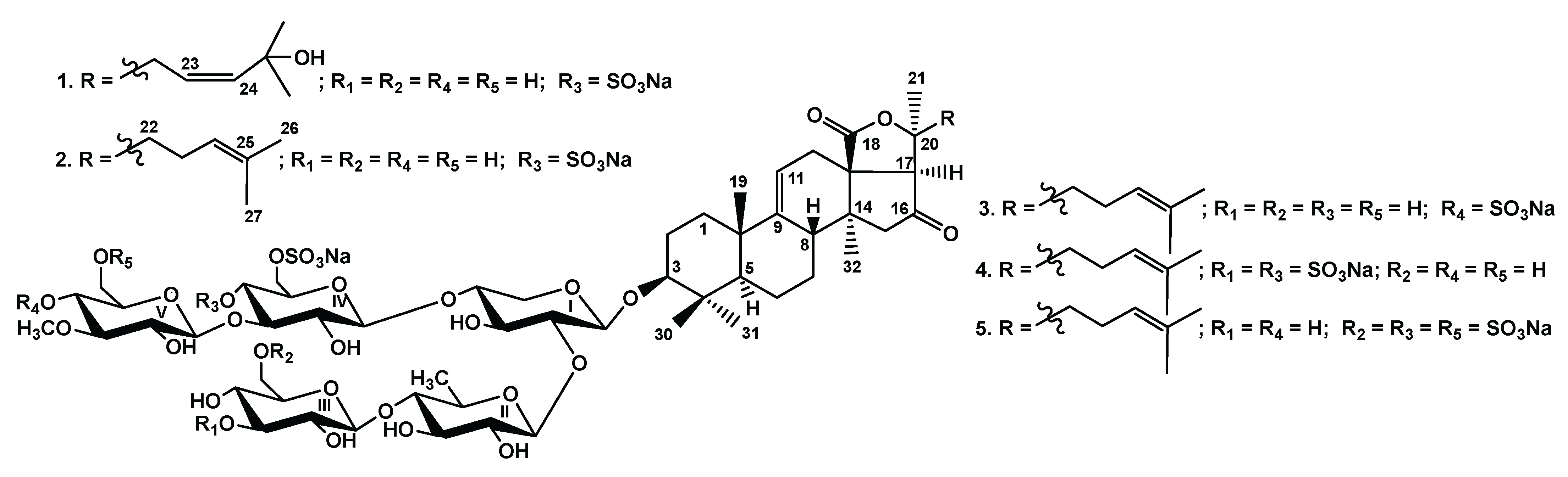
2.1. Structural Elucidation of the Glycosides
The crude glycosidic fraction of the sea cucumber
Paracaudina chilensis was obtained as a result of hydrophobic chromatography of the concentrated ethanolic extract on a Polychrom-1 column (powdered Teflon, Biolar, Latvia). Its subsequent separation by chromatography on Si gel columns with the stepped gradient of the system of eluents CHCl3/EtOH/H2O used in ratios (100:100:17), (100:125:25), and (100:150:50) gave the fractions I–III. Each of the obtained fractions was additionally purified on a Si gel column with the solvent system CHCl3/EtOH/H2O (100:125:25), which resulted in the isolation of five subfractions I.0, I.1, II, III.1 and III.2. The individual compounds
1–
5 (
Figure 1) were isolated through HPLC of these subfractions on the silica-based column Supelcosil LC-Si (4.6
× 150 mm), and reversed-phase columns Supelco Discovery HS F5-5 (10
× 250 mm) and Diasfer 110 C-8 (4.6 × 250 mm).
The configurations of the monosaccharide residues in the glycosides 1–5 were assigned as D based on the biogenetic analogies with the monosaccharides from all other known sea cucumber triterpene glycosides.
The molecular formula of chilensoside A (
1) was determined to be C
60H
92O
34S
2Na
2 from the [M
2Na–Na]
− ion peak at
m/z 1443.4800 (calc. 1443.4815), and [M
2Na–2Na]
2− ion peak at
m/z 710.2466 (calc. 710.2461) in the (
−) HR-ESI-MS (
Figure S8). The
13C NMR spectrum of the aglycone part of chilensoside A (
1) demonstrated the signals of quaternary oxygen-bearing carbons at δ
C 176.8 (C-18) and 82.8 (C-20), corresponding to 18(20)-lactone and the signals of olefinic carbons at δ
C 151.1 (C-9), 111.2 (C-11) (
Table 1,
Figures S1–S7), indicating the presence of 9(11)-double bond, typical of many sea cucumber glycosides. An additional deshielded signal at δ
C 214.6 was assigned to C-16 oxo-group in the holostane nucleus, confirmed by the singlet signal of H-17 at δ
H 2.89 with a corresponding carbon signal at δ
C 61.2 (C-17). The protons of the side chain H-22/H-23/H-24 formed the isolated spin system deduced by the COSY spectrum, indicating the presence of an additional 23
Z(24)-double bond (δ
H-23 5.90 (dd,
J = 6.3; 11.8 Hz), δ
H-24 5.90 (d,
J = 11.8 Hz)). The presence of the signal of quaternary oxygen-bearing carbon at δ
C 81.3 along with the coincidence of the signals of 26, 27-methyl groups to each other (δ
C 24.7 (C-26, C-27), δ
H 1.42 (s, H-26, H-27)) indicated the presence of hydroxyl at C-25. The side chain structure was confirmed by the HMBC correlations H-24/C-25; H-23/C-22; H-26(27)/C-24 (
Table 1). The same aglycone was found only once earlier in cladoloside A
5 from the sea cucumber
Cladolabes schmeltzii [
42].
The
1H and
13C NMR spectra of the carbohydrate chain of chilensosides A (
1) (
Table 2,
Figures S1–S7) and A
1 (
2) (
Table S1,
Figures S9–S15) were coincident to each other, indicating the identity of sugar moieties of
1,
2. The spectra of
1 demonstrated five characteristic doublets of anomeric protons at δ
H 4.66–5.20 (
J = 7.1–8.1 Hz) and the signals of anomeric carbons at δ
C 102.2–104.7, indicating the presence of a pentasaccharide chain and
β-configurations of glycosidic bonds. The coherent analysis of the
1H,
1H-COSY, 1D TOCSY, HSQC and ROESY spectra of
1 indicated the presence of one xylose (Xyl1), one quinovose (Qui2), two glucose (Glc3 and Glc4), and 3-
O-methylglucose (MeGlc5) residues. The ROE- and HMBC correlations showed the positions of glycosidic linkages (
Table 2,
Figures S1–S7), which indicated that the carbohydrate chain of
1 was branched by C-4 Xyl1 having bottom semi-chain composed of two sugar units and the upper semi-chain from three.
Noticeably, such architecture of carbohydrate chains is not common for the holothuroid glycosides, but similar sugar moieties have been found in some glycosides of recently studied species of sea cucumbers:
Thyonidium kurilensis [
43] and
Psolus chitonoides [
6] (order Dendrochirotida).
The availability of two sulfate groups in the sugar moiety of
1 was deduced on the basis of shifting effects observed in its
13C NMR spectrum. These were the signals of two hydroxy methylene groups of glucopyranose residues at δ
C 61.8 (C-6 Glc3) and 62.0 (C-6 MeGlc5), indicating the absence of sulfate groups in these positions and one signal at δ
C 68.2 (deshielded due to α-shifting effect of sulfate group) corresponding to sulfated at C-6 Glc4 residue. Additional shifting effects of the sulfate group became evident when the
13C NMR spectrum of
1 was compared with the spectrum of the carbohydrate part of kuriloside A
1 [
43]. The signals of all monosaccharides in the spectra of these glycosides were close to each other, with the exception of the signals of glucose residue in the upper semi-chain (Glc4). The signals of C-3 Glc4 and C-5 Glc4 were shielded in the spectrum of
1 (to δ
C 82.9 and 74.5, correspondingly) in comparison with the same signals in the spectrum of kuriloside A
1 (δ
C 86.9 and 75.7, correspondingly) due to the
β-shifting effect of the sulfate group, which was attached to C-4 Glc4 of
1. This was confirmed by an α-shifting effect: the signal of C-4 Glc4 in the spectrum of chilensoside A (
1) was deshielded (δ
C 75.6) when compared with the signal of C-4 Glc4 in the spectrum of kuriloside A
1 (δ
C 69.6). Therefore, two sulfate groups were attached to one monosaccharide unit (Glc4) in the sugar chain of
1. Such a structural feature was also recently found in psolusoside P from
Psolus fabricii [
44]. However, chilensoside A (
1) is a new combination of some unusual structural features: aglycone side chain structure, carbohydrate chain architecture and the positions of sulfate groups.
The (
−)ESI-MS/MS of
1 (
Figure S8) demonstrated the fragmentation of [M
2Na−Na]
− ion at
m/z 1443.5 with ion peaks observed at
m/z 1179.5 [M
2Na−Na−Glc–SO
3Na+2H]
−, 1135.5 [M
2Na−Na−Glc–Qui+H]
−, 1010.4 [M
2Na−Na−MeGlc−2HSO
4Na]
−, 417.1 [M
2Na−Na−MeGlc−Glc(OSO
3Na)
2−Agl]
−, and 255.0 [M
2Na−Na−MeGlc−Glc(OSO
3Na)
2−Glc−Agl]
−, corroborating the sequence of monosaccharides and the aglycone structure of
1.
These data indicate that chilensoside A (1) is 3β-O-{β-D-glucopyranosyl-(1 → 4)-β-D-quinovopyranosyl-(1 → 2)-[3-O-methyl-β-D-glucopyranosyl-(1 → 3)-4,6-O-sodium disulfate-β-D-glucopyranosyl-(1 → 4)]-β-D-xylopyranosyl}-16-oxo,25-hydroxyholosta-9(11),23Z(24)-diene.
The aglycones of chilensosides A
1 (
2), B (
3), C (
4) and D (
5) (
Table 3,
Tables S2–S4, Figures S9–S14, S17–S22, S25–S30 and S33–S38) were identical to each other and to those of cladoloside A
4 [
42] and psolusoside D
1 [
45]. This holostane aglycone has the same polycyclic system as
1 and differs in the side chain structure with a 24(25)-double bond.
The molecular formula of chilensoside A
1 (
2) was determined to be C
60H
92O
33S
2Na
2 from the [M
2Na−Na]
− ion peak at
m/z 1427.4928 (calc. 1427.4865), and [M
2Na–2Na]
2− ion peak at
m/z 702.2510 (calc. 702.2487) in the (−)HR-ESI-MS (
Figure S16). The (
−)ESI-MS/MS of
2 (
Figure S16) demonstrated the fragmentation of [M
2Na−Na]
− ion at
m/z 1427.5,
m/z: 1120.5 [M
2Na−Na−Glc−Qui+H]
−, 915.4 [M
2Na−Na−Glc−Qui−2SO
3Na+3H]
−, 667.1 [M
2Na−Na−Glc−Qui–Agl]
−, 417.1 [M
2Na−Na−MeGlc−Glc(OSO
3Na)
2−Agl]
−.
All these data indicate that chilensoside A1 (2) is 3β-O-{β-D-glucopyranosyl-(1 → 4)-β-D-quinovopyranosyl-(1 → 2)-[3-O-methyl-β-D-glucopyranosyl-(1 → 3)-4,6-O-sodium disulfate-β-D-glucopyranosyl-(1 → 4)]-β-D-xylopyranosyl}-16-oxoholosta-9(11),24(25)-diene.
The molecular formula of chilensoside B (
3) was determined to be C
60H
92O
33S
2Na
2 from the [M
2Na–Na]
− ion peak at
m/z 1427.4881 (calc. 1427.4865), and [M
2Na–2Na]
2− ion peak at
m/z 702.2499 (calc. 702.2487) in the (
−)HR-ESI-MS (
Figure S24). The
1H and
13C NMR spectra of the carbohydrate chain of chilensoside B (
3) (
Table 4,
Figures S17–S23) demonstrated five characteristic doublets of anomeric protons at δ
H 4.66–5.18 (
J = 7.1–8.1 Hz) and five signals of anomeric carbons at δ
C 102.3–104.7, indicating the presence of a pentasaccharide chain and
β-configurations of glycosidic bonds. The extensive analysis of the
1H,
1H-COSY, 1D TOCSY, HSQC, ROESY and HMBC spectra (
Table 4,
Figures S17–S23) of
3 indicated the same monosaccharide composition, positions of glycosidic linkages, and architecture established for the glycosides
1,
2. The differences in the chemical shifts of carbon signals of chilensosides A (
1) and B (
3) were attributed to the diverse positions of sulfate groups. The signal of C-4 Glc4 in the
13C NMR spectrum of
3 was shielded to δ
C 68.9 instead of δ
C 75.6 in
1 due to the absence of a sulfate group in this position of
3. Additionally, the signal of C-3 Glc4 was deshielded to 85.9 due to the glycosylation effect and the absence of the
β-shifting effect of sulfate group. The signal of C-6 Glc4 at δ
C 67.2 was characteristic for the sulfated hydroxy methylene group of the glucopyranose unit. Therefore, the glucose residue attached to C-4 Xyl1 of the carbohydrate chain of
3 bears one sulfate group at C-6. The comparison of the signals assigned to carbons of the 3-
O-methylglucose unit of the compounds
3 (
Table 4) and
1 (
Table 2) showed that the signal of C-4 MeGlc5 of
3 was deshielded by 6.1 ppm (to δ
C 76.1) and the signals of C-3 and C-5 MeGlc5 were shielded by 1.7 and 1.0 ppm, corresponding to the shifting effects of the sulfate group attached to C-4 MeGlc5 of chilensoside B (
3). Thus, the glycoside
3 is a new disulfated pentaoside having sulfate groups at C-6 Glc4 and C-4 MeGlc5. The compound with identical positions of sulfates but differing in the terminal xylose residue in the bottom semi-chain was chitonoidoside H, found recently in the sea cucumber
Psolus chitonoides [
6].
The (
−)ESI-MS/MS of
3 (
Figure S24) demonstrated the fragmentation of [M
2Na−Na]
− ion at
m/z 1427.5, resulting in the ion peaks appearance at
m/z 1307.5 [M
2Na−Na−NaHSO
4]
−, 1149.5 [M
2Na−Na−MeGlcOSO
3Na+H]
−, 987.4 [M
2Na−Na−MeGlcOSO
3Na−Glc+H]
−, 841.4 [M
2Na−Na−MeGlcOSO
3Na−Glc−Qui+H]
−, 667.1 [M
2Na−Na−Agl−Glc−Qui−H]
−. The fragmentation of [M
2Na−2Na]
2− ion at
m/z 702.2 led to the ion peak at
m/z 621.7 [M
2Na−2Na−Glc]
2−, and 548.2 [M
2Na−2Na−Glc−Qui]
2−, confirming the structure of
3.
These data indicate that chilensoside B (3) is 3β-O-{β-D-glucopyranosyl-(1 → 4)-β-D-quinovopyranosyl-(1 → 2)-[4-O-sodium sulfate-3-O-methyl-β-D-glucopyranosyl-(1 → 3)-6-O-sodium sulfate-β-D-glucopyranosyl-(1 → 4)]-β-D-xylopyranosyl}-16-oxoholosta-9(11),24(25)-diene.
The molecular formula of chilensoside C (
4) was determined to be C
60H
91O
36S
3Na
3 from the [M
3Na–Na]
− ion peak at
m/z 1529.4300 (calc. 1529.4253), [M
3Na–2Na]
2− ion peak at
m/z 753.2206 (calc. 753.2180) and [M
3Na–3Na]
3− ion peak at
m/z 494.4839 (calc. 494.4823) in the (
−)HR-ESI-MS (
Figure S32).
The
1H and
13C NMR spectra of the carbohydrate chain of chilensoside С (
4) (
Table 5,
Figures S25–S31) demonstrated five characteristic doublets of anomeric protons at δ
H 4.65–5.21 (
J = 6.5–8.5 Hz) and five signals of anomeric carbons at δ
C 102.4–104.7, indicating the presence of a pentasaccharide chain and
β-configurations of glycosidic bonds.
The extensive analysis of the 1H, 1H-COSY, 1D TOCSY, HSQC, ROESY, and HMBC spectra of 4 indicated the same monosaccharide composition, glycosidic bond locations and architecture of carbohydrate chains as in the previously discussed glycosides 1−3. Differences were found in the quantity of sulfate groups, which was also confirmed by MS data, where three-charged ions were registered, indicating the presence of three sulfate groups.
The comparison of the
13C NMR spectra of sugar moieties of
4 and
1 showed the coincidence of all the signals except the signals of glucose residue in the bottom semi-chain. The signal of C-3 Glc3 was deshielded to δ
C 84.3 in the spectrum of
4, which could be explained by the
α-shifting effect of the sulfate group as well as by the glycosylation effect. However, the latter was excluded due to the absence of the ROE- and HMBC correlations of H-3 Glc3 with any protons or carbons of neighboring monosaccharide residues (
Table 5). Moreover, the signals of C-2 Glc3 and C-4 Glc3 in the spectrum of
4 were shielded to δ
C 73.1 and 69.8, respectively, in comparison with the corresponding signals in the spectrum of
1 due to
β-shifting effect of sulfate group at C-3 Glc3. Therefore, the third sulfate group in chilensoside C (
4) was unique for the glycosides position at C-3 Glc3 instead of the characteristic glycosidic bond position in the glycosides with normal (consisting of three monosaccharide units) bottom semi-chain. Such a location of the sulfate group makes further elongation of the carbohydrate chain of
4 impossible. The rest of the sulfate groups were attached to C-4 Glc4 and C-6 Glc4 in chilensoside C (
4), by the same manner as in chilensosides A (
1), and A
1 (
2).
The (
−)ESI-MS/MS of
4 (
Figure S32) demonstrated the fragmentation of [M
3Na−Na]
− ion at
m/z 1529.5, which resulted in the ion peaks at
m/z 1015.4 [M
3Na−Na−GlcOSO
3Na−Qui−SO
3Na]
−, 987.4 [M
3Na−Na−MeGlc−Glc(OSO
3Na)
2]
−, 605.2 [M
3Na−Na−MeGlc−NaHSO
4]
−. The fragmentation of [M
3Na−2Na]
2− ion at
m/z 753.2 led to the presence of the ion peaks at
m/z 702.2 [M
3Na−2Na−SO
3Na]
2−, 605.2 [M
3Na−2Na−MeGlc−NaHSO
4]
2−.
These data indicate that chilensoside C (4) is 3β-O-{3-O-sodium sulfate-β-D-glucopyranosyl-(1 → 4)-β-D-quinovopyranosyl-(1 → 2)-[3-O-methyl-β-D-glucopyranosyl-(1 → 3)-4,6-O-sodium disulfate-β-D-glucopyranosyl-(1 → 4)]-β-D-xylopyranosyl}-16-oxoholosta-9(11),24(25)-diene.
The molecular formula of chilensoside D (
5) was determined to be C
60H
90O
39S
4Na
4 from the [M
4Na–Na]
− ion peak at
m/z 1631.3667 (calc. 1631.3641), [M
4Na–2Na]
2− ion peak at
m/z 804.1886 (calc. 804.1874), [M
4Na–3Na]
3− ion peak at
m/z 528.4631 (calc. 528.4619) and [M
4Na–4Na]
4− ion peak at
m/z 390.6005 (calc. 390.5991) in the (−) HR-ESI-MS (
Figure S40). Chilensoside D (
5), analogously to compounds
1–
4, has a pentasaccharide branched by C-4 Xyl1 chain consisting of xylose, quinovose, two glucose and 3-
O-methylglucose residues deduced from thorough analysis of its 1D and 2D NMR spectra (
Table 6,
Figures S33–S39). The availability of four-charged ion peaks in the ESI-MS spectra of
5 indicated four sulfate groups are present in its carbohydrate chain. The analysis of 1D TOCSY spectrum corresponding to Glc3 showed strongly deshielded signals of protons of the hydroxy methylene group at δ
H 4.61 (m) and 5.00 (d,
J = 11.9 Hz), which were assigned to the corresponding carbon signal at δ
C 67.6. These data indicate that the glucose residue in the bottom semi-chain was sulfated by C-6. The glucose unit (Glc4) attached to C-4 Xyl1 in chilensoside D (
5) had two sulfate groups at C-4 Glc4 and C-6 Glc4, deduced from the deshielding of its signals to δ
C 75.1 and 68.5, respectively. The fourth sulfate group was positioned at C-6 MeGlc5 because of the deshielding of the signals of hydroxy methylene group to δ
C 67.0 and δ
H 4.99 (brd,
J = 11.9 Hz); 4.78 (dd,
J = 5.1; 11.9 Hz). Therefore, chilensoside D (
5) is a new, sixth tetrasulfated glycoside found in sea cucumbers [
7,
44].
The (
−) ESI-MS/MS of chilensoside D (
5) (
Figure S40) demonstrated the fragmentation of [M
4Na−Na]
− ion at
m/z 1631.5, leading to the presence of the ion peak at
m/ 987.4 [M
4Na−Na−MeGlcOSO
3Na−Glc(OSO
3Na)
2]
−, of [M
4Na−2Na]
2− ion at
m/z 804.2 leading to the ion peak at
m/z 753.2 [M
4Na−2Na−SO
3Na+H]
2−, and of [M
4Na−3Na]
3− ion at
m/z 528.5 leading to the ion peak at
m/z 494.3 [M
4Na−3Na−SO
3Na+H]
3−.
These data indicate that chilensoside D (5) is 3β-O-{6-O-sodium sulfate-β-D-glucopyranosyl-(1 → 4)-β-D-quinovopyranosyl-(1 → 2)-[6-O-sodium sulfate-3-O-methyl-β-D-glucopyranosyl-(1 → 3)-4,6-O-sodium disulfate-β-D-glucopyranosyl-(1 → 4)]-β-D-xylopyranosyl}-16-oxoholosta-9(11),24(25)-diene.
The structural peculiarities of the glycosides of
P. chilensis showed similarity to the compounds of the representatives of the order Dendrochirotida, i.e., sea cucumbers of the species
Thyonidium kurilensis and
Psolus chitonoides (the same architecture of the carbohydrate chains),
Psolus fabricii (attachment of sulfates to C-4 Glc4 and C-6 Glc4) and
Cladolabes schmeltzii (the same aglycones). All these data significantly support the phylogenetic closeness of the order Molpadida to the order Dendrochirotida, rather than to the order Aspidochirotida (in accordance with the system of Pawson and Fell). This order is absent in the last revision of the system of the class Holothuroidea, and the families, which were part of it, are now included in the orders Holothuriida, Persiculida and Synallactida [
46]. The obtained structural data are in good agreement with the phylogenetic study of Holothuroidea using a multi-gene approach, which showed poor support of Molpadida as a sister group to Synallactida but demonstrated the close relationship of Molpadida to Dendrochirotida [
46].