1. Introduction
Boron cluster chemistry, which traces its roots to the seminal works of Stock [
1], Lipscomb [
2], Hawthorne [
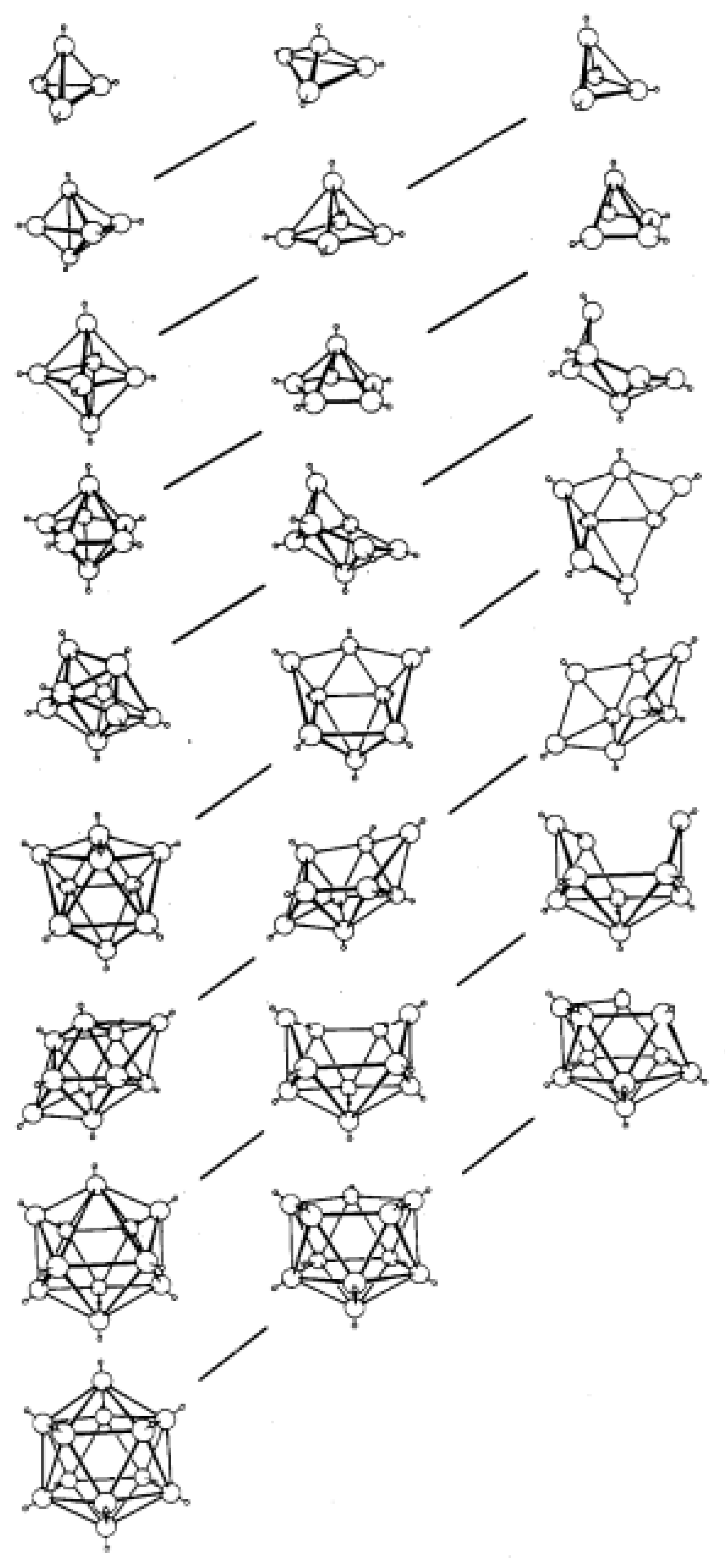
3] and many others, is now a mature but still developing field which sits at the intersection of organic, inorganic and organometallic chemistry. From the earliest times it was appreciated that the polyhedral nature of these species meant that the bonding within them could not be explained by simple 2-centre-2-electon interactions and that a delocalized model was required. The critical breakthroughs in rationalization of their structures occurred in the early 1970s. First, Williams recognized that the structures of the boranes fell into well-defined groups, being either closed polyhedra (given the prefix
closo) or open fragments thereof (
nido,
arachno,
hypho, etc.) [
4]. This was important because previously there had been the general belief that structurally the majority of the open boranes were simply fragments of an icosahedron. Williams identified that, e.g., the structure of B
10H
14 was in fact a
nido fragment of the 11-vertex octadecahedron, whilst that of B
5H
11 was an
arachno fragment of the 7-vertex pentagonal bipyramid. These structural relationships between the closed polyhedra and their
nido and
arachno fragments were later to be popularized in a classic figure by Rudolph [
5],
Figure 1. Second, and most importantly, Wade subsequently provided an explanation for these structural patterns in terms of the number of skeletal electron pairs (SEPs) available [
6]. In brief, for an
n vertex polyhedron a
closo species has (
n + 1) SEPs, a
nido species (
n + 2), an
arachno species (
n + 3), etc.
A particularly important feature of boron cluster chemistry is the number and diversity of heteroatom fragments, from across a broad spectrum of the periodic table, that can occupy vertices of the polyhedron. Thus, for example, in [3-(C
5H
5)-
closo-3,1,2-CoC
2B
9H
11] [
7,
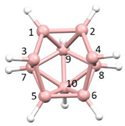
8] the Co and C atoms occupy vertices of the resulting icosahedron (
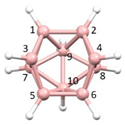
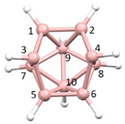
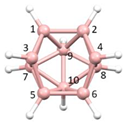
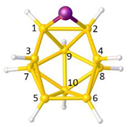
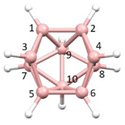
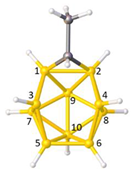
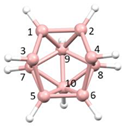
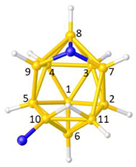
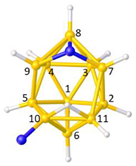
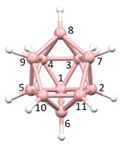
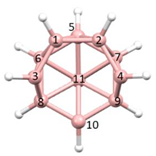
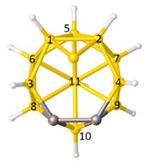
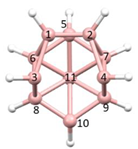
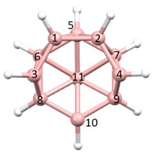
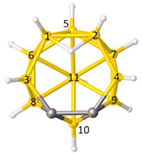
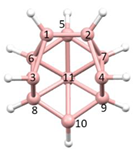
Figure 2a), whilst in [
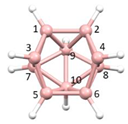
nido-6-SB
9H
11] [
9] the S atom occupies a vertex of the resulting
nido fragment of an octadecahedron (
Figure 2b). Importantly, the same electron-counting rules which describe the boranes apply to these heteroboranes, with the {CoCp} fragment providing 1 vertex and 2 skeletal electrons, the {CH} fragments 1 vertex and 3 skeletal electrons and the {S} fragment 1 vertex and 4 skeletal electrons.
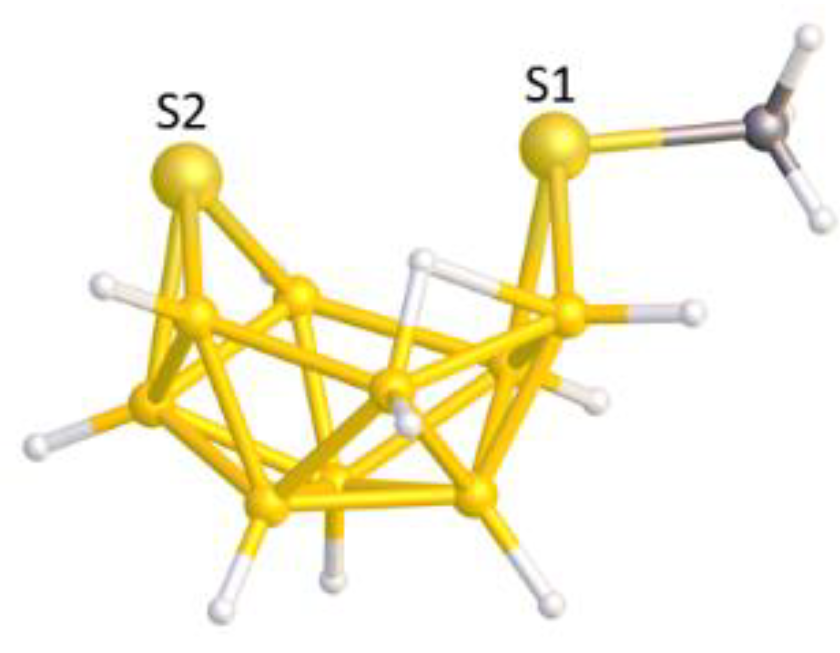
In this contribution we are concerned with heteroboranes in which a main group heteroatom
could either be considered as a vertex or as a bridge to the cluster, and we describe a method to distinguish between these alternatives based on the differing effects that a heteroatom bridge or vertex has on the structure of the {B
x} fragment of the molecule. To illustrate the problem, consider the species [Me
3NCB
10H
10PPh] [
10], assigned the refcode PACBOR in the Cambridge Structural Database (CSD) [
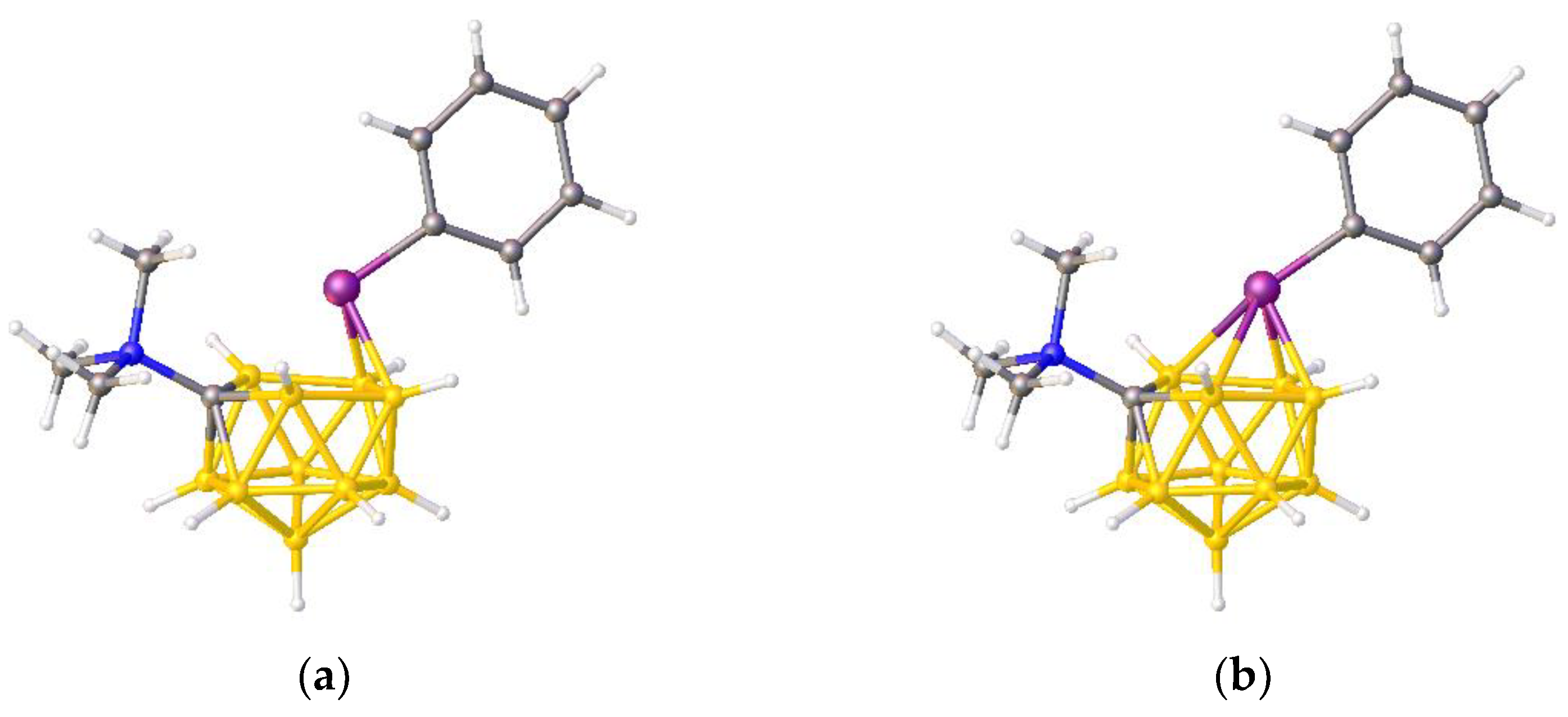
11]. As described in the publication, and as implied by
Figure 3a, PACBOR is considered to be an 11-vertex
nido CB
10 species with a {PPh} fragment bridging a B—B edge in the open face. In this description the P atom is not formally a vertex, the {PPh} unit contributes just 2e to the cluster bonding, and consequently the {B
10} residue would be an
arachno fragment of an icosahedron, characterized by 13 SEP. Alternatively, simply redrawing the molecule with two additional P—B connectivities (
Figure 3b) implies that the P atom is a cluster vertex. In this description PACBOR would be a 12-vertex
nido PCB
10 species in which the {PPh} fragment contributes 1 vertex and 4 skeletal electrons. Now the {B
10} residue would be described as a
hypho fragment of a 13-vertex parent (docosahedron), characterized by 14 SEP.
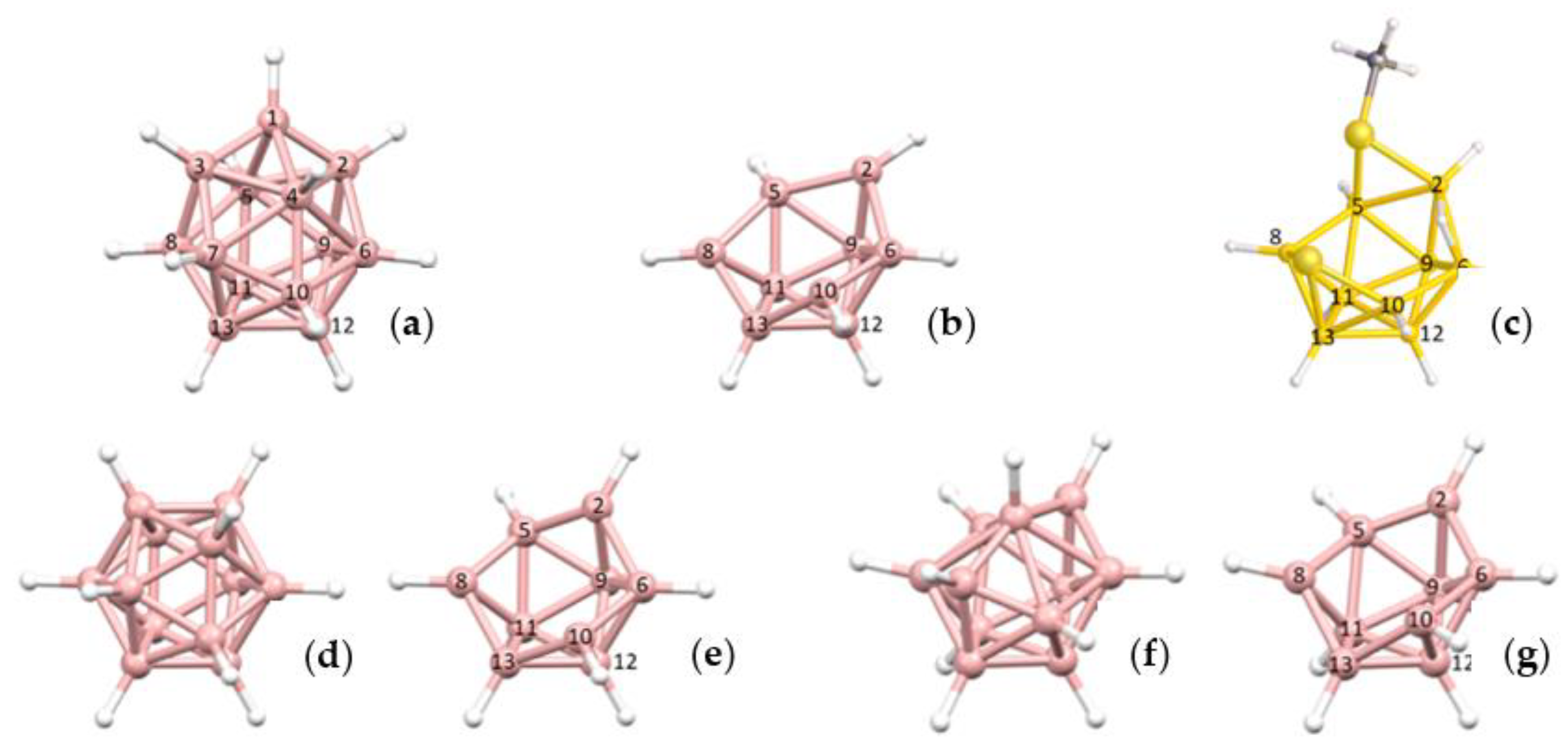
To distinguish between these alternatives, and hence to afford the more appropriate description of PACBOR, we need to see if the experimental {B
10} residue fits better with exemplar
arachno or
hypho {B
10} fragments, but first we need to establish if such exemplar fragments are themselves distinguishable. This is because, as shown in
Figure 4, these fragments are topologically identical and, moreover, they are further topologically identical with a 12-SEP
nido {B
10} fragment. So, faced with an experimental {B
10} fragment of this topology (from a crystallographic study) how do we know if it is best described as
nido,
arachno or
hypho? More generally, how do we distinguish between topologically equivalent {B
x} fragments of different parent polyhedra? One approach for the {B
10} fragments could be to use the dimensions of the fragment, since as we go from
nido {B
10} to
arachno {B
10} to
hypho {B
10} the cluster gets progressively shorter, deeper and broader (see
Figure S1 in Supplementary Materials for detail) but this is not the best method and certainly not general for other fragments. Rather we make use of
Structure Overlay (SO) calculations implemented in Mercury [
12], as described in the Method section. The present study complements our earlier one using the same principle of structure overlay to distinguish between transition- or post-transition-metal bridges and vertices in species of the type [
MB
10H
12] [
13].
3. Discussion
We have used
Structure Overlay calculations to analyze boron clusters which contain an open face onto which a main group heteroatom
X is bound by two short B—
X connectivities for a variety of atoms
X; B (BUPPEI [
24]), C (MMUHDB [
17] and YACRIE [
18]), P (PACBOR [
10]) and S (YELXES [
21]). Our approach is based on the fact that considering the atom
X as a bridge or a cluster vertex will result in different formal descriptions of the {B
x} residue of the cluster. We have overlaid the structure of the experimental {B
x} residue with those of exemplar {B
x} fragments having the same topology derived from
closo species [B
nH
n]
2− optimized by DFT calculation, and used the better or best fit to identify the better or best description for the experimental {B
x} residue and hence better or best description of
X as a bridge or vertex.
In describing their experimental results, some authors have simply assumed that X is a bridge and not a formal part of the cluster (PACBOR, MMUHDB and YACRIE, although in the last case the authors also cast some doubt about this assumption). Others have argued (as it turns out correctly) that X is a vertex whilst also discussing the possibility of it as a bridge (YELXES). Yet, others have assumed (as it turns out incorrectly) that X is a vertex without any explanation (BUPPEI). However, as far as we are aware no previous method to distinguish between bridge or vertex options has been proposed.
We find that, with the exception of BUPPEI, in all the cases we have considered
X is better described as a vertex and we have attempted to quantify this with the term
verticity. For MMUHDB, YACRIE, PACBOR and YELXES the calculated verticities are remarkably consistent, 60–65%. We recognize that these values are not particularly high, and certainly not as high as those (>90%) we have found when similarly analyzing heteroatoms bound by three short B connectivities (ZONCOU [
19] and FAFYAN [
20]) [
25]. Nevertheless, verticities of ca. 60% do suggest that the better description of the atom
X in MMUHDB, YACRIE, PACBOR and YELXES is as a cluster vertex. The exception is BUPPEI, in which the verticity of the bridging B atom is only ca. 28%, implying that this atom is better described as a bridge than a vertex, and that the correct formal description of the anion is as a
nido C
2B
11 species with a bridging B and not as a
nido C
2B
12 species [
24]. How can we rationalize these results?
Formally, electron donation to the cluster from all these ‘bridging’ units is from two sources; those electrons involved in the (radial) interaction between X and the cluster (i.e., in simple terms those electrons donated via the B—X connectivities) and those electrons in either a (tangential) lone pair or 2-centre-2-electron bond on X which is endo with respect to the cluster. The {C(H)Me} fragments of MMUHDB and YACRIE provide 2 radial electrons as does the {PPh} fragment of PACBOR, whilst the {SMe} group of YELXES provides 3 such electrons. In BUPPEI, however, only 1 radial electron is formally donated to the cluster. In all cases these contributions to the overall skeletal electron count are then formally enhanced by the endo electron pair. Note that in ZONCOU and FAFYAN the orientation of the Ph or Me substituent implies no endo lone pair on the N atoms, with all the (4) donated electrons being radial.
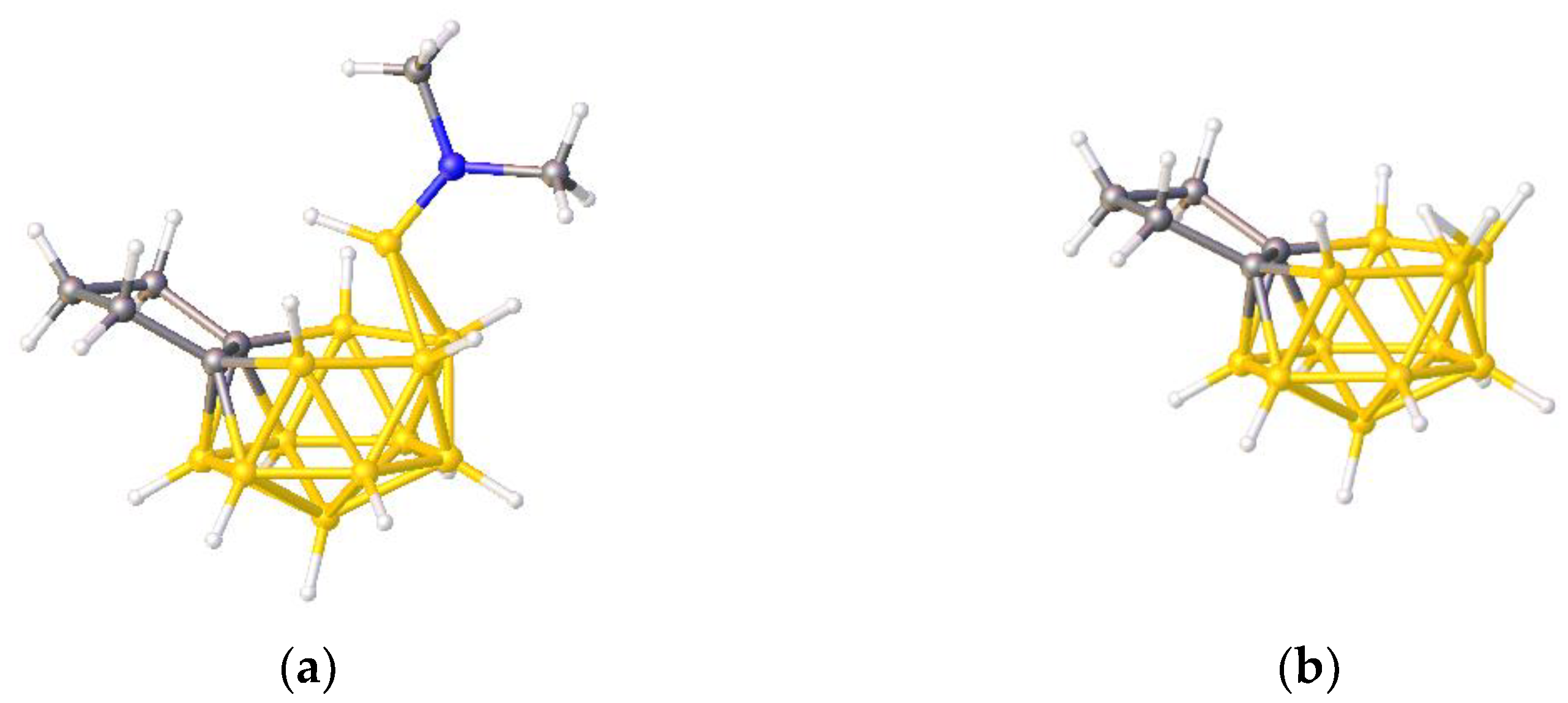
Our structure overlay results, and the conclusions regarding the formal descriptions of X as either a bridge or vertex which derive from them, may be understood at a simple level if we assume that the radial electron donation from X is more important than donation of the endo electron pair. In this way the 2- or 3-electron radial donations of {C(H)Me}, {PPh} and {SMe}, supplemented by relatively weak endo donation, achieve verticities > 50% (making the atom X formally a vertex) but they do not approach the very high verticities seen in ZONCOU and FAFYAN where the N heteroatoms are fully integrated into the cluster framework and the total skeletal electron count does not depend on endo electrons. In contrast weak endo donation is insufficient to raise the verticity in BUPPEI (which has only 1-electron radial donation) above the 50% threshold and consequently the {B(H)NMe2} group is formally described as a bridge. Note, however, that the {B(H)NMe2} in BUPPEI has a higher verticity than the simple bridging H atom in BUPPIM since in the latter case there are no endo electrons to be donated.
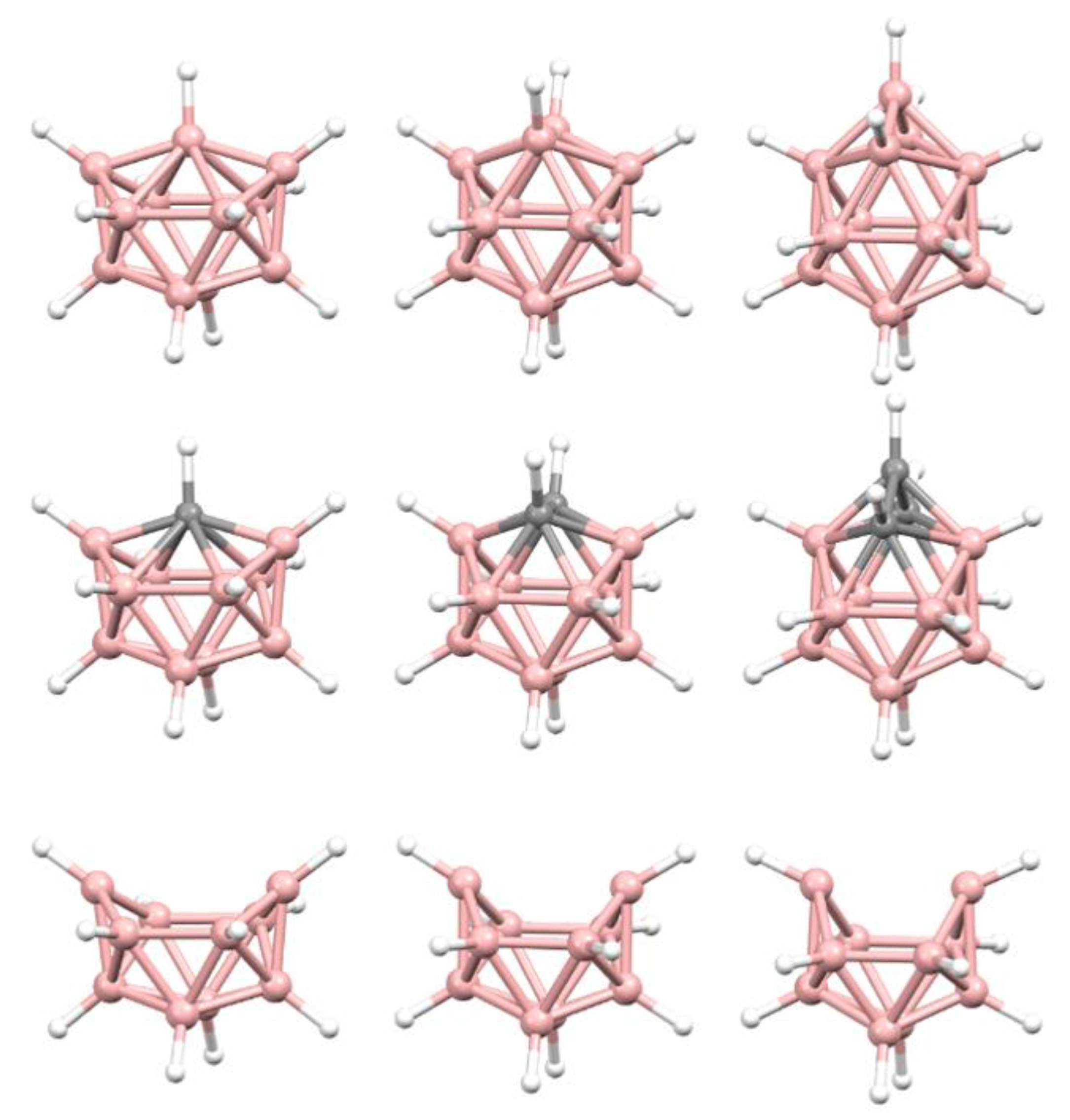
A useful test of our explanation would be consider the effect of a ‘bridging’ {B(H)NMe
3} unit as opposed to a bridging {B(H)NMe
2} unit, since the former would provide 2 radial electrons as opposed to just 1 and should, therefore, assume more vertex character. Since we have been unable to locate a (hetero)borane with a ‘bridging’ {B(H)NMe
3} fragment in the CSD we have constructed one from BUPPEI. Initially (to check the validity of the process) we have simply optimized the anion BUPPEI by DFT calculation and in
Table S3 of Supplementary Materials are presented the results of structure overlay calculations of the {B
11} fragment of the optimized model and the unprimed and primed experimental {B
11} fragments. In both cases the very small rms misfit values of 0.019 Å confirm the validity of the optimization. Consequently, we have constructed the neutral species [(CH
2)
3C
2B
11H
11{B(H)NMe
3}] (compound
1) from the coordinates of BUPPEI and optimized its structure.
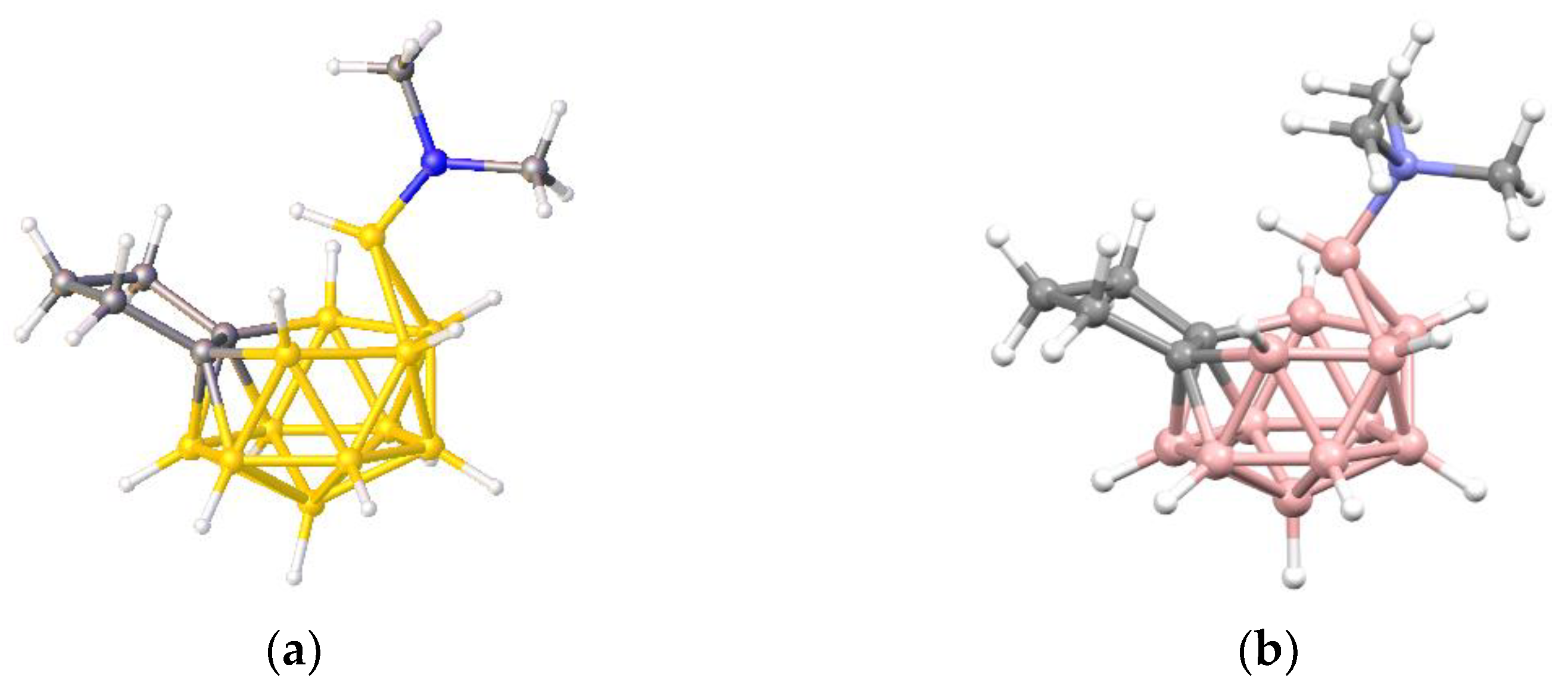
Figure 10b shows a perspective view of
1 (since this is an optimized model and not a real molecule B atoms are shown in pink) adjacent to BUPPEI (
Figure 10a). Note that, in terms of their overall skeletal electron count, the anion BUPPEI and neutral species
1 are isoelectronic, the anionic charge of BUPPEI being compensated by an additional radial electron from the bridge in
1. Initial comparison of the two structures does suggest a somewhat greater degree of vertex character for the bridging B of
1 since, in addition to the two short B—B distances of 1.788 and 1.790 Å (c.f. 1.868–1.890 Å in BUPPEI) the bridging B is only 2.155 and 2.168 Å from the other two B atoms in the open face (c.f. 2.388–2.442 Å in BUPPEI).
Table 13 reports the results of structure overlay calculations of the {B
11} fragment of
1 with both the
hypho and
klado {B
11} exemplars. Although the rms misfit with the
hypho exemplar is still the smaller (meaning that the bridging B is still better described as a bridge) it is greater than that for BUPPEI and, correspondingly, the rms misfit with the
klado exemplar is smaller than that for BUPPEI. For the bridging B atom the verticity is 44.0% in compound
1 compared to only ca. 28% in BUPPEI, supporting the idea that formally increasing the radial electron donation of the bridging unit from 1 to 2 electrons significantly increases the vertex character.