis of 26-Thiodiosgenin 3β-4-Nitrobenzoate (3a)
26-Thiodiosgenin (3) (200 mg, 0.47 mmol) was dissolved in a mixture of dry pyridine (25 mL) and dichloromethane (10 mL), and then 4-nitrobenzoyl chloride (106 mg, 0.56 mmol) was added. The reaction mixture was stirred at room temperature. After completion of the reaction (7 days), it was poured into water, and extracted with dichloromethane (3 × 100 mL). The combined organic extracts were dried over anhydrous Na2SO4, and the solvent was evaporated in vacuo. The crude product was purified by dry flash chromatography with a hexane/ethyl acetate (99:1) mixture to afford ester 3a (245 mg, 91%).
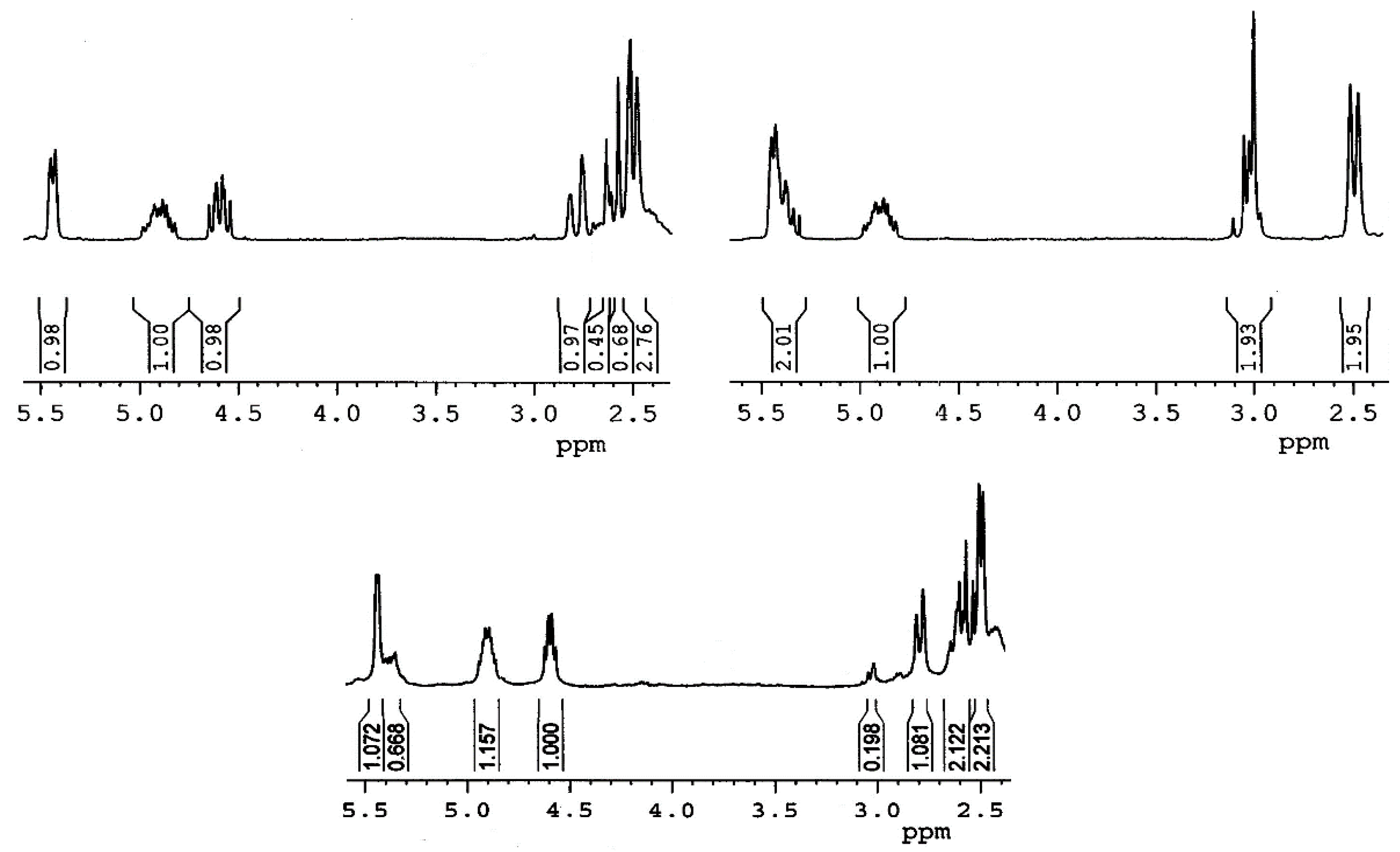
Compound 3a: colorless crystals (hexane/ethyl acetate); mp 257–258 °C; IR (ATR) νmax 1601, 1520, 1449, 1337, 1270, 1106, 1013 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.28 (2H, d, J = 8.7 Hz, H-Ar), 8.21 (2H, d, J = 8.7 Hz, H-Ar), 5.44 (1H, m, H-6), 4.90 (1H, m, H-3α), 4.65 (1H, m, H-16), 2.55 (1H, t, J = 12.6 Hz, H-26α), 2.30 (1H, d, J = 12.6 Hz, H-26β), 1.10 (3H, s, H-19), 1.03 (3H, d, J = 6.9 Hz, H-21), 0.94 (3H, d, J = 6.5 Hz, H-27), 0.83 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 164.0 (C), 150.4 (C), 139.3 (C), 136.2 (C), 130.6 (2 × CH), 123.4 (2 × CH), 122.9 (CH), 97.5 (C), 81.6 (CH), 75.7 (CH), 62.8 (CH), 56.5 (CH), 49.9 (CH), 44.4 (CH), 40.3 (C), 39.7 (CH2), 38.5 (CH2), 38.0 (CH2), 36.9 (C), 36.7 (CH2), 33.3 (CH), 32.1 (CH2), 32.0 (CH2), 31.7 (CH2), 31.4 (CH), 31.4 (CH2), 27.7 (CH2), 22.5 (CH3), 20.8 (CH2), 19.4 (CH3), 16.5 (CH3), 16.2 (CH3); HRMS m/z 580.3104 (calcd for C34H46NO5S+, 580.3091).
3.1.3. Preparation of 26-Thiodiosgenin 3β-t-Butyldimethylsilyl Ether (3b)
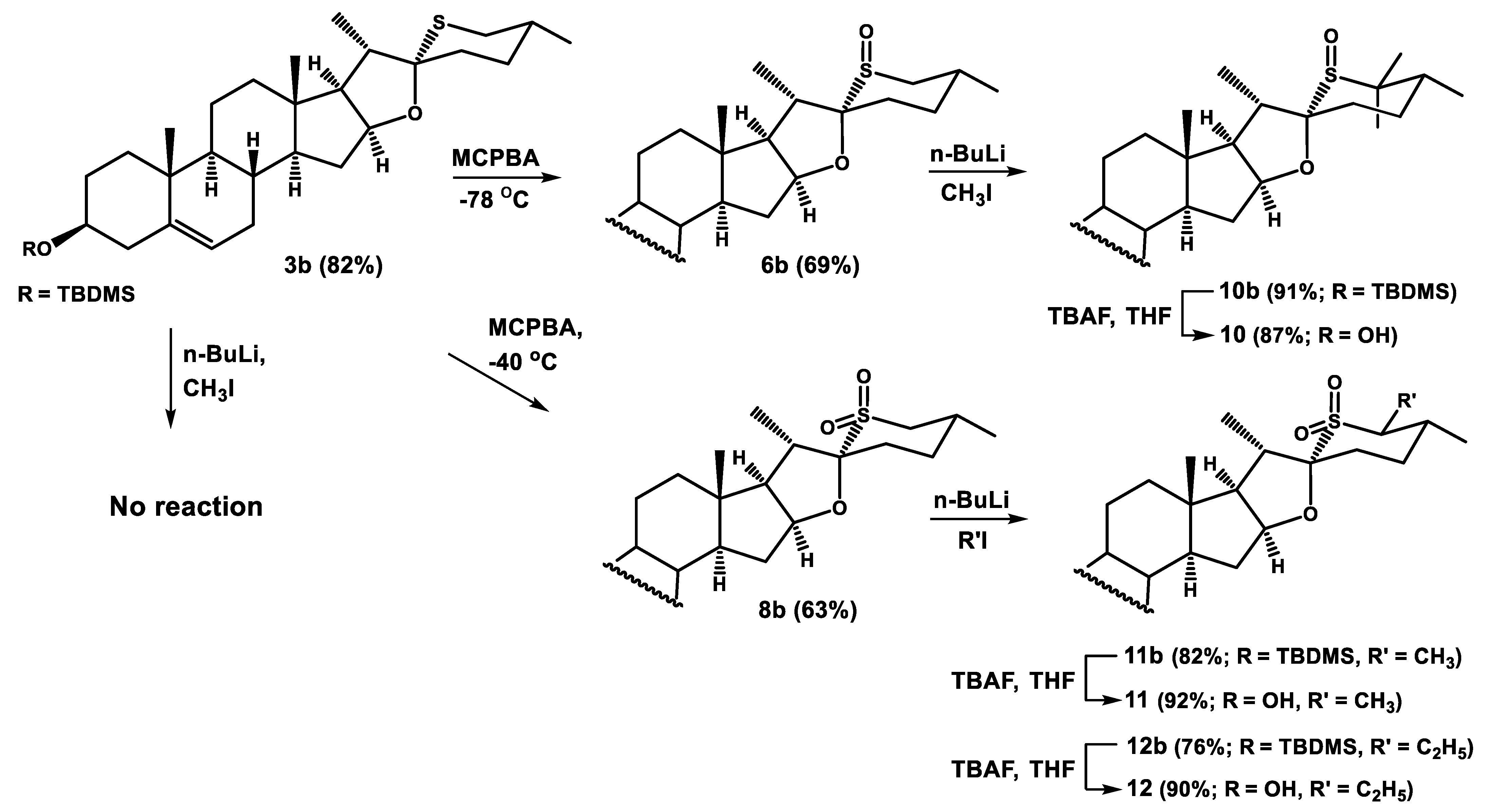
Imidazole (48 mg, 0.7 mmol) and t-butyldimethylsilyl chloride (106 mg, 0.7 mmol) were added to a solution of 26-thiodiosgenin (3) (200 mg, 0.47 mmol) in dry dimethylformamide (20 mL). The reaction mixture was stirred for 24 h at room temperature, then poured into water, and extracted with diethyl ether (3 × 100 mL). The combined organic extracts were dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product was purified by dry flash chromatography with hexane/ethyl acetate (97:3) elution to afford ether 3b (207 mg, 82%).
Compound 3b: colorless crystals (hexane/ethyl acetate); mp 235–237 °C; IR (ATR) νmax 1458, 1373, 1248, 1092, 1014 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.32 (1H, m, H-6), 4.64 (1H, dd, J = 15.4 Hz, J = 7.4 Hz, H-16), 3.49 (1H, m, H-3α), 2.55 (1H, dd, J = 12.7 Hz, J = 11.7 Hz, H-26α), 2.30 (1H, d, J = 12.7 Hz, H-26β), 1.027 (3H, d, J = 6.8 Hz, H-21), 1.026 (3H, s, H-19), 0.94 (3H, d, J = 6.5 Hz, H-27), 0.90 (9H, s, t-Bu-Si), 0.81 (3H, s, H-18), 0.07 (6H, s, (CH3)2Si); 13C NMR (CDCl3, 100 MHz) δ 141.6 (C), 120.9 (CH), 97.5 (C), 81.7 (CH), 72.6 (CH), 62.8 (CH), 56.7 (CH), 50.1 (CH), 44.4 (CH), 42.8 (CH2), 40.3 (C), 39.8 (CH2), 38.5 (CH2), 37.3 (CH2), 36.7 (C), 33.3 (CH), 32.10 (CH2), 32.09 (CH2), 32.06 (CH2), 31.7 (CH2), 31.5 (CH), 31.4 (CH2), 25.9 (3 x CH3), 22.5 (CH3), 20.8 (CH2), 19.4 (CH3), 18.3 (C), 16.5 (CH3), 16.2 (CH3), −4.6 (2 × CH3); HRMS m/z 545.3829 (calcd for C33H57O2SSi+, 545.3843).
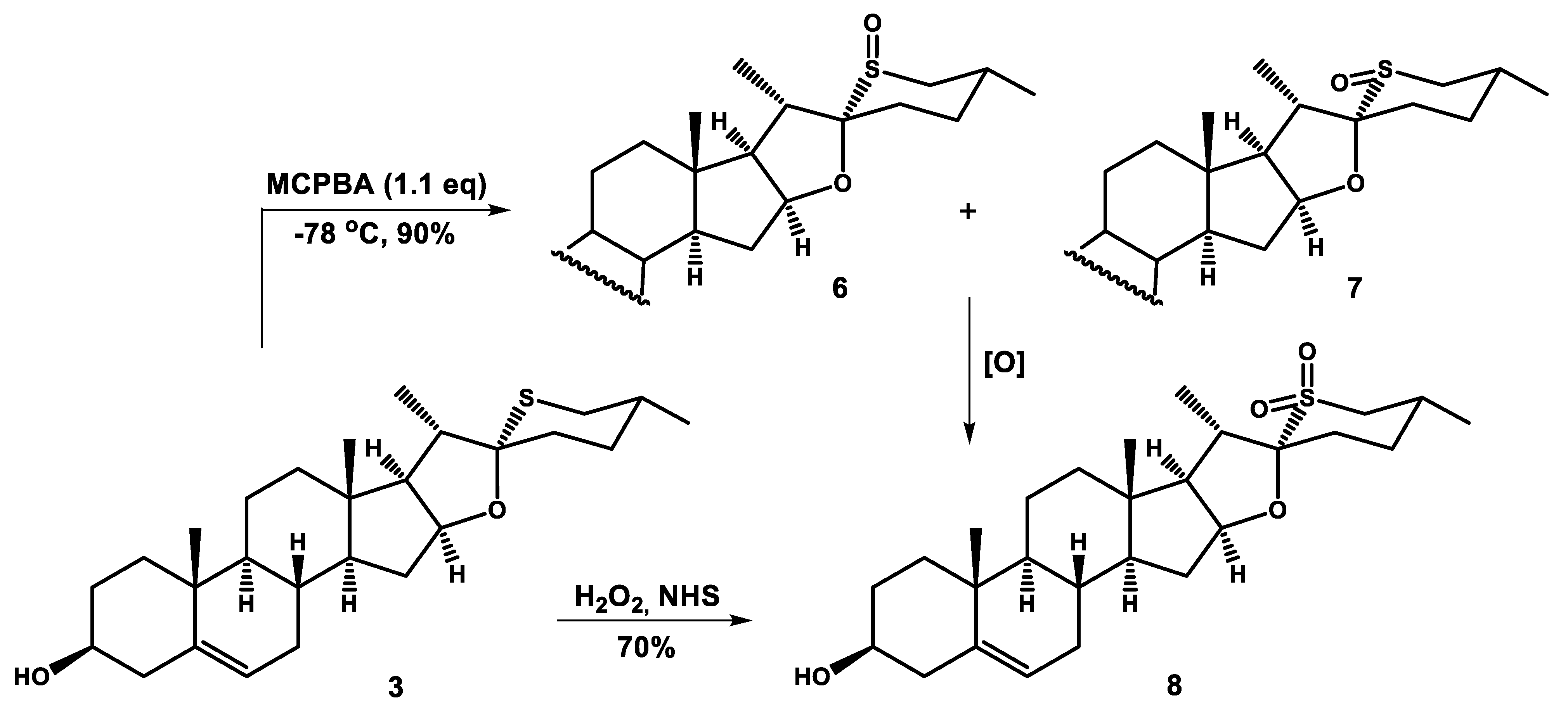
3.1.4. General Procedure for S-Oxides Formation
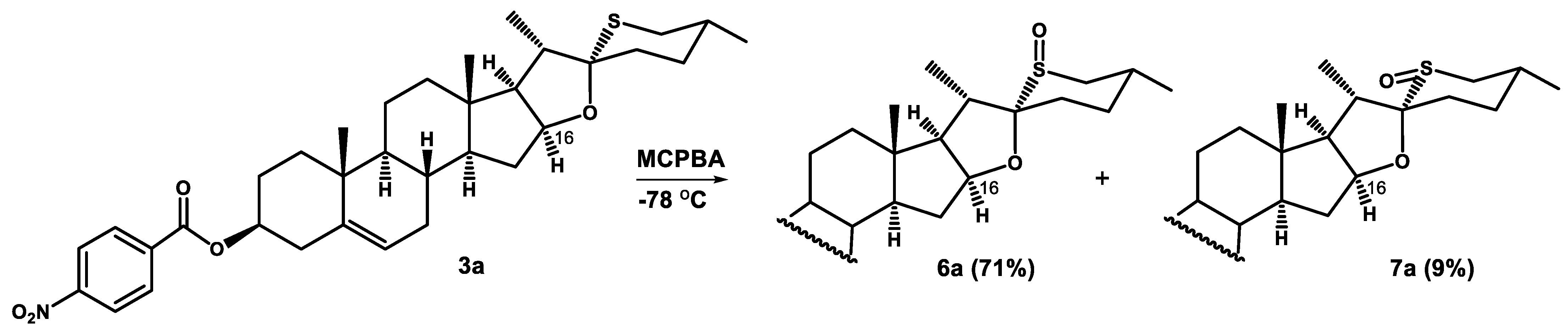
Steroidal sulfide (3, 3a or 3b) (0.23, mmol) was dissolved in dry dichloromethane (20 mL), cooled to −78 °C (dry ice/acetone cooling bath), and m-chloroperoxybenzoic acid (56 mg, 0.25 mmol) was added. The solution was stirred at −78 °C for 2–3 h and monitored by TLC. When the reaction was completed, dimethyl sulfide (0.1 mL, 1.3 mmol) was added. The reaction mixture was poured into saturated aqueous solution of NaHCO3, and extracted with dichloromethane (3 × 100 mL). The combined organic extracts were dried over anhydrous Na2SO4, and evaporated to dryness in vacuo. The residue was subjected to gravity flow column chromatography on silica gel 60 Å (230–400 mesh), which resulted in separation of compounds 6/7 (as a mixture), 6a and 7a, or 8b.
Compound 6a: was eluted with benzene/ethyl acetate (1:1) mixture in 71% yield. Colorless crystals (benzene/ethyl acetate); mp 150–151 °C; IR (ATR) νmax 1600, 1521, 1452, 1345, 1270, 1168, 1107, 1017 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.24 (2H, d, J = 9.0 Hz, H-Ar), 8.17 (2H, d, J = 9.0 Hz, H-Ar), 5.39 (1H, m, H-6), 4.84 (1H, m, H-3α), 4.55 (1H, m, H-16), 2.74 (1H, d, J = 13.3 Hz, H-26β), 2.53 (1H, t, J = 13.3 Hz, H-26α), 1.32 (3H, d, J = 7.2 Hz, H-21), 1.06 (3H, s, H-19), 0.94 (3H, d, J = 6.7 Hz, H-27), 0.80 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 163.9 (C), 150.3 (C), 139.3 (C), 136.0 (C), 130.5 (2 × CH), 123.3 (2 × CH), 122.5 (CH), 99.6 (C), 84.2 (CH), 75.5 (CH), 63.6 (CH), 56.2 (CH), 49.7 (CH), 49.0 (CH2), 43.7 (CH), 40.8 (C), 39.1 (CH2), 37.9 (CH2), 36.8 (CH2), 36.6 (C), 32.5 (CH2), 31.8 (CH2), 31.2 (CH), 29.7 (CH2), 27.65 (CH2), 27.60 (CH2), 21.3 (CH3), 20.6 (CH2), 19.9 (CH), 19.2 (CH3), 16.7 (CH3), 16.1 (CH3); HRMS m/z 596.3020 (calcd for C34H46NO6S+, 596.3040).
Compound 7a: was eluted with benzene/ethyl acetate (1:1) mixture in 9% yield. Colorless crystals (benzene/ethyl acetate); mp 175–176 °C; IR (ATR) νmax 1522, 1453, 1342, 1268, 1163, 1108, 1038 cm−1; 1H NMR (CDCl3, 400 MHz) δ 8.28 (2H, d, J = 9.0 Hz, H-Ar), 8.20 (2H, d, J = 9.0 Hz, H-Ar), 5.43 (1H, m, H-6), 5.38 (1H, m, H-16), 4.89 (1H, m, H-3α), 3.06-2.98 (2H, m, H-26), 1.46 (3H, d, J = 7.0 Hz, H-21), 1.09 (3H, s, H-19), 1.05 (3H, d, J = 6.6 Hz, H-27), 0.82 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 164.0 (C), 150.4 (C), 139.2 (C), 136.2 (C), 130.6 (2 × CH), 123.4 (2 × CH), 122.8 (CH), 104.4 (C), 87.3 (CH), 75.6 (CH), 63.0 (CH), 56.0 (CH), 52.4 (CH2), 49.8 (CH), 43.9 (CH), 40.7 (C), 39.4 (CH2), 38.0 (CH2), 36.8 (CH2), 36.7 (C), 33.6 (CH2), 33.2 (CH2), 32.0 (CH2), 31.3 (CH), 30.0 (CH2), 29.7 (CH), 27.7 (CH2), 21.5 (CH3), 20.8 (CH2), 19.3 (CH3), 16.4 (2 x CH3); HRMS m/z 596.3023 (calcd for C34H46NO6S+, 596.3040).
Compound 6b: was eluted with hexane/ethyl acetate (7:3) mixture in 69% yield. Colorless crystals (hexane/ethyl acetate); mp 205–207 °C; IR (ATR) νmax 1571, 1455, 1376, 1248, 1083, 1031 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.32 (1H, m, H-6), 4.58 (1H, m, H-16), 3.49 (1H, m, H-3α), 2.78 (1H, d, J = 13.0 Hz, H-26β), 2.57 (1H, t, J = 13.0 Hz, H-26α), 1.36 (3H, d, J = 7.3 Hz, H-21), 1.03 (3H, s, H-19), 0.98 (3H, d, J = 6.7 Hz, H-27), 0.90 (9H, s, t-Bu-Si), 0.82 (3H, s,H-18), 0.07 (6H, s, (CH3)2Si); 13C NMR (CDCl3, 100 MHz) δ 141.7 (C), 120.6 (CH), 99.7 (C), 84.4 (CH), 72.5 (CH), 63.8 (CH), 56.5 (CH), 50.0 (CH), 49.1 (CH2), 43.8 (CH), 42.7 (CH2), 40.9 (C), 39.3 (CH2), 37.3 (CH2), 36.7 (C), 32.6 (CH2), 31.99 (CH2), 31.97 (CH2), 31.4 (CH), 29.8 (CH2), 27.8 (CH2), 25.9 (3 × CH3), 21.4 (CH3), 20.7 (CH2), 20.0 (CH), 19.4 (CH3), 18.2 (C), 16.8 (CH3), 16.2 (CH3), −4.6 (2 × CH3); HRMS m/z 561.3784 (calcd for C33H57O3SSi+, 561.3792).
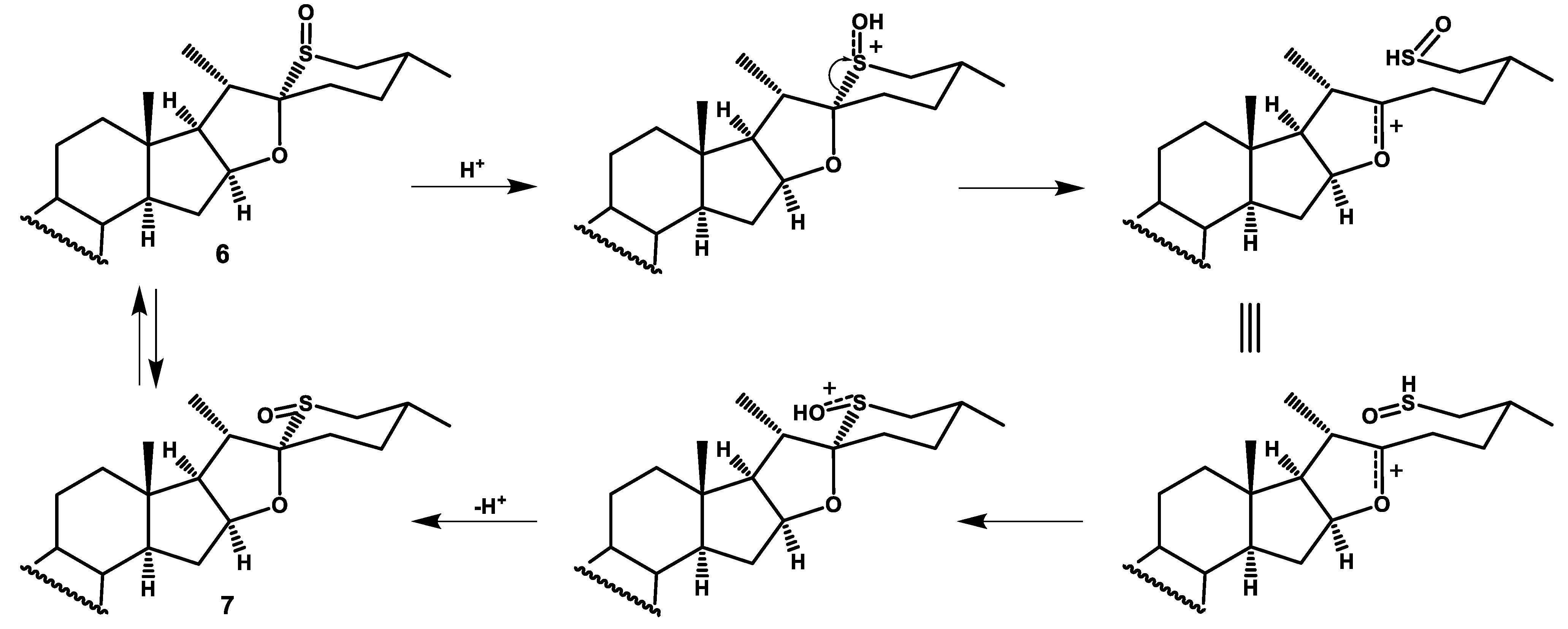
The mixture of compounds 6 and 7 proved inseparable. These compounds were obtained in their pure forms by removing p-nitrobenzoyl groups from separated compounds 6a and 7a, what is described below.
3.1.5. General Procedure for Removing the 3β-4-Nitrobenzoyl Group
To a solution of steroidal sulfoxide (6a or 7a) (50 mg) (0.08 mmol) in dry methanol (10 mL) NaOH (80 mg, 2 mmol) was added. The reaction was stirred for 24 h. Then, the solvent was evaporated in vacuo, the crude product was dissolved in dichloromethane and washed by water. The organic extract was dried over anhydrous Na2SO4, and concentrated under reduced pressure. The crude product (6 or 7) was purified by column chromatography on silica gel with hexane/ethyl acetate (3:7) mixture elution.
Compound 6: 89% yield; colorless crystals (hexane/ethyl acetate); mp 143–146 °C; IR (ATR) νmax 3331, 1449, 1377, 1348, 1164, 1139, 1052, 1017 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.35 (1H, m, H-6), 4.58 (1H, m, H-16), 3.53 (1H, m, H-3α), 2.78 (1H, d, J = 13.0 Hz, H-26β), 2.57 (1H, t, J = 13.0 Hz, H-26α), 1.36 (3H, d, J = 7.3 Hz, H-21), 1.04 (3H, s, H-19), 0.98 (3H, d, J = 6.7 Hz, H-27), 0.83 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 140.9 (C), 121.1 (CH), 99.7 (C), 84.4 (CH), 71.6 (CH), 63.8 (CH), 56.5 (CH), 50.0 (CH), 49.2 (CH2), 43.9 (CH), 42.2 (CH2), 40.9 (C), 39.3 (CH2), 37.2 (CH2), 36.6 (C), 32.6 (CH2), 31.9 (CH2), 31.6 (CH2), 31.4 (CH), 29.8 (CH2), 27.8 (CH2), 21.4 (CH3), 20.7 (CH2), 20.0 (CH), 19.4 (CH3), 16.8 (CH3), 16.3 (CH3); HRMS m/z 447.2921 (calcd for C27H43O3S+, 447.2927).
Compound 7: 87% yield; colorless crystals (hexane/ethyl acetate); mp 165–166 °C; IR (ATR) νmax 3420, 1455, 1377, 1341, 1156, 1138, 1074, 1033, 1011 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.40–5.34 (2H, m, H-6, H-16), 3.53 (1H, m, H-3α), 3.07-2.98 (2H, m, H-26), 1.46 (3H, d, J = 8.8 Hz, H-21), 1.05 (3H, d, J = 6.6 Hz, H-27), 1.03 (3H, s, H-19), 0.82 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 140.9 (C), 121.3 (CH), 104.5 (C), 87.4 (CH), 71.7 (CH), 63.1 (CH), 56.2 (CH), 52.6 (CH2), 50.1 (CH), 44.1 (CH), 42.3 (CH2), 40.7 (C), 39.6 (CH2), 37.3 (CH2), 36.7 (C), 33.7 (CH2), 33.3 (CH2), 32.0 (CH2), 31.7 (CH2), 31.5 (CH), 30.1 (CH2), 29.7 (CH), 21.5 (CH3), 20.9 (CH2), 19.4 (CH3), 16.4 (CH3), 16.3 (CH3); HRMS m/z 447.2984 (calcd for C27H43O3S+, 447.2927).
3.1.6. Procedures for Preparation of Sulfones
Procedure 1 (with H2O2 and NHS)
30% hydrogen peroxide (45 µL, 0.4 mmol) and NHS (N-hydroxysuccinimide) (23 mg, 0.2 mmol) were added to a solution of 26-thiodiosgenin (3) (40 mg, 0.1 mmol) in acetone (5 mL). The mixture was heated at 50 °C for 10 h. Then, the reaction mixture was poured into aqueous solution of NaHSO3, and extracted with ethyl acetate (3 × 50 mL). The combined organic extracts were dried over anhydrous Na2SO4, and evaporated to dryness in vacuo. The crude product was purified by column chromatography on silica gel with hexane/ethyl acetate (3:1) elution to afford sulfone (8) (230 mg, 70%).
Compound 8: was eluted with hexane/ethyl acetate (3:1) mixture in 70% yield. Colorless crystals (hexane/ethyl acetate); mp 205–207 °C; IR (ATR) νmax 3573, 3431, 1464, 1291, 1261, 1050, 1021 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.36 (1H, m, H-6), 5.10 (1H, m, H-16), 3.54 (1H, m, H-3α), 3.16 (1H, t, J = 13.2 Hz, H-26α), 2.55 (1H, d, J = 13.2 Hz, H-26β), 1.42 (3H, d, J = 7.3 Hz, H-21), 1.04 (3H, s, H-19), 1.02 (3H, d, J = 6.7 Hz, H-27), 0.80 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 140.8 (C), 121.2 (CH), 103.3 (C), 85.5 (CH), 71.6 (CH), 63.9 (CH), 56.3 (CH), 55.2 (CH2), 49.9 (CH), 42.9 (CH), 42.2 (CH2), 40.8 (C), 39.3 (CH2), 37.2 (CH2), 36.6 (C), 35.2 (CH2), 32.6 (CH2), 31.9 (CH2), 31.6 (CH2), 31.4 (CH), 30.9 (CH), 29.1 (CH2), 21.1 (CH3), 20.8 (CH2), 19.4 (CH3), 16.2 (CH3), 16.0 (CH3); HRMS m/z 463.2856 (calcd for C27H43O4S+, 463.2877).
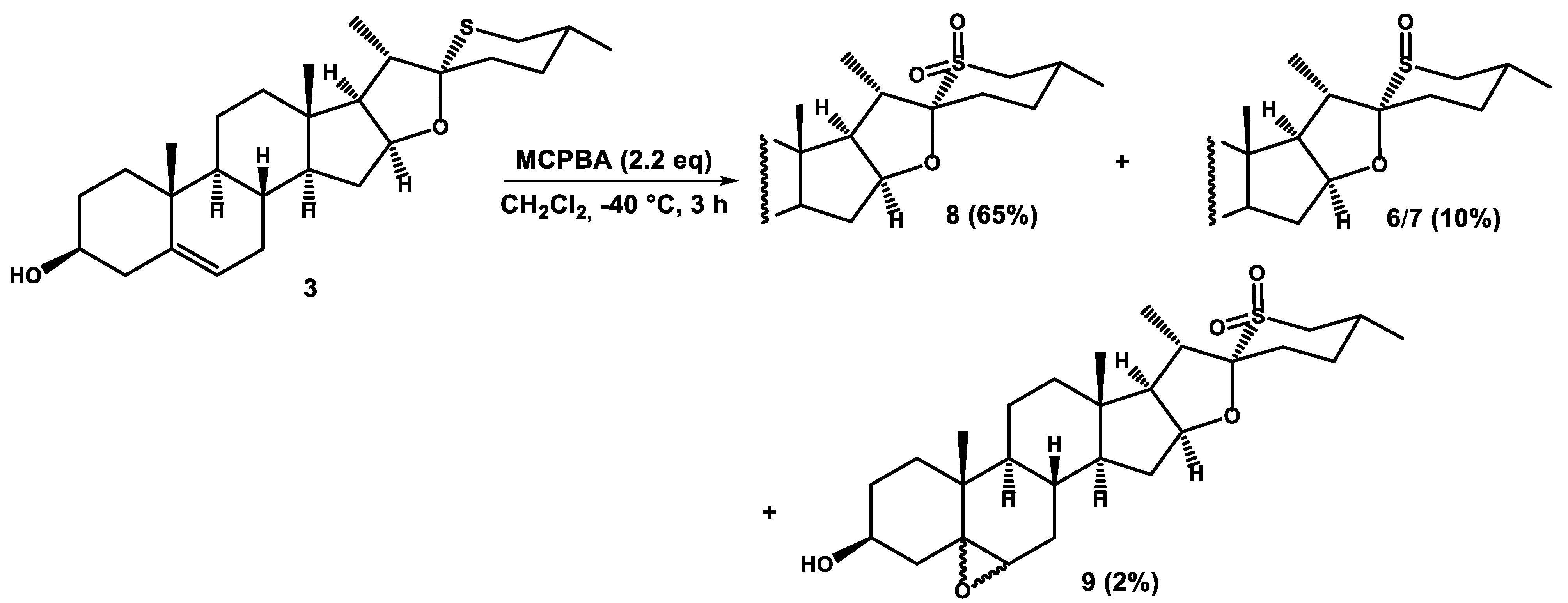
Procedure 2 (with MCPBA)
Steroidal sulfide (3 or 3b) (0.23 mmol) was dissolved in dry dichloromethane (20 mL) cooled to −40 °C (dry ice/acetonitrile cooling bath) and m-chloroperoxybenzoic acid (112 mg, 0.50 mmol) was added. The solution was stirred at −40 °C for 2–3 h and monitored by TLC. When the reaction was completed, dimethyl sulfide (0.2 mL, 2.6 mmol) was added. Then, the reaction mixture was poured into saturated aqueous solution of NaHCO3, and extracted with dichloromethane (3 × 100 mL). The combined organic extracts were dried over anhydrous Na2SO4, and evaporated to dryness in vacuo. The residue was chromatographed on a silica gel column to afford pure compounds 8 or 8b from 3 and 3b, respectively.
In the reaction of 3, in addition to 8 (65%), small amounts of 5,6-epoxides 9 (2%) and sulfoxides 6/7 (10%) were isolated. Compounds 6/7 and 8 were described above. Epoxides 9 were eluted with hexane/ethyl acetate (6:4) as a mixture of 5,6α and 5,6β epimers in the ratio of 7:3. Only major product (5,6α-epoxide) is described below.
5,6α-Epoxide 9: IR (ATR) νmax 3698, 3626, 3589, 3523, 1572, 1450, 1290, 1126, 1055 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.08 (1H, m, H-16), 3.93 (1H, m, H-3α), 3.15 (1H, t, J = 12.7 Hz, H-26α), 2.92 (1H, d, J = 4.4 Hz, H-6), 2.64 (1H, d, J = 12.7 Hz, H-26β), 1.40 (3H, d, J = 7.3 Hz, H-21), 1.09 (3H, s, H-19), 1.01 (3H, d, J = 6.8 Hz, H-27), 0.74 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 103.3 (C), 85.3 (CH), 68.7 (CH), 65.6 (C), 63.7 (CH), 58.9 (CH), 56.5 (CH), 55.2 (CH2), 42.9 (CH), 42.3 (CH), 40.8 (C), 39.7 (CH2), 38.9 (CH2), 35.2 (CH2), 35.0 (C), 32.4 (CH2), 32.3 (CH2), 31.0 (CH2), 30.9 (CH), 29.5 (CH), 29.2 (CH2), 28.9 (CH2), 21.1 (CH3), 20.4 (CH2), 16.2 (CH3), 16.03 (CH3), 15.95 (CH3); HRMS m/z 479.2822 (calcd for C27H43O5S+, 479.2826).
Compound 8b: was eluted with hexane/ethyl acetate (3:1) in 63% yield. Colorless crystals (hexane/ethyl acetate); mp 228–230 °C; IR (ATR) νmax 1454, 1374, 1284, 1249, 1081 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.31 (1H, m, H-6), 5.10 (1H, m, H-16), 3.48 (1H, m, H-3α), 3.16 (1H, t, J = 13.2 Hz, H-26a), 2.63 (1H, dd, J = 13.2 Hz, J = 1.6 Hz, H-26b), 1.41 (3H, d, J = 7.3 Hz, H-21), 1.02 (3H, s, H-19), 1.01 (3H, d, J = 6.6 Hz, H-27), 0.89 (9H, s, t-Bu-Si), 0.79 (3H, s, H-18), 0.06 (6H, s, (CH3)2Si); 13C NMR (CDCl3, 100 MHz) δ 141.6 (C), 120.7 (CH), 103.3 (C), 85.5 (CH), 72.4 (CH), 63.8 (CH), 56.4 (CH), 55.2 (CH2), 49.9 (CH), 42.9 (CH), 42.7 (CH2), 40.7 (C), 39.3 (CH2), 37.3 (CH2), 36.6 (C), 35.1 (CH2), 32.6 (CH2), 32.0 (CH2), 31.9 (CH2), 31.4 (CH), 30.9 (CH), 29.1 (CH2), 25.9 (3 × CH3), 21.1 (CH3), 20.7 (CH2), 19.4 (CH3), 18.2 (C), 16.2 (CH3), 16.0 (CH3), −4.6 (2 × CH3); HRMS m/z 577.3755 (calcd for C33H57O4SSi+, 577.3741).
3.1.7. General Procedure for α-Alkylation
n-BuLi (0.08 mL, 2.5 M in hexane, 0.2 mmol) was added to a solution of steroidal sulfoxide or sulfone (6b or 8b) (0.08 mmol) in dry THF (10 mL), and the mixture was stirred at room temperature for 15 min. After this time, alkyl iodide (CH3I or C2H5I) (0.2 mmol) was added and stirring was continued for 45–90 min. Then, the reaction mixture was poured into water, and extracted with dichloromethane (3 × 50 mL). The combined organic extracts were dried over anhydrous Na2SO4, and evaporated to dryness in vacuo. The residue was subjected to chromatography on a silica gel column to afford pure compounds 10b, 11b, or 12b.
Compound 10b: was eluted with hexane/ethyl acetate (3:1) mixture in 91% yield. Colorless crystals (hexane/ethyl acetate); mp 198–200 °C; IR (ATR) νmax 1426, 1359, 1219, 1091 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.31 (1H, m, H-6), 4.65 (1H, m, H-16), 3.48 (1H, m, H-3α), 1.32 (3H, d, J = 7.4 Hz, H-21), 1.31 (3H, s, CH3-C26), 1.23 (3H, s, CH3-C26), 1.02 (3H, s, H-19), 0.90 (3H, d, J = 6.9 Hz, H-27), 0.89 (9H, s, t-Bu-Si), 0.81 (3H, s, H-18), 0.06 (6H, s, (CH3)2Si); 13C NMR (CDCl3, 100 MHz) δ 141.7 (C), 120.6 (CH), 103.2 (C), 84.0 (CH), 72.5 (CH), 63.5 (CH), 57.5 (C), 56.5 (CH), 50.1 (CH), 46.0 (CH), 42.8 (CH2), 40.8 (C), 39.5 (CH2), 37.3 (CH2), 36.7 (C), 32.4 (CH2), 32.0 (2 × CH2), 31.3 (CH), 30.7 (CH), 28.5 (CH2), 27.4 (CH2), 26.2 (CH3), 25.9 (3 × CH3), 20.8 (CH2), 19.4 (CH3), 18.2 (C), 16.7 (CH3), 16.2 (CH3), 15.9 (CH3), 15.6 (CH3), −4.6 (2 x CH3); HRMS m/z 589.4089 (calcd for C35H61O3SSi+, 589.4105).
Compound 11b: was eluted with hexane/ethyl acetate (93:7) mixture in 82% yield. Colorless crystals (hexane/ethyl acetate); mp 198–200 °C; IR (ATR) νmax 1459, 1375, 1290, 1251, 1123, 1081 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.31 (1H, m, H-6), 5.07 (1H, m, H-16), 3.49 (1H, m, H-3α), 3.14 (1H, dk, J = 11.1 Hz, J = 6.9 Hz, H-26α), 1.42 (3H, d, J = 7.4 Hz, H-21), 1.29 (3H, d, J = 6.9 Hz, CH3-C26), 1.02 (3H, s, H-19), 0.98 (3H, d, J = 6.6 Hz, H-27), 0.89 (9H, s, t-Bu-Si), 0.80 (3H, s, H-18), 0.06 (6H, s, (CH3)2Si); 13C NMR (CDCl3, 100 MHz) δ 141.6 (C), 120.7 (CH), 103.5 (C), 85.5 (CH), 72.5 (CH), 63.8 (CH), 57.9 (CH), 56.4 (CH), 50.0 (CH), 43.4 (CH), 42.8 (CH2), 40.7 (C), 39.3 (CH2), 37.3 (CH2), 36.7 (C), 35.9 (CH), 34.8 (CH2), 32.6 (CH2), 32.03 (CH2), 31.98 (CH2), 31.5 (CH), 29.8 (CH2), 25.9 (3 × CH3), 20.7 (CH2), 19.4 (CH3), 18.9 (CH3), 18.2 (C), 16.2 (CH3), 16.0 (CH3), 7.3 (CH3), −4.6 (2 × CH3); HRMS m/z 591.3928 (calcd for C34H59O4SSi+, 591.3898).
Compound 12b: was eluted with hexane/ethyl acetate (99:1) mixture in 76% yield. Colorless crystals (hexane/ethyl acetate); mp 215–217 °C; IR (ATR) νmax 1457, 1375, 1286, 1254, 1082 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.31 (1H, m, H-6), 5.07 (1H, m, H-16), 3.49 (1H, m, H-3α), 2.99 (1H, dt, J = 11.4 Hz, J = 4.6 Hz, H-26α), 1.41 (3H, d, J = 7.3 Hz, H-21), 1.13 (3H, t, J = 7.6 Hz, CH3CH2-C26), 1.02 (3H, s, H-19), 1.01 (3H, d, J = 6.5 Hz, H-27), 0.89 (9H, s, t-Bu-Si), 0.79 (3H, s, H-18), 0.06 (6H, s, (CH3)2Si); 13C NMR (CDCl3, 100 MHz) δ 141.6 (C), 120.7 (CH), 103.7 (C), 85.4 (CH), 72.5 (CH), 63.8 (CH), 62.7 (CH), 56.4 (CH), 50.0 (CH), 43.4 (CH), 42.8 (CH2), 40.7 (C), 39.4 (CH2), 37.3 (CH2), 36.7 (C), 34.8 (CH2), 33.8 (CH), 32.6 (CH2), 32.03 (CH2), 31.98 (CH2), 31.4 (CH), 29.9 (CH2), 25.9 (3 × CH3), 20.8 (CH2), 19.4 (CH3), 19.0 (CH3), 18.2 (C), 16.6 (CH2), 16.2 (CH3), 16.0 (CH3), 11.8 (CH3), −4.6 (2 × CH3); HRMS m/z 605.4031 (calcd for C35H61O4SSi+, 605.4054).
3.1.8. General Procedure for the Removal of the TBDMS Group
Steroidal 3β-t-butyldimethylsilyl ether (10b, 11b, or 12b) (0.08 mmol) was dissolved in dry THF (5 mL) and then tetra-n-butylammonium fluoride (0.8 mL, 1 M in THF, 0.8 mmol) was added dropwise. After stirring at room temperature for 2 h, the reaction mixture was poured into water and extracted with diethyl ether (3 × 50 mL). The combined organic layers were dried over Na2SO4, and evaporated in vacuo. The residue was purified by column chromatography on silica gel afforded compound 10, 11, or 12.
Compound 10: was eluted with hexane/ethyl acetate (7:13) mixture as a white amorphous solid in 87% yield. IR (ATR) νmax 3338, 1445, 1346, 1250, 1139, 1062, 1014 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.35 (1H, m, H-6), 4.66 (1H, m, H-16), 3.53 (1H, m, H-3α), 1.33 (3H, d, J = 7.4 Hz, H-21), 1.32 (3H, s, CH3-C26), 1.23 (3H, s, CH3-C26), 1.04 (3H, s, H-19), 0.91 (3H, d, J = 6.9 Hz, H-27), 0.82 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 141.0 (C), 121.2 (CH), 103.2 (C), 84.0 (CH), 71.7 (CH), 63.6 (CH), 57.5 (C), 56.6 (CH), 50.1 (CH), 46.1 (CH), 42.3 (CH2), 40.8 (C), 39.6 (CH2), 37.2 (CH2), 36.7 (C), 32.5 (CH2), 31.7 (2 × CH2), 31.4 (CH), 30.7 (CH), 28.6 (CH2), 27.4 (CH2), 26.2 (CH3), 20.8 (CH2), 19.4 (CH3), 16.7 (CH3), 16.2 (CH3), 15.9 (CH3), 15.6 (CH3); HRMS m/z 475.3253 (calcd for C29H47O3S+, 475.3240).
Compound 11: was eluted with hexane/ethyl acetate (3:1) mixture in 92% yield. Colorless crystals (hexane/ethyl acetate); mp 225–227 °C; IR (ATR) νmax 3514, 3355, 1452, 1378, 1348, 1290, 1279, 1117, 1049 cm−1; 1H NMR (1H NMR (CDCl3, 400 MHz) δ 5.35 (1H, m, H-6), 5.07 (1H, m, H-16), 3.53 (1H, m, H-3α), 3.14 (1H, dq, J = 11.1 Hz, J = 7.0 Hz, H-26α), 1.43 (3H, d, J = 7.4 Hz, H-21), 1.29 (3H, d, J = 7.0 Hz, CH3-C26), 1.03 (3H, s, H-19), 0.98 (3H, d, J = 6.6 Hz, H-27), 0.80 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 140.9 (C), 121.2 (CH), 103.6 (C), 85.5 (CH), 71.7 (CH), 63.9 (CH), 57.9 (CH), 56.4 (CH), 49.9 (CH), 43.5 (CH), 42.3 (CH2), 40.8 (C), 39.4 (CH2), 37.2 (CH2), 36.6 (C), 35.9 (CH), 34.9 (CH2), 32.6 (CH2), 32.0 (CH2), 31.6 (CH2), 31.5 (CH), 29.8 (CH2), 20.8 (CH2), 19.4 (CH3), 18.9 (CH3), 16.2 (CH3), 16.0 (CH3), 7.3 (CH3); HRMS m/z 477.3039 (calcd for C28H45O4S+, 477.3033).
Compound 12: was eluted with hexane/ethyl acetate (3:1) mixture in 90% yield. Colorless crystals (hexane/ethyl acetate); mp 210–212 °C; IR (ATR) νmax 3503, 1450, 1377, 1349, 1276, 1225, 1152, 1117, 1072, 1051 cm−1; 1H NMR (CDCl3, 400 MHz) δ 5.35 (1H, m, H-6), 5.07 (1H, m, H-16), 3.53 (1H, m, H-3α), 2.99 (1H, dt, J = 11.1 Hz, J = 4.6 Hz, H-26α), 1.41 (3H, d, J = 7.2 Hz, H-21), 1.13 (3H, t, J = 7.5 Hz, CH3CH2-C26), 1.03 (3H, s, H-19), 1.02 (3H, d, J = 6.8 Hz, H-27), 0.80 (3H, s, H-18); 13C NMR (CDCl3, 100 MHz) δ 140.8 (C), 121.2 (CH), 103.7 (C), 85.4 (CH), 71.7 (CH), 63.7 (CH), 62.7 (CH), 56.3 (CH), 49.9 (CH), 43.4 (CH), 42.2 (CH2), 40.7 (C), 39.3 (CH2), 37.1 (CH2), 36.6 (C), 34.8 (CH2), 33.8 (CH), 32.6 (CH2), 31.9 (CH2), 31.6 (CH2), 31.4 (CH), 29.9 (CH2), 20.8 (CH2), 19.4 (CH3), 19.0 (CH3), 16.6 (CH2), 16.2 (CH3), 16.0 (CH3), 11.8 (CH3); HRMS m/z 491.3197 (calcd for C29H47O4S+, 491.3190).