Biological Activity and Chemical Composition of Propolis from Various Regions of Poland
Abstract
1. Introduction
2. Results and Discussion
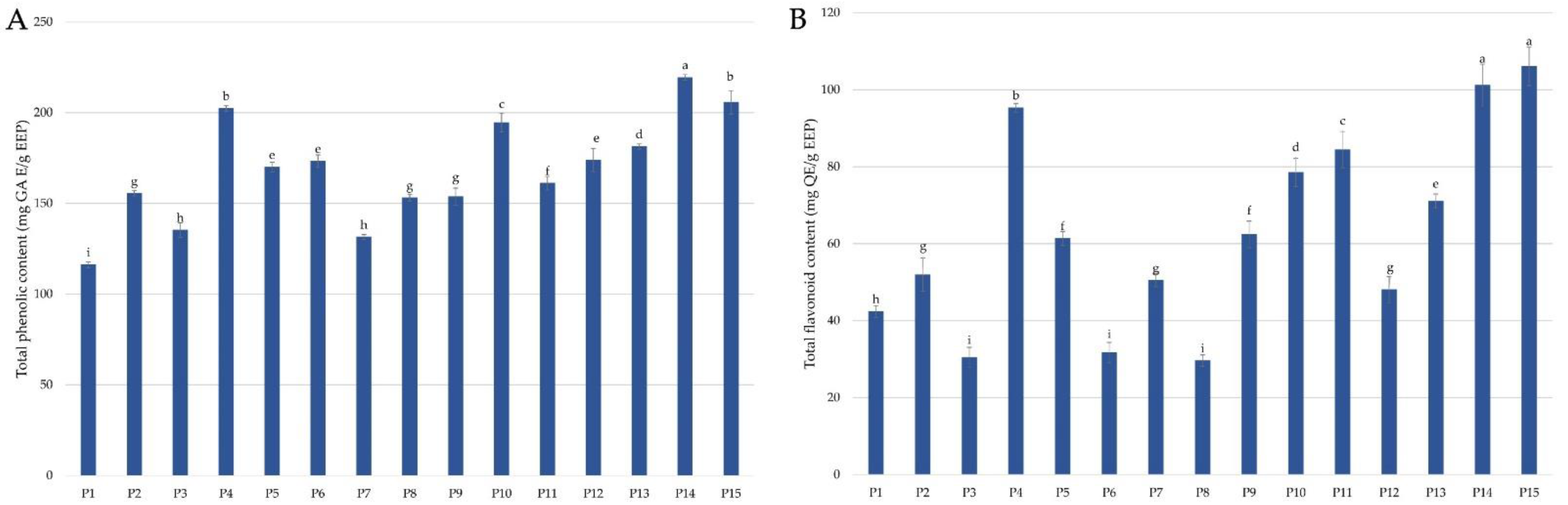
2.1. The Concentration of Phenolic Compounds
2.1.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.1.2. Profile of Phenolic Compounds
2.2. Biological Activity of Propolis Extracts
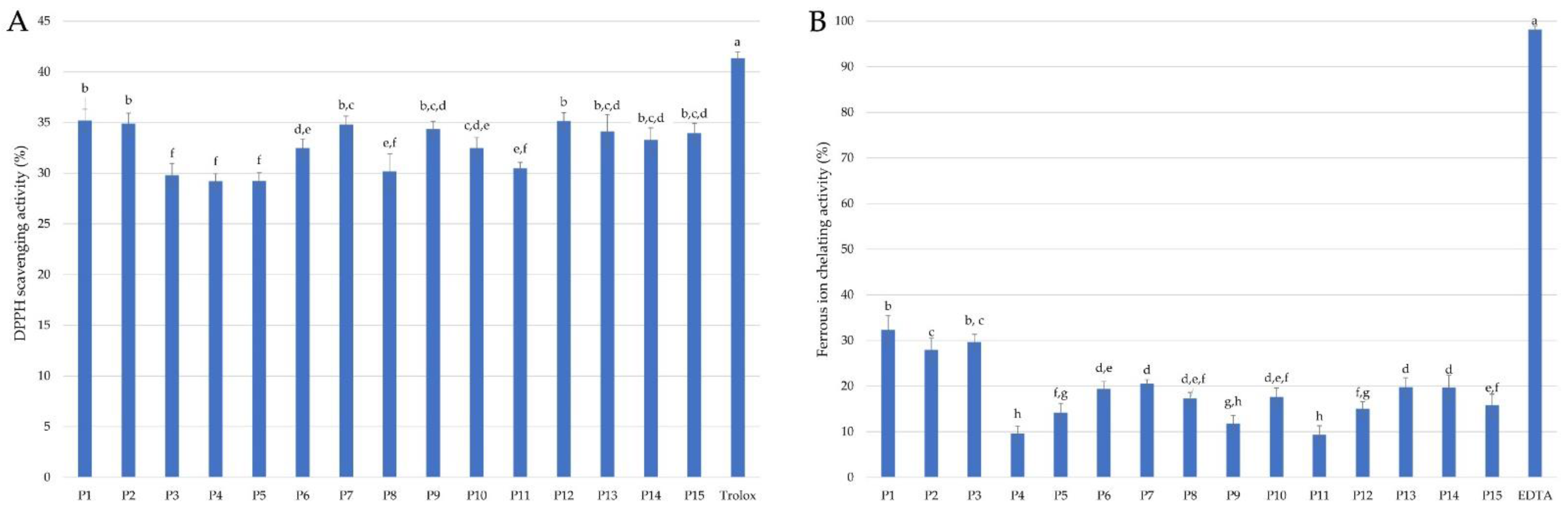
2.2.1. Antioxidant Activity
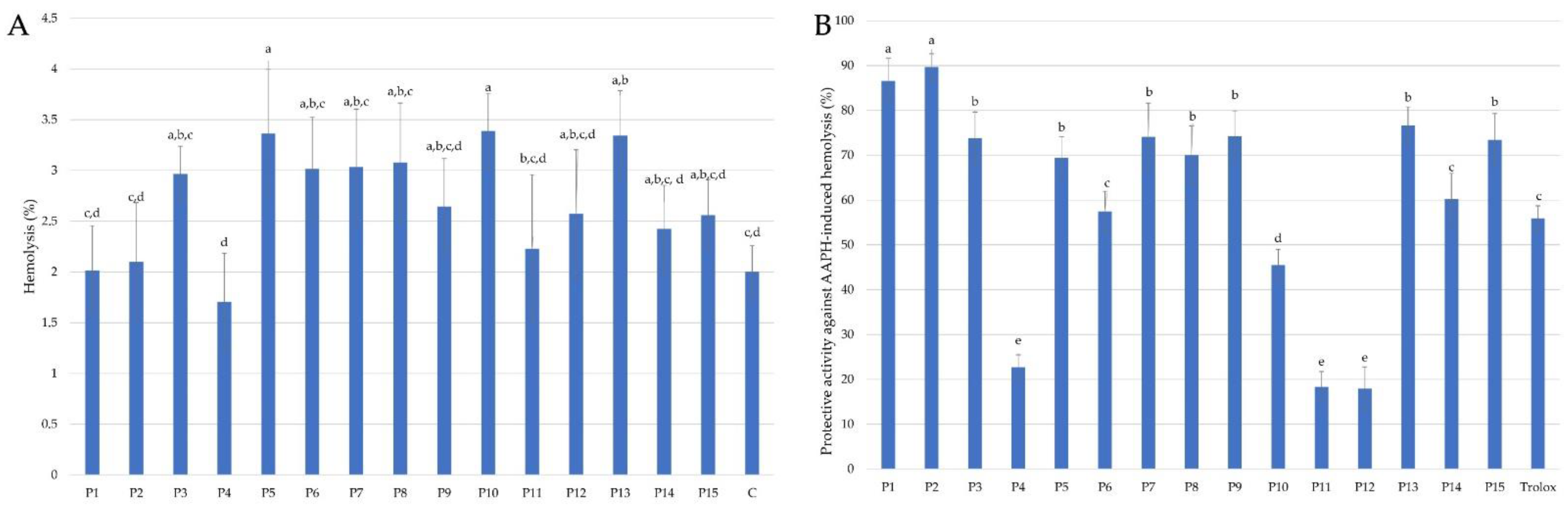
2.2.2. Effect of Propolis Extracts on Human Red Blood Cells (RBCs) Membrane under In Vitro Physiological and Oxidative Stress Conditions
2.2.3. Antibacterial and Antifungal Activity
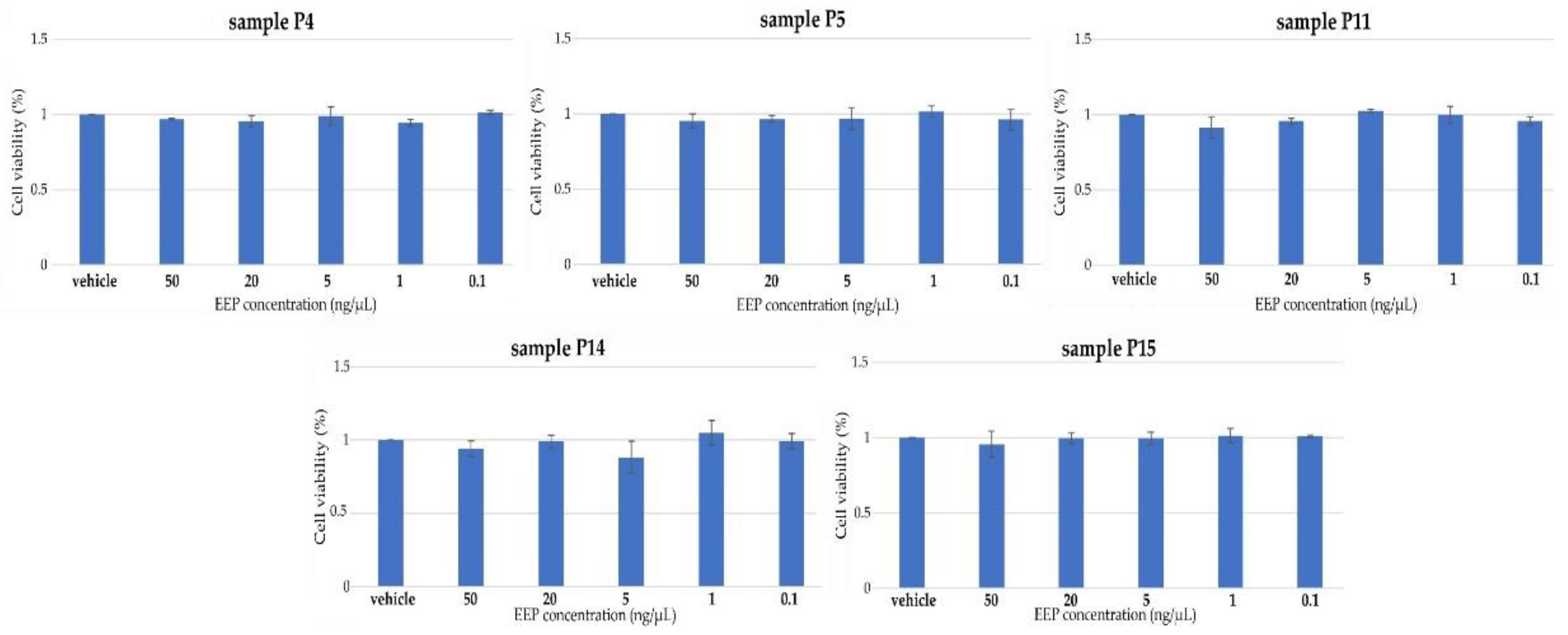
2.2.4. Antiviral Activity
3. Materials and Methods
3.1. Propolis Samples and Preparation of Propolis Extracts
3.2. Determination of Total Phenolic Content (TPC)
3.3. Determination of Total Flavonoid Content (TFC)
3.4. Phenolic Profile of Propolis Extracts
3.5. Antioxidant Activity of Propolis Extracts
3.6. Hemolytic Activity of Propolis Extracts
3.7. Inhibition of Oxidative Stress-Induced Hemolysis of Human RBC by Propolis Extracts
3.8. Antibacterial and Antifungal Activity of Propolis Extracts
3.9. Antiviral Activity of Propolis Extracts
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Popova, M.; Giannopoulou, E.; Skalicka-Wózniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bȩben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- Okinczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Zbikowska, B.; Krzyzanowska, B.; Sroka, Z. Profile of polyphenolic and essential oil composition of polish propolis, black poplar and aspens buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chinese Med. 2022, 17, 1–60. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products. Foods 2018, 7, 41. [Google Scholar] [CrossRef]
- dos Santos, H.F.; Campos, J.F.; dos Santos, C.M.; Balestieri, J.B.P.; Silva, D.B.; Carollo, C.A.; Souza, K. de P.; Estevinho, L.M.; dos Santos, E.L. Chemical profile and antioxidant, anti-inflammatory, antimutagenic and antimicrobial activities of geopropolis from the stingless bee Melipona orbignyi. Int. J. Mol. Sci. 2017, 18, 953. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Inmaculada González-Martín, M.; Escuredo, O.; Revilla, I.; Vivar-Quintana, A.M.; Carmen Coello, M.; Riocerezo, C.P.; Moncada, G.W. Determination of the mineral composition and toxic element contents of propolis by near infrared spectroscopy. Sensors 2015, 15, 27854–27868. [Google Scholar] [CrossRef]
- Godhi, B.S.; Beeraka, N.M.; Buddi, J.S.H.P.; Mahadevaiah, S.; Madhunapantula, S.V. Updates in the Analytical Isolation of Indian Propolis Chemical Constituents and Their Role in Dental Pharmacology—A Review. Nat. Prod. J. 2022, 11, 77–78. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, O. Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants 2020, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef]
- Pang, Z.; Chen, J.; Wang, T.; Gao, C.; Li, Z.; Guo, L.; Xu, J.; Cheng, Y. Linking Plant Secondary Metabolites and Plant Microbiomes: A Review. Front. Plant Sci. 2021, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.; Alenzi, N.; Alenazi, F.; Tabassum, H.; Watson, D. Chemical characterization of Saudi propolis and its antiparasitic and anticancer properties. Sci. Rep. 2021, 11, 5390. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid GC/MS determination of botanical precursors of Eurasian propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, D.; Papotti, G.; Bortolotti, L.; Marcazzan, G.L.; Plessi, M. 1H-NMR simultaneous identification of health-relevant compounds in propolis extracts. Phytochem. Anal. 2012, 23, 260–266. [Google Scholar] [CrossRef]
- Kasote, D.M.; Pawar, M.V.; Bhatia, R.S.; Nandre, V.S.; Gundu, S.S.; Jagtap, S.D.; Kulkarni, M.V. HPLC, NMR based chemical profiling and biological characterisation of Indian propolis. Fitoterapia 2017, 122, 52–60. [Google Scholar] [CrossRef]
- Lv, L.; Cui, H.; Ma, Z.; Liu, X.; Yang, L. Recent progresses in the pharmacological activities of caffeic acid phenethyl ester. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1327–1339. [Google Scholar] [CrossRef]
- Krol, W.; Szliszka, E.; Czuba, Z.P.; Bronikowska, J.; Mertas, A.; Paradysz, A. Ethanolic extract of propolis augments TRAIL-induced apoptotic death in prostate cancer cells. Evid.-Based Complement. Altern. Med. 2011, 2011, 535172. [Google Scholar] [CrossRef]
- Tolba, M.F.; Azab, S.S.; Khalifa, A.E.; Abdel-Rahman, S.Z.; Abdel-Naim, A.B. Caffeic acid phenethyl ester, a promising component of propolis with a plethora of biological activities: A review on its anti-inflammatory, neuroprotective, hepatoprotective, and cardioprotective effects. IUBMB Life 2013, 65, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and functional characterization of Italian propolis obtained by different harvesting methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.M.; Marcos, P.; Azofra, P.; de Pablo, A.; Fernández-Muíño, M.Á.; Sancho, M.T. Phenolic Profile, Antioxidant Capacities and Enzymatic Inhibitory Activities of Propolis from Different Geographical Areas: Needs for Analytical Harmonization. Antioxidants 2020, 9, 75. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Sokół-Łętowska, A.; Kucharska, A.Z.; Jaworska, D.; Czuba, Z.P.; Król, W. Ethanolic extract of polish propolis: Chemical composition and TRAIL-R2 death receptor targeting apoptotic activity against prostate cancer cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 757628. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Nowak, P.; Ratajczak, I. Effect of the solvent on propolis phenolic profile and its antifungal, antioxidant, and in vitro cytoprotective activity in human erythrocytes under oxidative stress. Molecules 2020, 25, 4266. [Google Scholar] [CrossRef]
- Markiewicz-Zukowska, R.; Car, H.; Naliwajko, S.K.; Sawicka, D.; Szynaka, B.; Chyczewski, L.; Isidorov, V.; Borawska, M.H. Ethanolic extract of propolis, chrysin, CAPE inhibit human astroglia cells. Adv. Med. Sci. 2012, 57, 208–216. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Żubernik, J.; Witrowa-Rajchert, D.; Gniewosz, M. Growth biocontrol of foodborne pathogens and spoilage microorganisms of food by Polish propolis extracts. Molecules 2019, 24, 2965. [Google Scholar] [CrossRef]
- Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Bielec, B.; Wojtyczka, R.D.; Bułdak, R.J.; Wyszyńska, M.; Stawiarska-Pięta, B.; Szaflarska-Stojko, E. The Ethanol Extract of Polish Propolis Exhibits Anti-Proliferative and/or Pro-Apoptotic Effect on HCT 116 Colon Cancer and Me45 Malignant Melanoma Cells In Vitro Conditions. Adv. Clin. Exp. Med. 2015, 24, 203–212. [Google Scholar] [CrossRef]
- Dudoit, A.; Mertz, C.; Chillet, M.; Cardinault, N.; Brat, P. Antifungal activity of Brazilian red propolis extract and isolation of bioactive fractions by thin-layer chromatography-bioautography. Food Chem. 2020, 327, 127060. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Ye, S.R.; Ting, C.; Yu, Y.H. Antibacterial activity of propolins from Taiwanese green propolis. J. Food Drug Anal. 2018, 26, 761–768. [Google Scholar] [CrossRef]
- Franchin, M.; Freires, I.A.; Lazarini, J.G.; Nani, B.D.; da Cunha, M.G.; Colón, D.F.; de Alencar, S.M.; Rosalen, P.L. The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur. J. Med. Chem. 2018, 153, 49–55. [Google Scholar] [CrossRef]
- Belmehdi, O.; El Menyiy, N.; Bouyahya, A.; El Baaboua, A.; El Omari, N.; Gallo, M.; Montesano, D.; Naviglio, D.; Zengin, G.; Skali Senhaji, N.; et al. Recent Advances in the Chemical Composition and Biological Activities of Propolis. Food Rev. Int. 2022, 1–51. [Google Scholar] [CrossRef]
- Magnavacca, A.; Sangiovanni, E.; Racagni, G.; Dell’Agli, M. The antiviral and immunomodulatory activities of propolis: An update and future perspectives for respiratory diseases. Med. Res. Rev. 2022, 42, 897–945. [Google Scholar] [CrossRef] [PubMed]
- Yosri, N.; El-Wahed, A.A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-Viral and Immunomodulatory Properties of Propolis: Chemical Diversity, Pharmacological Properties, Preclinical and Clinical Applications, and In Silico Potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.P.; Amorim, J.M.; Lima, W.G.; Brito, J.C.M.; da Cruz Nizer, W.S. Effect of honey and propolis, compared to acyclovir, against Herpes Simplex Virus (HSV)-induced lesions: A systematic review and meta-analysis. J. Ethnopharmacol. 2022, 287, 114939. [Google Scholar] [CrossRef]
- da Silva, C.C.F.; Salatino, A.; da Motta, L.B.; Negri, G.; Salatino, M.L.F. Chemical characterization, antioxidant and anti-HIV activities of a Brazilian propolis from Ceará state. Rev. Bras. Farmacogn. 2019, 29, 309–318. [Google Scholar] [CrossRef]
- Wieczyńska, A.; Wezgowiec, J.; Wiȩckiewicz, W.; Czarny, A.; Kulbacka, J.; Nowakowska, D.; Gancarz, R.; Wilk, K.A. Antimicrobial activity, cytotoxicity and total phenolic content of different extracts of propolis from the west pomeranian region in Poland. Acta Pol. Pharm.-Drug Res. 2017, 74, 715–722. [Google Scholar]
- Szliszka, E.; Krol, W. Polyphenols isolated from propolis augment TRAIL-induced apoptosis in cancer cells. Evid.-Based Complement. Altern. Med. 2013, 2013, 731940. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. The role of seasonality on the chemical composition, antioxidant activity and cytotoxicity of Polish propolis in human erythrocytes. Brazilian J. Pharmacogn. 2019, 29, 301–308. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. Phenolic profile and antioxidant activity of propolis extracts from Poland. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Wojtyczka, R.D.; Kabała-Dzik, A.; Tanasiewicz, M.; Morawiec, T. The antibacterial effect of ethanol extract of Polish propolis on mutans Streptococci and Lactobacilli isolated from saliva. Evid.-Based Complement. Altern. Med. 2013, 2013, 681891. [Google Scholar] [CrossRef]
- Regueira Neto, M.S.; Tintino, S.R.; da Silva, A.R.P.; Costa, M. do S.; Boligon, A.A.; Matias, E.F.F.; de Queiroz Balbino, V.; Menezes, I.R.A.; Melo Coutinho, H.D. Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect and phytochemical screening. Food Chem. Toxicol. 2017, 107, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Wezgowiec, J.; Wieczyńska, A.; Wieckiewicz, W.; Kulbacka, J.; Saczko, J.; Pachura, N.; Wieckiewicz, M.; Gancarz, R. Polish propolis—Chemical composition and biological effects in tongue cancer cells and macrophages. Molecules 2020, 25, 2426. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial activities of European propolis collected from various geographic origins alone and in combination with antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef]
- Wang, X.; Sankarapandian, K.; Cheng, Y.; Woo, S.O.; Kwon, H.W.; Perumalsamy, H.; Ahn, Y.J. Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea. BMC Complement. Altern. Med. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Touzani, S.; Imtara, H.; Katekhaye, S.; Mechchate, H.; Ouassou, H.; Alqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Fearnley, H.; Fearnley, J.; et al. Determination of Phenolic Compounds in Various Propolis Samples Collected from an African and an Asian Region and Their Impact on Antioxidant and Antibacterial Activities. Molecules 2021, 26, 4589. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Sci. Technol. 2014, 34, 16–23. [Google Scholar] [CrossRef]
- Kumazawa, S.; Bonvehí, J.S.; Torres, C.; Mok-Ryeon, A.; Bermejo, F.J.O. Chemical and functional characterisation of propolis collected from East Andalusia (Southern Spain). Phytochem. Anal. 2013, 24, 608–615. [Google Scholar] [CrossRef]
- Silva, J.C.; Rodrigues, S.; Feás, X.; Estevinho, L.M. Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food Chem. Toxicol. 2012, 50, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.R.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Kis, B.; Avram, S.; Pavel, I.Z.; Lombrea, A.; Buda, V.; Dehelean, C.A.; Soica, C.; Yerer, M.B.; Bojin, F.; Folescu, R.; et al. Recent Advances Regarding the Phytochemical and Therapeutic Uses of Populus nigra L. Buds. Plants 2020, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Isidorov, V.A.; Bakier, S.; Pirożnikow, E.; Zambrzycka, M.; Swiecicka, I. Selective Behaviour of Honeybees in Acquiring European Propolis Plant Precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef]
- Stanciauskaite, M.; Marksa, M.; Liaudanskas, M.; Ivanauskas, L.; Ivaskiene, M.; Ramanauskiene, K. Extracts of Poplar Buds (Populus balsamifera L., Populus nigra L.) and Lithuanian Propolis: Comparison of Their Composition and Biological Activities. Plants 2021, 10, 828. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Konteles, S.J.; Troullidou, E.; Mourtzinos, I.; Karathanos, V.T. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009, 116, 452–461. [Google Scholar] [CrossRef]
- Barbarić, M.; Mišković, K.; Bojić, M.; Lončar, M.B.; Smolčić-Bubalo, A.; Debeljak, Ž.; Medić-Šarić, M. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J. Ethnopharmacol. 2011, 135, 772–778. [Google Scholar] [CrossRef]
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS methods for metabolite profiling of propolis extracts. J. Pharm. Biomed. Anal. 2011, 55, 934–948. [Google Scholar] [CrossRef]
- Medana, C.; Carbone, F.; Aigotti, R.; Appendino, G.; Baiocchi, C. Selective analysis of phenolic compounds in propolis by HPLC-MS/MS. Phytochem. Anal. 2008, 19, 32–39. [Google Scholar] [CrossRef]
- Volpi, N.; Bergonzini, G. Analysis of flavonoids from propolis by on-line HPLC-electrospray mass spectrometry. J. Pharm. Biomed. Anal. 2006, 42, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, L.L.; Nazareno, M.A. Chemical variability in propolis from Santiago del Estero, Argentina, related to the arboreal environment as the sources of resins. J. Sci. Food Agric. 2009, 89, 978–983. [Google Scholar] [CrossRef]
- Bonamigo, T.; Campos, J.F.; Oliveira, A.S.; Torquato, H.F.V.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; Souza, K. de P.; Dos Santos, E.L. Antioxidant and cytotoxic activity of propolis of Plebeia droryana and Apis mellifera (Hymenoptera, Apidae) from the Brazilian Cerrado biome. PLoS ONE 2017, 12, e0183983. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evid.-Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I.; Bursal, E.; Şehitoĝlu, M.H.; Bilsel, M.; Gören, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Mrówczyńska, L.; Hägerstrand, H. Platelet-activating factor interaction with the human erythrocyte membrane. J. Biochem. Mol. Toxicol. 2009, 23, 345–348. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. Microbiological and calorimetric investigations on the antimicrobial actions of different propolis extracts: An in vitro approach. Thermochim. Acta 2004, 422, 115–124. [Google Scholar] [CrossRef]
- Silici, S.; Ünlü, M.; Vardar-Ünlü, G. Antibacterial activity and phytochemical evidence for the plant origin of Turkish propolis from different regions. World J. Microbiol. Biotechnol. 2007, 23, 1797–1803. [Google Scholar] [CrossRef]
- Agüero, M.B.; Svetaz, L.; Baroni, V.; Lima, B.; Luna, L.; Zacchino, S.; Saavedra, P.; Wunderlin, D.; Feresin, G.E.; Tapia, A. Urban propolis from San Juan province (Argentina): Ethnopharmacological uses and antifungal activity against Candida and dermatophytes. Ind. Crop. Prod. 2014, 57, 166–173. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kepa, M.; Kubina, R.; Kabała-Dzik, A.; Smoleń-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wasik, T.J. Susceptibility of Staphylococcus aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.; Kosti´ckosti´c, A.Ž.; Luo, L.; Wang, K.; Tasmia Asma, S.; Bobi¸sbobi¸s, O.; Victori¸ta Bonta, V.; Acaroz, U.; Rizwan, S.; Shah, A.; et al. General Nutritional Profile of Bee Products and Their Potential Antiviral Properties against Mammalian Viruses. Nutrients 2022, 14, 3579. [Google Scholar] [CrossRef]
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Reyes-Reali, J.; Mendoza-Ramos, M.I.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Effects of Propolis on Infectious Diseases of Medical Relevance. Biology 2021, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R. Analysis of total phenols and other oxidatioon substrates and antioxidant substrates and antioxidants by means oof Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Buck, C.B.; Pastrana, D.V.; Lowy, D.R.; Schiller, J.T. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 2005, 119, 445–462. [Google Scholar] [CrossRef]
- Broniarczyk, J.; Bergant, M.; Goździcka-Józefiak, A.; Banks, L. Human papillomavirus infection requires the TSG101 component of the ESCRT machinery. Virology 2014, 460, 83–90. [Google Scholar] [CrossRef]
- Bugnon Valdano, M.; Massimi, P.; Broniarczyk, J.; Pim, D.; Myers, M.; Gardiol, D.; Banks, L. Human Papillomavirus Infection Requires the CCT Chaperonin Complex. J. Virol. 2021, 95, e01943-20. [Google Scholar] [CrossRef]

| Concentration (mg/g EEP) | Propolis Samples | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | |
| Apigenin | nd | nd | 0.328 c ± 0.019 | nd | 1.194 a ± 0.073 | 0.145 d ± 0.010 | nd | nd | nd | 0.106 d,e ± 0.013 | 0.386 c ± 0.022 | 0.123 d,e ± 0.011 | 0.056 e,f ± 0.008 | nd | 0.477 b ± 0.033 |
| Catechin | 0.214 h ± 0.011 | nd | nd | nd | Nd | 0.029 h ± 0.002 | 3.049 e ± 0.085 | 5.492 b ± 0.031 | 4.313 c ± 0.037 | 2.986 e ± 0.138 | 3.052 e ± 0.156 | 3.751 d ± 0.154 | 6.262 a ± 0.170 | 1.479 g ± 0.130 | 2.077 f ± 0.103 |
| Chrysin | nd | nd | nd | nd | Nd | nd | 0.047 ± 0.004 | nd | 0.060 ± 0.008 | nd | nd | 0.179 ± 0.018 | nd | nd | nd |
| Epicatechin | nd | 66.008 a ± 2.928 | nd | 32.574 c ± 3.010 | 23.489 d ± 1.820 | 42.262 b ± 3.130 | 2.999 e,f ± 0.140 | nd | nd | nd | nd | nd | 6.578 e ± 0.191 | nd | nd |
| Galangin | nd | nd | 0.360 c ± 0.034 | nd | 0.034 f ± 0.004 | nd | 0.550 a ± 0.034 | 0.111 e ± 0.012 | 0.206 d ± 0.016 | 0.115 e ± 0.012 | nd | 0.093 e ± 0.007 | nd | 0.135 e ± 0.012 | 0.475 b ± 0.021 |
| Kaempferol | nd | nd | 0.273 d ± 0.025 | nd | 0.282 d ± 0.027 | nd | nd | 0.317 c,d ± 0.019 | nd | 0.075 e ± 0.004 | 0.468 b ± 0.032 | 0.258 d ± 0.014 | 0.286 d ± 0.015 | 0.389 c ± 0.019 | 0.840 a ± 0.048 |
| Myricetin | 0.192 h ± 0.012 | 6.735 a ± 0.371 | 1.363 f,g ± 0.092 | 5.223 b ± 0.224 | 1.964 d,e ± 0.103 | 3.302 c ± 0.238 | 3.868 c ± 0.102 | nd | 3.293 c ± 0.150 | 1.562 e,f ± 0.125 | 0.972 g ± 0.092 | 0.349 h ± 0.032 | 2.339 d ± 0.215 | nd | nd |
| Naringenin | nd | nd | 0.561 d ± 0.033 | 0.769 b ± 0.036 | 0.955 a ± 0.084 | nd | 0.642 c ± 0.038 | nd | 0.574 c,d ± 0.031 | 0.331 e ± 0.035 | 0.669 b,c ± 0.043 | 0.058 f,g ± 0.008 | nd | 0.130 f ± 0.004 | nd |
| Pinobanksin | 1.983 d ± 0.182 | 5.608 b ± 0.352 | nd | nd | 6.995 b ± 0.336 | 0.362 e ± 0.033 | 0.286 e ± 0.024 | 2.109 c,d ± 0.341 | 2.857 c,d ± 0.115 | 10.216 a ± 0.801 | 6.684 b ± 0.338 | 9.555 a ± 0.616 | 3.412 c ± 0.384 | 0.175 e ± 0.028 | 10.418 a ± 0.672 |
| Pinocembrin | nd | 0.253 b ± 0.026 | nd | nd | Nd | nd | nd | 0.493 a ± 0.029 | 0.022 f ± 0.002 | nd | nd | 0.137 d ± 0.010 | 0.085 e ± 0.007 | nd | 0.212 c ± 0.014 |
| Pinostrobin | nd | nd | 0.284 c ± 0.016 | 0.652 a ± 0.037 | Nd | nd | nd | nd | nd | 0.609 a,b ± 0.021 | 0.585 b ± 0.015 | nd | nd | 0.591 b ± 0.014 | nd |
| Quercetin | 0.729 a ± 0.037 | nd | 0.031 e,f ± 0.002 | nd | Nd | nd | nd | nd | 0.031 e,f ± 0.005 | 0.097 e ± 0.014 | 0.486 b ± 0.045 | 0.348 c ± 0.035 | 0.191 d ± 0.017 | 0.760 a ± 0.04 | 0.102 e ± 0.013 |
| Sum of flavonoids | 3.118 | 78.604 | 3.200 | 39.218 | 34.913 | 46.100 | 11.441 | 8.522 | 11.356 | 16.097 | 13.302 | 14.851 | 19.209 | 3.659 | 14.601 |
| Caffeic acid | nd | 0.048 a ± 0.003 | nd | nd | Nd | nd | nd | nd | nd | 0.003 b ± 0.001 | nd | nd | nd | nd | nd |
| Cinnamic acid | 0.029 d,e ± 0.003 | nd | nd | nd | 0.182 c ± 0.014 | 0.037 d ± 0.003 | nd | nd | nd | nd | nd | nd | 0.422 a ± 0.025 | 0.038 d ± 0.002 | 0.235 b ± 0.017 |
| Coumaric acid | 3.504 a ± 0.159 | nd | 0.102 b ± 0.007 | 0.024 b ± 0.003 | 0.020 b ± 0.002 | 0.078 b ± 0.003 | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 3-hydroxycinnamic | 4.963 a ± 0.188 | 0.331 b ± 0.028 | 0.015 c ± 0.002 | nd | Nd | nd | 0.004 c ± 0.001 | nd | nd | 0.022 c ± 0.002 | nd | nd | nd | nd | nd |
| Ferulic acid | 0.956 b ± 0.068 | nd | nd | 0.019 f ± 0.002 | Nd | nd | 0.135 e,f ± 0.010 | 0.021 f ± 0.002 | 0.169 d,e ± 0.024 | 0.344 c ± 0.033 | 0.077 e,f ± 0.008 | 0.123 e,f ± 0.011 | 0.159 d,e ± 0.022 | 1.848 a ± 0.115 | 0.280 c,d ± 0.018 |
| Syringic acid | 2.122 b ± 0.108 | 2.873 a ± 0.112 | 2.143 b ± 0.113 | 0.599 d ± 0.009 | 0.787 c ± 0.011 | 0.591 d ± 0.009 | 0.157 g,h ± 0.005 | 0.097 h ± 0.011 | 0.150 g,h ± 0.003 | 0.279 f,g,h ± 0.017 | 0.439 d,e,f ± 0.008 | 0.126 h± 0.006 | 0.099 h ± 0.002 | 0.318 e,f,g ± 0.026 | 0.477 d,e ± 0.020 |
| Sinapic acid | 0.157 b ± 0.004 | 0.073 c,d ± 0.003 | 0.034 c,d,e ± 0.004 | 0.031 d,e ± 0.001 | 0.103 b,c ± 0.005 | nd | nd | nd | nd | nd | nd | 0.014 d,e ± 0.002 | nd | nd | 2.909 a ± 0.074 |
| Vanillic acid | nd | 0.304 g,h ± 0.009 | 0.236 g,h ± 0.012 | 0.055 h ± 0.003 | Nd | 0.221 g,h ± 0.005 | nd | 5.812 a ± 0.137 | 4.264 b ± 0.180 | 3.583 c ± 0.194 | 1.306 f ± 0.204 | 2.003 e ± 0.133 | 2.808 d ± 0.196 | 1.446 f ± 0.040 | 0.567 g ± 0.010 |
| Sum of phenolic acid | 11.731 | 3.629 | 2.530 | 0.728 | 1.092 | 0.927 | 0.296 | 5.930 | 4.583 | 4.231 | 1.822 | 2.266 | 3.488 | 3.650 | 4.468 |
| CAPE | 0.983 a ± 0.053 | 0.878 a ± 0.049 | 0.198 d,e,f ± 0.023 | 0.211 d,e,f ± 0.017 | 0.554 b ± 0.026 | 0.848 a ± 0.046 | 0.035 e,f ± 0.004 | 0.310 c,d ± 0.027 | 0.228 d,e ± 0.014 | 0.979 a ± 0.097 | 0.467 b,c ± 0.022 | 0.213 d,e,f ± 0.025 | 0.319 c,d ± 0.026 | 1.014 a ± 0.120 | 0.056 e,f ± 0.013 |
| Microbial Strain | Zone of Inhibition (mm) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | |
| Listeria monocytogenes | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 12 | 12 | 10 | 10 | 12 | 10 | 10 | 10 |
| Staphylococcus aureus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bacillus cereus | 15 | 17 | 15 | 20 | 18 | 14 | 15 | 15 | 15 | 15 | 24 | 12 | 13 | 25 | 27 |
| Lacticaseibacillus paracesei | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Escherichia coli | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Salmonella Enteritidis | 10 | 10 | 10 | 10 | 10 | 10 | 12 | 11 | 12 | 13 | 15 | 14 | 11 | 15 | 14 |
| Pseudomonas aeruginosa | 13 | 14 | 14 | 15 | 14 | 10 | 14 | 13 | 13 | 15 | 14 | 12 | 13 | 12 | 14 |
| Klebsiella pneumonia | 13 | 13 | 13 | 14.5 | 13 | 10 | 13 | 13 | 12 | 13 | 14 | 13 | 12 | 11 | 15 |
| Saccharomyces cerevisiae | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 11 | 11 | 11 | 11 | 16 |
| Candida albicans | 0 | 0 | 0 | 18 | 15 | 0 | 10 | 10 | 12 | 14 | 12 | 11 | 12 | 15 | 15 |
| Symbol of Propolis Samples | City of Apiary Localization | Provinces | Extraction Yield (%) |
|---|---|---|---|
| P1 | Trójca | Lower Silesia | 71.13 |
| P2 | Dąbrowa Dolna | Lower Silesia | 76.81 |
| P3 | Stary Majdan | Lublin | 64.20 |
| P4 | Kutno | Lódźkie | 71.06 |
| P5 | Trzebinia | Lesser Poland | 53.91 |
| P6 | Cegłów | Masovia | 77.25 |
| P7 | Kozłowiec | Masovia | 44.03 |
| P8 | Sanok | Subcarpathia | 73.63 |
| P9 | Lewki | Podlaskie | 51.19 |
| P10 | Sierakowo | Pomerania | 60.98 |
| P11 | Kąpino | Pomerania | 77.47 |
| P12 | Sosnówka | Świętokrzyskie | 86.63 |
| P13 | Iława | Warmia-Masuria | 68.76 |
| P14 | Kąpiel | Greater Poland | 54.32 |
| P15 | Tychowo | West Pomerania | 44.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, M.; Sip, A.; Mrówczyńska, L.; Broniarczyk, J.; Waśkiewicz, A.; Ratajczak, I. Biological Activity and Chemical Composition of Propolis from Various Regions of Poland. Molecules 2023, 28, 141. https://doi.org/10.3390/molecules28010141
Woźniak M, Sip A, Mrówczyńska L, Broniarczyk J, Waśkiewicz A, Ratajczak I. Biological Activity and Chemical Composition of Propolis from Various Regions of Poland. Molecules. 2023; 28(1):141. https://doi.org/10.3390/molecules28010141
Chicago/Turabian StyleWoźniak, Magdalena, Anna Sip, Lucyna Mrówczyńska, Justyna Broniarczyk, Agnieszka Waśkiewicz, and Izabela Ratajczak. 2023. "Biological Activity and Chemical Composition of Propolis from Various Regions of Poland" Molecules 28, no. 1: 141. https://doi.org/10.3390/molecules28010141
APA StyleWoźniak, M., Sip, A., Mrówczyńska, L., Broniarczyk, J., Waśkiewicz, A., & Ratajczak, I. (2023). Biological Activity and Chemical Composition of Propolis from Various Regions of Poland. Molecules, 28(1), 141. https://doi.org/10.3390/molecules28010141