Voltammetric and Fluorimetric Studies of Dibenzoylmethane on Glassy Carbon Electrodes and Its Interaction with Tetrakis (3,5-Dicarboxyphenoxy) Cavitand Derivative
Abstract
1. Introduction
2. Results
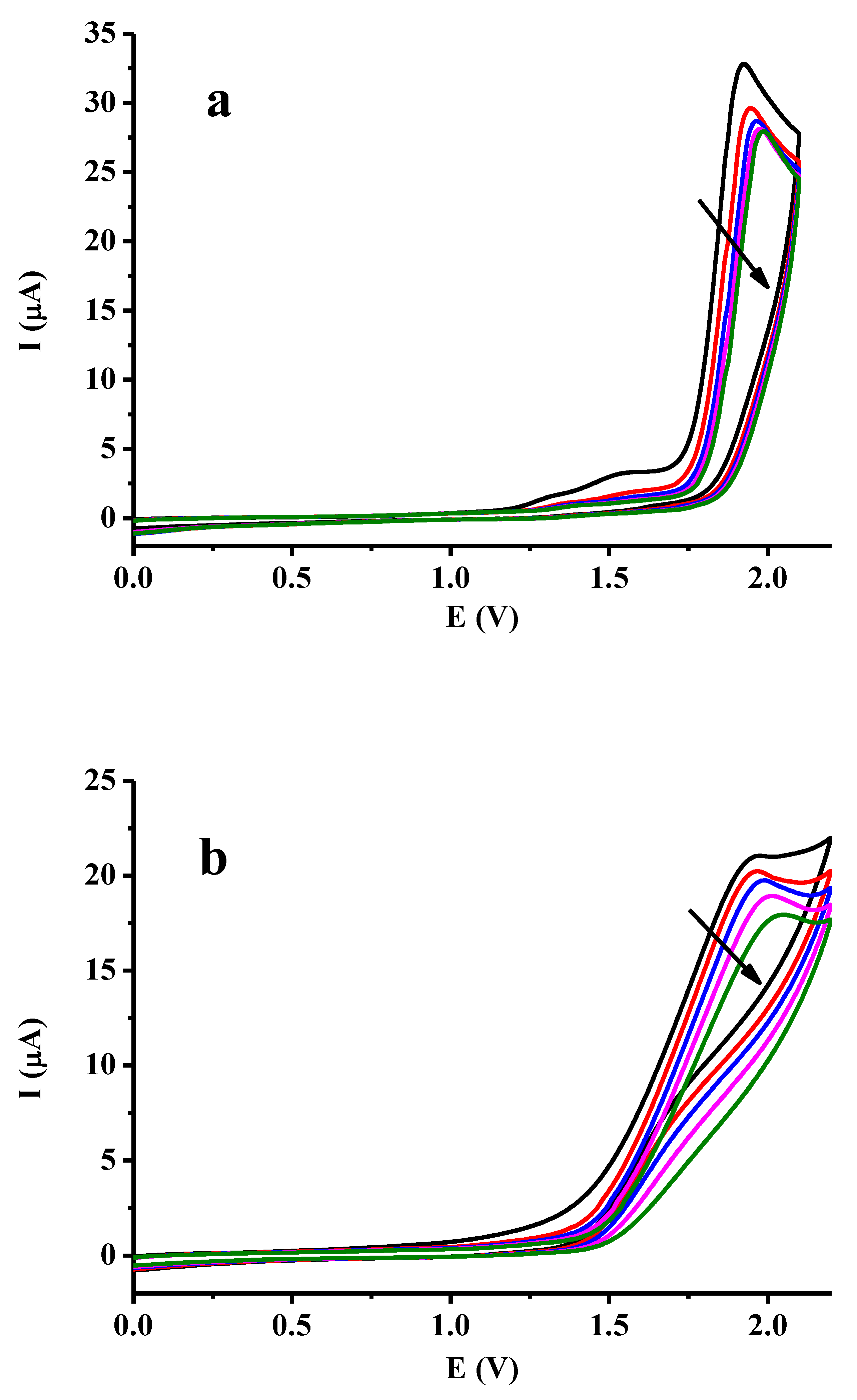
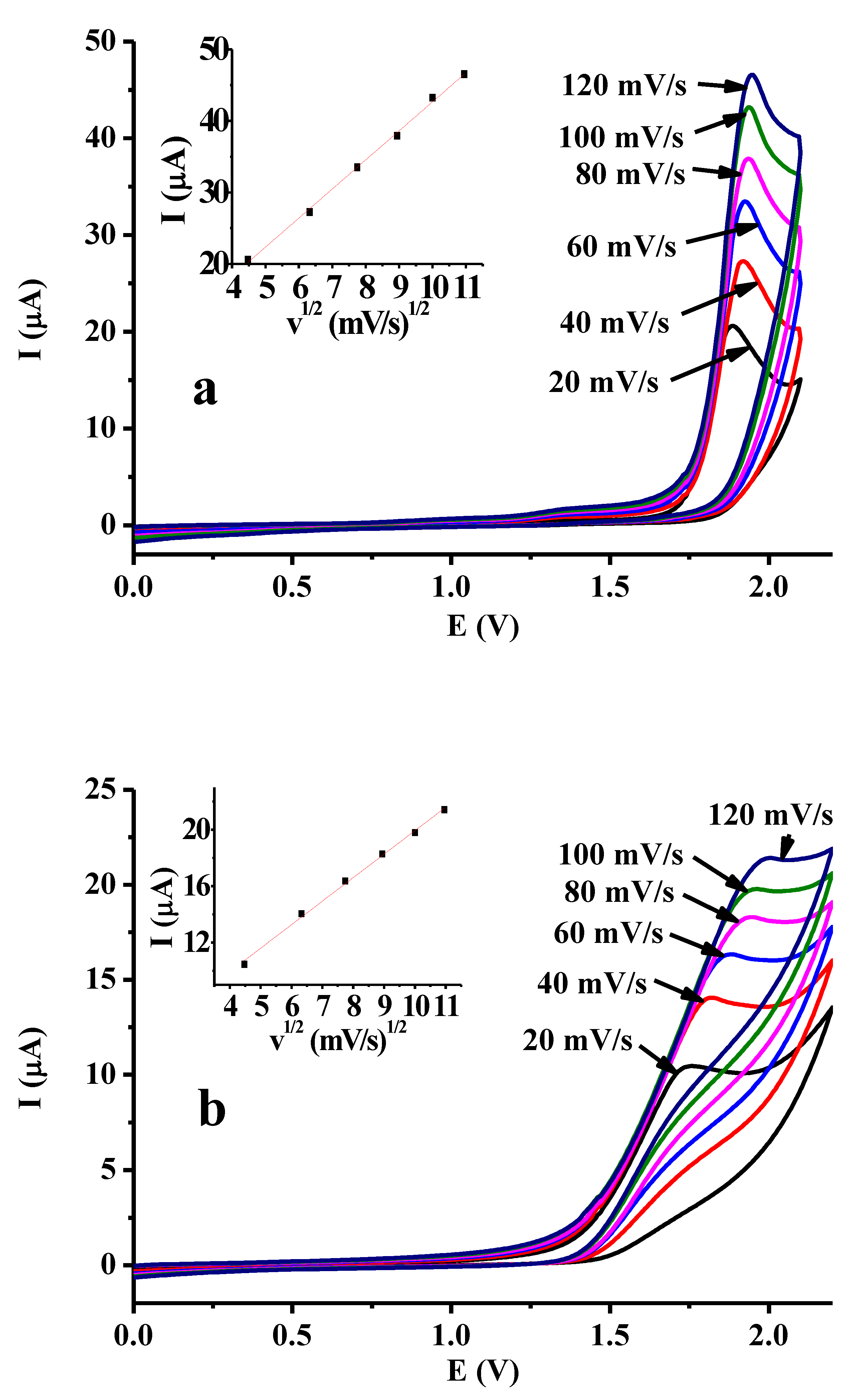
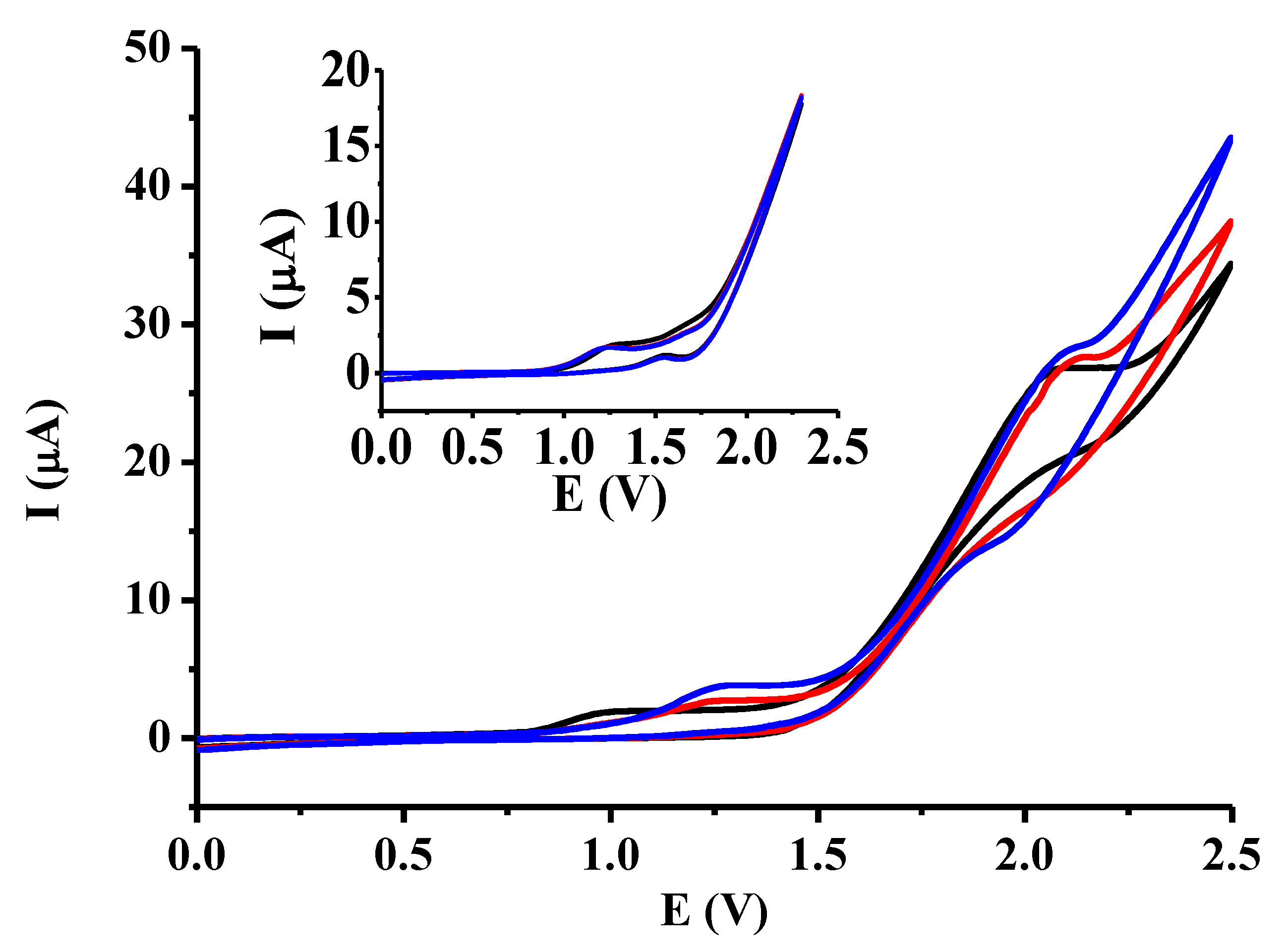
2.1. Voltammetric Characterization of Dibenzoylmethane and Effect of Some Additives
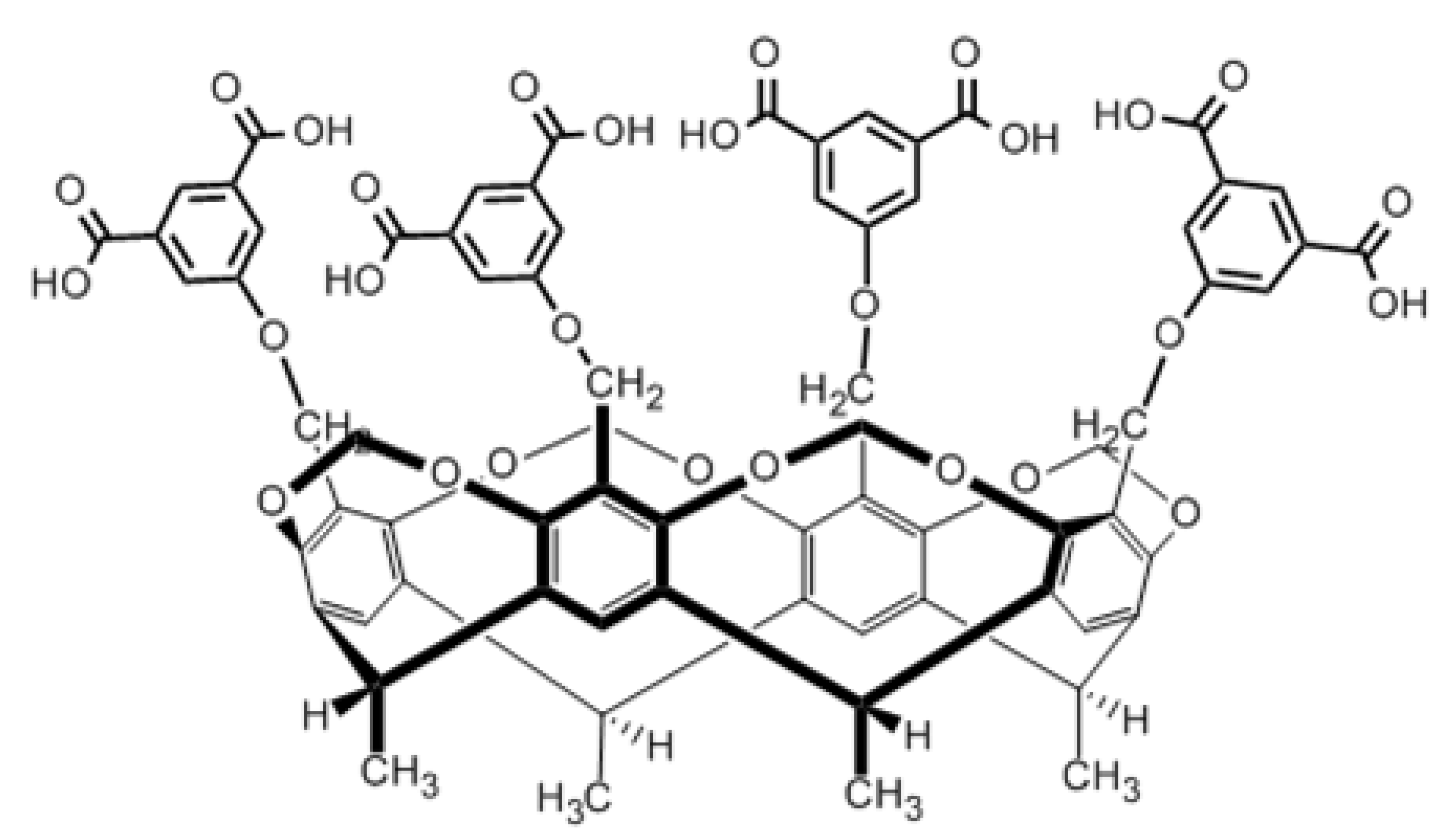
2.2. Titration Experiments
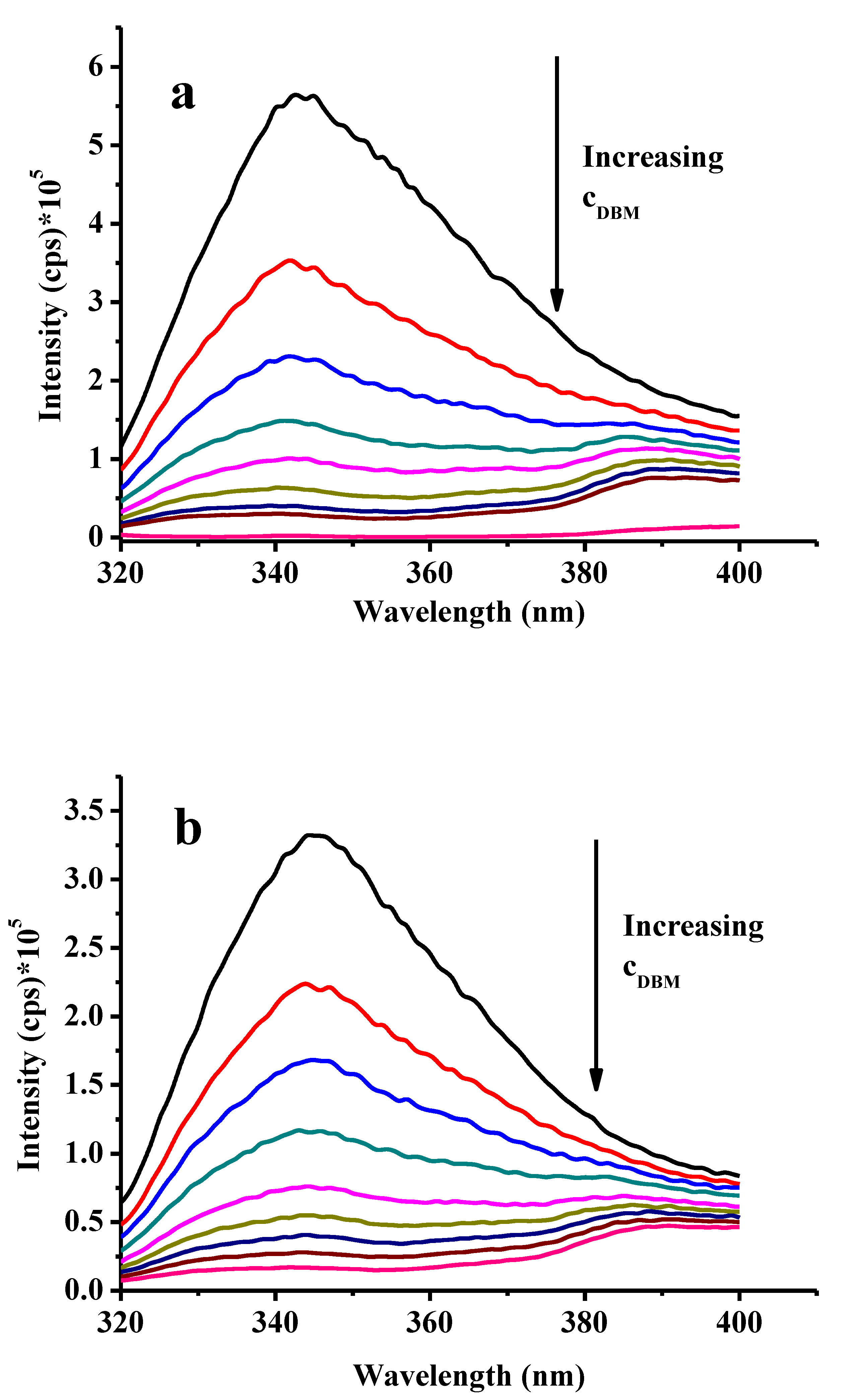
2.3. Fluorimetric Investigations in Dimethyl Sulfoxide
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hegedűs, C.; Lakatos, P.; Kiss-Szikszai, A.; Patonay, T.; Gergely, S.; Gregus, A.; Bai, P.; Haskó, G.; Szabó, É.; Virág, L. Cytoprotective dibenzoylmethane derivatives protect cells from oxidative stress-induced necrotic cell death. Pharmacol. Res. 2013, 72, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Greci, L.; Parsons, R.; Knowland, J. Nitroxide radicals protect DNA from damage when illuminated in vitro in the presence of dibenzoylmethane and a common sunscreen ingredient. Free Radic. Biol. Med. 1999, 26, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Frazier, M.C.; Jackson, K.M.; Janowska-Stephens, E.; Anderson, M.G.; Harris, W.B. Proteomic analysis of proteins altered by dibenzoylmethane in human prostatic cancer LNCaP cells. Proteomics 2004, 4, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Murakami, R.; Katayama, T.; Hibino, S.; Choshi, T.; Yamada, M. Nuclear factor κB inhibition by dibenzoylmethane derivatives. J. Health Sci. 2009, 55, 311–313. [Google Scholar] [CrossRef][Green Version]
- Shishu, A.K.; Singla, I.P.K. Inhibitory effect of dibenzoylmethane on mutagenicity of food-derived heterocyclic amine mutagens. Phytomedicine 2003, 10, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Yang, Y.; Ho, C.; Yen, G. Mechanisms of apoptotic effects induced by resveratrol, dibenzoylmethane, and their analogues on human lung carcinoma cells. J. Agric. Food Chem. 2009, 57, 5235–5243. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.M.; DeLeon, M.; Verret, C.R.; Harris, W.B. Dibenzoylmethane induces cell cycle deregulation in human prostate cancer cells. Cancer Lett. 2002, 178, 161–165. [Google Scholar] [CrossRef]
- Ashana, P.; Amanpreet, K.; Kaisar, R.; Shishu, G.; Om, P.K. Development and evaluation of topical microemulsion of dibenzoylmethane for treatment of UV induced photoaging. J. Drug Deliv. Sci. Technol. 2017, 37, 1–12. [Google Scholar]
- Jutta, K.; Michael, O.; Sherryl, R.; Beverley, D.G. Influence of titanium dioxide particle size on the photostability of the chemical UV-filters butyl methoxy dibenzoylmethane and octocrylene in a microemulsion. Cosmetics 2014, 1, 128–139. [Google Scholar]
- Silvina, Q.L.; Federico, S.; Gabriel, S.; Lelia, D. Absorption and photo-stability of substituted dibenzoylmethanes and chalcones as UVA filters. Cosmetics 2018, 5, 33–45. [Google Scholar]
- Hornyák, I.; Erostyák, J.; Buzády, A.; Kaszás, A.; Kozma, L. Enhanced fluorimetric determination of samarium with dibenzoylmethane and diphenylguanidine by gadolinium. Spectrosc. Lett. 1997, 30, 1475–1483. [Google Scholar] [CrossRef]
- Srivastava, M.M.; Srivastava, S.N. Polarographic studies on kinetics of electrode reaction of dibenzoylmethane. Electrochim. Acta 1985, 30, 1481–1483. [Google Scholar] [CrossRef]
- Mendez-Arroyo, J.; d’Aquino, I.A.; Chinen, A.B.; Manraj, Y.D.; Mirkin, C.A. Reversible and selective encapsulation of dextromethorphan and β-estradiol using an asymmetric molecular capsule assembled via the weak-link approach. J. Am. Chem. Soc. 2017, 139, 1368–1371. [Google Scholar] [CrossRef] [PubMed]
- Nagymihály, Z.; Lemli, B.; Kollár, L.; Kunsági-Máté, S. Solvent switched weak interaction of a 4-quinazolinone with a cavitand derivative. Molecules 2020, 25, 1915. [Google Scholar] [CrossRef]
- Preisz, Z.; Nagymihály, Z.; Lemli, B.; Kollár, L.; Kunsági-Máté, S. Weak interaction of the antimetabolite drug methotrexate with a cavitand derivative. Int. J. Mol. Sci. 2020, 21, 4345. [Google Scholar] [CrossRef]
- Peles-Lemli, B.; Peles-Lemli, J.; Bitter, I.; Kollár, L.; Nagy, G.; Kunsági-Máté, S. Competitive thermodynamic and kinetic processes during dissociation of some host-guest complexes of calix[4]arene derivatives. J. Incl. Phenom. Macrocycl. Chem. 2007, 59, 251–256. [Google Scholar] [CrossRef]
- Khrizanforova, V.V.; Knyazeva, I.R.; Nizamiev, I.R.; Gryaznova, T.V.; Sokolova, V.I.; Krasnov, S.A.; Kadirov, M.K.; Burilov, A.R.; Budnikova, Y.G.; Sinyashin, O.G. Electrochemical synthesis of the calix[4]resorcinol nickel complexes modified with thiophosphoryl fragments. Russ. J. Gen. Chem. 2013, 83, 663–669. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Lu, K.; Ye, B. A simple but highly sensitive and selective calixarene-based voltammetric sensor for serotonin. Electrochim. Acta 2013, 87, 756–762. [Google Scholar]
- Vishwanath, D.V.; Ashwini, K.S. Electrochemical behavior of folic acid at calixarene based chemically modified electrodes and its determination by adsorptive stripping voltammetry. Electrochim. Acta 2007, 53, 1713–1721. [Google Scholar]
- Wang, F.; Chi, C.; Yu, B.; Ye, B. Simultaneous voltammetric determination of dopamine and uric acidbased on Langmuir-Blodgett film of calixarene modified glassy carbon electrode. Sens. Actuators B Chem. 2015, 221, 1585–1593. [Google Scholar] [CrossRef]
- Preisz, Z.; Nagymihály, Z.; Kollár, L.; Kálai, T.; Kunsági-Máté, S. Weak interactions of the isomers of phototrexate and two cavitand derivatives. Int. J. Mol. Sci. 2021, 22, 10764. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.; Kunsági-Máté, S. Estimation of the usefulness of glassy carbon electrode in non-aqueous solvents polarized to higher anodic potentials. Stud. Univ. Babes-Bol. 2020, 65, 81–88. [Google Scholar] [CrossRef]
- Shirley, S.W.; Stewart, B.H.; Mirelman, S. Dimethyl sulfoxide in treatment of inflammatory genitourinary disorders. Urology 1978, 11, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Riccardo, P.; Fabio, M.; Agnese, P.; Claudio, P.; Giulio, L.; Belén, F.; Antonio, R.D.; Giorgio, S.; Massimo, N. Ruthenium(II)-arene complexes with dibenzoylmethane induce apoptotic cell death in multiple myeloma cell lines. Inorg. Chim. Acta 2017, 454, 139–148. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, L.; Nagymihály, Z.; Kollár, L.; Kunsági-Máté, S. Voltammetric and Fluorimetric Studies of Dibenzoylmethane on Glassy Carbon Electrodes and Its Interaction with Tetrakis (3,5-Dicarboxyphenoxy) Cavitand Derivative. Molecules 2023, 28, 185. https://doi.org/10.3390/molecules28010185
Kiss L, Nagymihály Z, Kollár L, Kunsági-Máté S. Voltammetric and Fluorimetric Studies of Dibenzoylmethane on Glassy Carbon Electrodes and Its Interaction with Tetrakis (3,5-Dicarboxyphenoxy) Cavitand Derivative. Molecules. 2023; 28(1):185. https://doi.org/10.3390/molecules28010185
Chicago/Turabian StyleKiss, László, Zoltán Nagymihály, László Kollár, and Sándor Kunsági-Máté. 2023. "Voltammetric and Fluorimetric Studies of Dibenzoylmethane on Glassy Carbon Electrodes and Its Interaction with Tetrakis (3,5-Dicarboxyphenoxy) Cavitand Derivative" Molecules 28, no. 1: 185. https://doi.org/10.3390/molecules28010185
APA StyleKiss, L., Nagymihály, Z., Kollár, L., & Kunsági-Máté, S. (2023). Voltammetric and Fluorimetric Studies of Dibenzoylmethane on Glassy Carbon Electrodes and Its Interaction with Tetrakis (3,5-Dicarboxyphenoxy) Cavitand Derivative. Molecules, 28(1), 185. https://doi.org/10.3390/molecules28010185





