Abstract
Welsh onion (Allium fistulosum L.) is usually used to enhance the flavor characteristics of various foods. Volatile compounds in Welsh onions, including sulfur-containing compounds, may vary during heat process and storage. Accordingly, the changes in the volatile compounds in Welsh onions, subjected to heat and antioxidant (ascorbic acid and glutathione) treatments during storage, are investigated in the present study. The majority of sulfur-containing compounds in Welsh onions showed significant differences between the untreated Welsh onions and heated Welsh onions. During the heating of the Welsh onions, some sulfur-containing compounds, such as 2-methylthiirane, 1-(methyldisulfanyl)prop-1-ene, 1-[[(E)-prop-1-enyl]disulfanyl]propane, 1-(propyltrisulfanyl)propane, 1-[[(E)-prop-1-enyl]trisulfanyl]propane, and (methyltetrasulfanyl)methane, showed significant differences between the untreated and heated Welsh onions (p < 0.05). In addition, partial least square discriminant analysis (PLS-DA) was applied to discriminate the heated Welsh onion samples added with different antioxidants. The heated Welsh onion samples added with ascorbic acid was mainly associated with 2-phenylacetaldehyde, acetic acid, methylsulfanylmethane, prop-2-ene-1-thiol, undecan-2-one, and (2E,4E)-deca-2,4-dienal. Moreover, the key volatile compounds in the heated Welsh onion samples added with glutathione were 3-ethylthiophene, 1-(methyldisulfanyl)-1-methylsulfanylpropane, 1-methylsulfanylpentane, 2-prop-2-enylsulfanylpropane, and 1-propan-2-ylsulfanylbutane.
1. Introduction
Allium plants are widely used to improve flavor characteristics in several dishes, such as soups and stews, as ingredients and seasoning agents [1]. These Allium plants, such as Welsh onion, garlic, and onion, contain various volatile compounds, which can produce a unique taste and flavor [2]. In particular, sulfur-containing compounds in Welsh onions (Allium fistulosum L.), such as 1-(methyldisulfanyl)propane, (1E)-1-(methyldisulfanyl)-1-propene, (1E)-1-(propyldisulfanyl)-1-propene, and dipropyltrisulfane, can be produced from several precursors, including 3-[(1E)-1-propen-1-ylsulfinyl]-L-alanine (isoalliin), 3-[(S)-methylsulfinyl]-L-alanine (methiin), and 3-(allylsulfinyl)-L-alanine (alliin), through the enzymatic hydrolysis and non-enzymatic decomposition [3].
Some studies found that these sulfur-containing compounds in the Allium genus can be changed by processing methods, such as high-temperature heating, drying, roasting, crushing, and aging [2,3,4,5,6,7,8]. In addition, Allium sulfides can be produced by thermal decomposition during the heating process, and their structural changes can occur by thermal reactions [7]. Accordingly, organosulfur compounds in Welsh onions can be changed during processing and storage, leading to the chemical transformation of various volatile components that can significantly affect the flavor quality [9,10].
Several studies have been conducted to improve the shelf life and quality of Welsh onions [11]. The volatile compounds in Welsh onions were affected by oxidizing conditions, which are inevitable in food systems [12]. These oxidizing conditions can be caused by various factors, such as temperature, moisture, gas, and pH levels during processing and storage, which spontaneously create oxidizing agents [13]. Reactive oxygen species and radicals produced under oxidizing conditions react with them, and can then lead to the loss of their original properties, resulting in a deterioration in the quality of food systems [14]. Therefore, antioxidants can be used to maintain the quality properties of Welsh onions during storage.
Ascorbic acid, which prevents browning and improves the nutritional value, can be found in many food systems, either naturally or as an antioxidant additive [15]. In addition, ascorbic acid is an antioxidant that can be classified as a singlet oxygen or oxygen scavenger, and it can react with free radicals to remove it [16]. Glutathione, which is well known as an antioxidant, transforms itself into oxidized glutathione (glutathione disulfide, GSSG), which breaks the disulfide bond of the surrounding substances and reduces it to cysteine [17]. This oxidized glutathione (GSSG) undergoes a cycle that is reduced again by a reductase or chemical reaction [17]. As a non-enzymatic mechanism, glutathione turns into an oxidized form and prevents the oxidation of other substances, or forms disulfide by combining sulfur with itself to prevent oxidation [17].
Various studies have been conducted to investigate the changes in the volatile compounds in the Allium plant after the heat process [18,19]. However, to date, studies on the effects of antioxidants on the changes in the volatile compounds in heated Welsh onion have not been conducted. Therefore, the objectives of this study are to investigate the effects of heat treatment and the application of antioxidants on the changes in the volatile compounds in Welsh onions during storage.
2. Results and Discussion
2.1. The Changes in the Volatile Compounds in Welsh Onions by Heat Treatment
The volatile compounds in untreated and heat-treated Welsh onion samples were analyzed using gas chromatography-mass spectrometry (GC-MS) combined with solid-phase microextraction (SPME). A total of 81 identified volatile compounds in the untreated and heat-treated Welsh onion samples are listed in Table 1. Among these volatile compounds, sulfur-containing compounds, such as 2-methylthiirane (propylene sulfide); 1-(methyldisulfanyl)prop-1-ene; 1-[[I-prop-1-enyl]disulfanyl]propane (propI(E)-1-propenyl disulfide); 1-(propyltrisulfanyl)propane; 1-[[(E)-prop-1-enyl]trisulfanyl]propane; and (methyltetrasulfanyl)methane (dimethyl tetrasulfide), were significantly increased (p < 0.05) after the heat treatment.

Table 1.
Volatile compounds identified in the untreated and heated Welsh onion samples.
In addition, some sulfur-containing compounds, such as 5-methylthiophene-2-carbaldehyde, 1-(methyldisulfanyl)propan-2-one (methyl-2-oxo-propyl disulfide), and 1-(methyldisulfanyl)-1-methylsulfanylpropane (methyl 1-(methylthio)propyl disulfide), were only detected in the heated Welsh onion samples. In particular, the levels of 1-(methyldisulfanyl)prop-1-ene; 1-[[(E)-prop-1-enyl]disulfanyl]propane; 1-[[(E)-prop-1-enyl]trisulfanyl]propane; 1-(propyltrisulfanyl)propane; (methyltrisulfanyl)methane; (methyltetrasulfanyl)methane; and 2,5-dimethylthiophene were the most abundant in both the untreated and heated Welsh onion samples, and their levels significantly increased (p < 0.05) (except methyltrisulfanyl methane and 2,5-dimethylthiophene) after the heat treatment. It is known that a thermal reaction can affect the formation of sulfur-containing compounds in Welsh onions through their thermal decomposition and rearrangement [19]. Previous studies showed that sulfides are formed in Welsh onion samples when the Welsh onions are exposed to heat during distillation [19,20]. Block et al. [8] also demonstrated that bis(1-propenyl)disulfide, a common compound of Allium distilled oil, rearranged to thiophene at a high temperature (85 °C). This compound could then form dimethyl thiophene or dimethyl disulfides [21,22]. Another study reported that cyclic sulfur compounds can be formed through polymerization or degradation at room temperature and polymerization at higher temperature [22], in accordance with the results of our study. In addition, these sulfur-containing compounds, such as (methyltrisulfanyl)methane(dimethyl trisulfide) (sulfurous, cooked, onion, savory, meaty odor notes); 1-(methyldisulfanyl)prop-1-ene(methyl (E)-1-propenyl disulfide) (baked, garlic, onion odor notes); and 1-[[(E)-prop-1-enyl]disulfanyl]propane ((E)-propenyl propyl disulfide) (sulfurous, cooked, onion odor notes) can contribute to the strong odor characteristics of heated Welsh onions [23,24]. This result indicates that these sulfur-containing compounds are considered to be the major contributors to the change in the aroma characteristic of Welsh onions by heat treatment.
Some volatile compounds, such as propanoic acid; heptanoic acid; pent-1-en-3-ol; (E)-hex-2-en-1-ol; tridecan-2-ol; (2E)-3,7-dimethylocta-2,6-dien-1-ol; methyl acetate; methyl propanoate; (2E,4E)-hexa-2,4-dienal; (E)-2-ethylbut-2-enal; (2E,6Z)-nona-2,6-dienal; (E)-non-2-enal; (2E,4E)-nona-2,4-dienal; butane-2,3-dione; 3-(methyltrisulfanyl)prop-1-ene; and 3-methylthiophene-2-carbaldehyde, were only detected in untreated Welsh onion samples. In addition, most of the aldehydes tended to decrease or disappear after heating. It can be considered that those aldehydes underwent thermal decomposition or oxidation during heating [18].
2.2. The Effects of Antioxidants on the Change in the Volatile Compounds in Heated Welsh Onions during Storage Periods
The volatile compounds in the heated Welsh onion samples added with antioxidants, such as ascorbic acid and glutathione, during storage periods are listed in Table 2. In total, 65 volatile compounds were identified: 2 acids, 5 alcohols, 16 aldehydes, 1 ester, 2 furans, 5 hydrocarbons, 3 ketones, and 31 sulfur-containing compounds (3 thiols; 7 sulfides; 6 disulfides; 4 trisulfides; 1 tetrasulfide; 8 cyclic polysulfides; and 2 others).

Table 2.
Volatile compounds identified in the heated Welsh onion samples added with antioxidants during storage.
The abundances of most volatile compounds in heated Welsh onion samples tended to decrease according to the storage time. Most of the aldehydes showed such behavior; however, some of them, such as (E)-oct-2-enal and decanal, were not changed significantly in the sample added with glutathione, compared to the other samples during 3–7 days of storage time. In addition, benzaldehyde, (2E,4E)-hepta-2,4-dienal, and (2E,4E)-deca-2,4-dienal were not detected in the samples added with glutathione during the storage periods.
The contents of most sulfur-containing compounds, except for thiols, decreased significantly according to storage periods. The contents of some linear and branched polysulfides, such as 1-(methyldisulfanyl)prop-1-ene, 1-[(E)-prop-1-enyl]disulfanyl]propane, (methyltrisulfanyl)methane, 1-(propyltrisulfanyl)propane, 1-[(E)-prop-1-enyl]trisulfanyl]propane, and (methyltetrasulfanyl)methane, which were the most abundant volatile compounds in the heated Welsh onion samples, decreased with the storage periods. These are known to be the predominant compounds in Welsh onions, and can also be related to the flavor characteristics of Allium plants [19,20,21,22,23]. The disulfide bonds of sulfur compounds can be cleaved to produce thio radicals and these radicals are attached to the double bond of another disulfide molecule to form a polysulfide [25]. In addition, the loss of a hydrogen molecule from the alkylthio group can form branched sulfides. In contrast with these sulfur compounds, the contents of some sulfides, such as methylsulfanylmethane (dimethyl sulfide), 3-prop-2-enylsulfanylprop-1-ene (diallyl sulfide), and (methyldisulfanyl)methane (dimethyl disulfide), were relatively low and decreased according to the storage periods. These sulfides can significantly influence the odor characteristics of Welsh onions, due to the low thresholds of such odor notes (unique cooked onion, cooked cabbage, and garlic-like odor notes), ranging from 0.16 to 1.2 ppb [26].
However, cyclic sulfur compounds, such as 2,4-dimethylthiophene, 2,3-dimethylthiophene, 2,5-dimethylthiophene, and 3,4-dimethylthiophene, slightly increased during the initial storage periods (1 and 2 days), possibly due to the rearrangement and cyclization of the fragments from other sulfur-containing compounds. In addition, 3,5-dimethyl-2-(methylthio)-thiophene was increased in the samples added with antioxidants (ascorbic acid and glutathione), according to the storage periods in this study, possibly because highly reactive radicals can attack the linear structure of compounds to form another ring structure [25].
The contents of most sulfur compounds showed a tendency to decrease in the heated Welsh onion samples without antioxidants, according to the storage periods. On the other hand, the thiols and sulfides showed a tendency to increase in the samples added with antioxidants at the initial periods of storage (up to 3 days), whereas they decreased at later periods of storage. Some sulfur compounds, such as 1-methylsulfanylpentane (amyl methyl sulfide) and 1-propan-2-ylsulfanylbutane (butyl isopropyl sulfide), were identified only in the samples added with glutathione, possibly because hydrogen sulfide released from glutathione can participate in the formation of these sulfur compounds by the polymerization and rearrangement of non-enzymatic mechanisms [27]. In addition, it can be assumed that, when a large amount of hydrogen sulfide was produced according to the storage periods, it would react with unstable free radicals to produce other compounds.
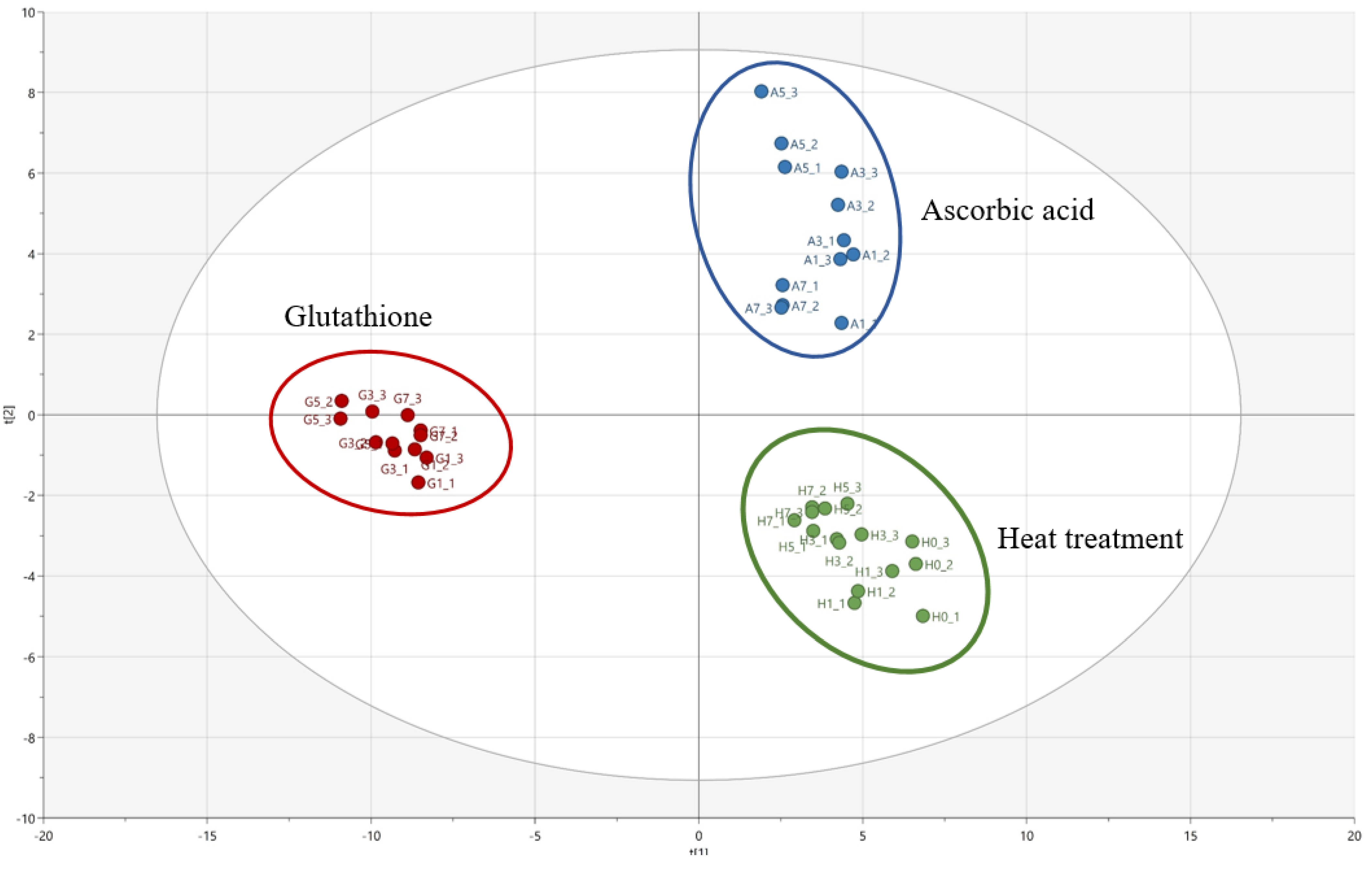
Partial least square discriminant analysis (PLS-DA) was conducted to investigate the effects of different antioxidants on the change in the volatile compounds in the heated Welsh onion samples during storage. Figure 1 shows the PLS-DA score plot for the comparison of the volatile compounds in heated Welsh onion samples added with antioxidants (ascorbic acid and glutathione). PLS (partial least square) component 1 (PLS 1) and PLS component 2 (PLS 2) explained 38.0% and 26.3% of the variance, respectively, and, hence, together explained 64.3% of the total variance. The parameters of the cross-validation modeling were component 3, R2X = 0.49, R2Y = 0.93, and Q2Y = 0.40. A permutation test involving 100 iterations was also conducted to validate the model, which yielded R2 = 0.056 and Q2 = −0.282.
Figure 1.
PLS-DA score plot of the volatile compounds identified in the heated Welsh onion samples added with antioxidants during storage.
The heated Welsh onion samples without antioxidants (control) and heated Welsh onion samples added with ascorbic acid were located on the positive PLS 1 axis, while heated Welsh onion samples added with glutathione were located on the negative PLS 1 axis. In addition, the heated Welsh onion samples added with ascorbic acid were located on the positive PLS 2 axis, whereas heated Welsh onion samples without antioxidants (control) were located on the negative PLS 2 axis. Table 3 and Table 4 list the major volatile compounds identified samples with a criterion of the variable importance plot (VIP) > 1.0.

Table 3.
The major volatile compounds identified in the heated Welsh onion samples added with different antioxidants, based on the variable importance plot (VIP > 1.0) list for PLS component 1 of PLS-DA.

Table 4.
The major volatile compounds identified in the heated Welsh onion samples added with different antioxidants, based on the variable importance plot (VIP > 1.0) list for PLS component 2 of PLS-DA.
The negative PLS 1 axis was related to some compounds, such as 3-ethylthiophene; 1-(methyldisulfanyl)-1-methylsulfanylpropane; 1-methylsulfanylpentane; 2-prop-2-enylsulfanylpropane; 1-propan-2-ylsulfanylbutane; 1,3-dithiane; methanethiol; 1-(propyldisulfanyl)propane; propane-1-thiol; (Z)-1-methylsulfanylprop-1-ene; and undecan-2-one, which could be considered as the major contributors to the heated Welsh onion sample added with glutathione (Table 3). On the other hand, 2-phenylacetaldehyde; acetic acid; methylsulfanylmethane; prop-2-ene-1-thiol; undecan-2-one; (2E,4E)-deca-2,4-dienal; 1-[[(E)-prop-1-enyl]trisulfanyl]propane; 2-prop-2-enylsulfanylpropane; 1,3-dithiane; 1-(propyltrisulfanyl)propane; 1-methyl-2-(3,5-dimethylthien-4-yl)disulfide; 3,4-dimethylthiophene; and propane-1-thiol were also associated with the positive PLS 2 axis. These results demonstrate that these compounds could considerably influence the heated Welsh onion samples added with ascorbic acid (Table 4).
3. Materials and Methods
3.1. Materials
Welsh onions (Allium fistulosum) were purchased from Nonghyup (Goyang-si, Gyeonggi-do, Korea). The Welsh onions were cultivated in Jangseong-gun, Jeollanam-do in South Korea (2020). All the samples were stored at 25 °C in a temperature and humid chamber (Han Baek Scientific Co., Bucheon-si, Korea), before they were analyzed. The solid-phase microextraction (SPME) fibers and holders were purchased from Supelco (Bellefonte, PA, USA), whereas the vial and screw caps (Ultraclean 18 mm) were purchased from Agilent Technologies (Santa Clara, CA, USA). β-damascone was purchased from Sigma-Aldrich (St. Louis, MO, USA). The mineral oil was purchased from Sigma-Aldrich (St. Louis, MO, USA). The methanol and water were purchased from J.T.Baker (Phillipsburg, NJ, USA). The antioxidants, L-ascorbic acid and L-glutathione, were purchased from Sigma-Aldrich (St. Louis, MO, USA).
3.2. The Preparation of the Welsh Onion Samples by Heating
A total of 800 g of washed Welsh onions and 800 g of purified water were ground in a blender, and 1.5 kg of Welsh onion juice was obtained. The Welsh onion sample was placed into a 1 L flask and heated in a 2 L oil bath. After preheating at 70 °C for 5 min, it was heated at 100 °C for 10 min.
3.3. The Storage Conditions of the Heated Welsh Onion Samples
Each 20 mL of heated Welsh onion samples in a 50 mL amber laboratory bottle was stored at 25 °C in constant temperature and humidity chamber (Han Baek Scientific Co., Bucheon-si, Korea). The different antioxidants, L-ascorbic acid and L-glutathione, were added to the heated Welsh onion samples at a concentration of 0.05g/100g (w/w). The Welsh onion samples were sealed with a PBT screw cap and coated silicon PTFE gasket to prevent the loss of volatile compounds. During the storage periods, the samples were obtained at 0, 1, 3, 5, and 7 days.
3.4. The Extraction of Volatile Compounds Using SPME
A sample of Welsh onions (5 g) was placed in a 20 mL vial; β-damascone (1 mg/mLin β-damascone/methanol solvent mixture (1:200, v/v)) was added as an internal standard, and the vial was sealed with a screw cap. SPME was used to obtain the volatile compounds of Welsh onions. The sample was maintained at 60 °C for 30 min to reach a state of equilibrium. An SPME fiber coated with carboxen/polydimethylsiloxane (CAR/PDMS) was used to adsorb volatile compounds at 40 °C for 20 min, and desorption was executed at 230 °C in a GC injector for 5 min.
3.5. GC-MS Analysis
GC-MS analysis was performed using a 7890A series gas chromatograph (Agilent Technologies) and a 5975C mass detector (Agilent Technologies) equipped with a DB-5MS column (30 m length × 0.25 mm i.d. × 0.25 μm film thickness, J&W Scientific, Folsom, CA, USA). The GC oven temperature was programed as follows: the initial temperature was maintained at 40 °C for 6 min, raised to 170 °C at a rate of 5 °C/min and held for 3 min, and increased to 220 °C at a rate of 5 °C/min. The flow rate of helium, the carrier gas, was constant at 0.8 mL/min, whereas the mass spectra was obtained with a mass scan rage of 35–350 atomic mass units (a.m.u.) at a rate of 4.5 scans/s, and the electron impact (EI) mode was 70 eV. All the samples were prepared and analyzed in triplicate.
3.6. The Identification and Semi-Quantification of the Volatile Compounds
The identification of each volatile compound was positively confirmed by a comparison of the retention time and mass spectral data with those of the authentic standard compounds. When the standard compounds were not available, each volatile compound was identified on the basis of its mass spectral data using the NIST.08 and Wiley.9 mass spectral libraries and the retention index (RI) values from the previous literature [1,2,20,26,27]. The RI value of the volatile compounds was calculated with the n-alkanes from C6 to C30 as the external standards. The quantification of the volatile compounds was performed by comparing their peak areas with that of the internal standard compound on the total ion chromatogram of GC-MS.
3.7. The Statistical Analysis
The significance of the differences (p < 0.05) between the untreated Welsh onion samples and the heated Welsh onion samples were evaluated by Duncan’s t-test using the SPSS program (version 12.0, Chicago, IL, USA). Multivariate statistical analysis, partial least square discriminant analysis (PLS-DA), was conducted using SIMCA-P (version 11.0, Umetrics, Umea, Sweden) to determine the key volatile compounds related to the discrimination of the heated Welsh onions added with antioxidants.
4. Conclusions
This study investigated the changes in the volatile compounds in Welsh onions by heat treatment and the effects of antioxidants (ascorbic acid and glutathione) on the changes in the volatile compounds during storage. Some sulfur-containing compounds, such as 2-methylthiirane (propylene sulfide); 1-(methyldisulfanyl)prop-1-ene; 1-[[(E)-prop-1-enyl]disulfanyl]propane (propyl (E)-1-propenyl disulfide); 1-(propyltrisulfanyl)propane; 1-[[(E)-prop-1-enyl]trisulfanyl]propane; and (methyltetrasulfanyl)methane (dimethyl tetrasulfide), showed significant differences between the untreated Welsh onion and heated Welsh onion samples. These results demonstrate that heat treatment can affect the flavor profiles of Welsh onions, through their thermal decomposition and rearrangement.
The application of PLS-DA to the data sets of volatile compounds revealed that heated Welsh onion samples can be distinguished according to different antioxidants, such as ascorbic acid and glutathione. 3-ethylthiophene; 1-(methyldisulfanyl)-1-methylsulfanylpropane; 1-methylsulfanylpentane; 2-prop-2-enylsulfanylpropane; and 1-propan-2-ylsulfanylbutane were strongly correlated with the heated Welsh onion samples added with glutathione. On the other hand, 2-phenylacetaldehyde; acetic acid; methylsulfanylmethane; prop-2-ene-1-thiol; undecan-2-one; and (2E,4E)-deca-2,4-dienal were related to the heated Welsh onion samples added with ascorbic acid. These findings indicate that the profiles of the volatile compounds of the heated Welsh onion samples added with different antioxidants can significantly change the volatile profiles during storage. These results can be used to improve the quality of Welsh onion-based products and develop processed foods.
Author Contributions
Conceptualization, S.M.L. and Y.-S.K.; methodology, D.K. and Y.-S.K.; analysis, S.M.L. and D.K.; investigation, S.M.L. and Y.-S.K.; data curation, D.K. and S.M.L.; writing—original draft preparation, S.M.L.; writing—review and editing, S.M.L. and Y.-S.K.; visualization, D.K.; supervision, Y.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Innovative Food Product and Natural Food Materials Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) [grant number (319048-3)] and BK21 FOUR (Fostering Outstanding Universities for Research) (No. 4299990914600) funded by the Korea government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the Innovative Food Product and Natural Food Materials Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (319048-3) and project BK21 FOUR (Fostering Outstanding Universities for Research) (No. 4299990914600) funded by the Korea government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuo, M.C.; Chien, M.; Ho, C.T. Novel Polysulfides Identified in the Volatile Components from Welsh Onions (Allium fistulosum L. Var. Maichuon) and Scallions (Allium fistulosum L. Var. Caespitosum). J. Agric. Food Chem. 1990, 38, 1378–1381. [Google Scholar] [CrossRef]
- Kuo, M.C.; Ho, C.T. Volatile Constituents of the Solvent Extracts of Welsh Onions (Allium Fistulosum L. Variety Maichuon) and Scallions (A. fistulosum L. Variety Caespitosum). J. Agric. Food Chem. 1992, 40, 1906–1910. [Google Scholar] [CrossRef]
- Aristizabal, L.; Ángel, M.; Orozco, C.; Ruiz, P.; Quijano, J.; Notario, R. Computational Study of the Thermal Decomposition and the Thermochemistry of Allyl Ethers and Allyl Sulfides. Struct. Chem. 2018, 29, 897–907. [Google Scholar] [CrossRef]
- Aware, R.; Thorat, B. Garlic under various drying study and its impact on allicin retention. Drying Technol. 2011, 29, 1510–1518. [Google Scholar] [CrossRef]
- Barril, C.; Clark, A.C.; Scollary, G.R. Chemistry of Ascorbic Acid and Sulfur Dioxide as an Antioxidant System Relevant to White Wine. Anal. Chim. Acta 2012, 732, 186–193. [Google Scholar] [CrossRef]
- Benkeblia, N.; Lanzotti, V. Allium Thiosulfinates: Chemistry, Biological Properties and their Potential Utilization in Food Preservation. Food 2007, 1, 193–201. [Google Scholar]
- Benkeblia, N.; Varoquaux, P. Effect of Nitrous Oxide (N2O) on Respiration Rate, Soluble Sugars and Quality Attributes of Onion Bulbs Allium Cepa Cv. Rouge Amposta during Storage. Postharvest Biol. Technol. 2003, 30, 161–168. [Google Scholar] [CrossRef]
- Block, E.; Naganathan, S.; Putman, D.; Zhao, S.H. Allium Chemistry: HPLC Analysis of Thiosulfinates from Onion, Garlic, Wild Garlic (Ramsoms), Leek, Scallion, Shallot, Elephant (Great-Headed) Garlic, Chive, and Chinese Chive. Uniquely High Allyl to Methyl Ratios in some Garlic Samples. J. Agric. Food Chem. 1992, 40, 2418–2430. [Google Scholar] [CrossRef]
- Boelens, H.; Brandsma, L. Formation of Dialkylthiophenes by Thermolysis of Di (1-alkenyl) Disulfides and Alkyl 1-propenyl Disulfides. Recl. Trav. Chim. Pays-Bas 1972, 91, 141–145. [Google Scholar] [CrossRef]
- Cecchi, L.; Ieri, F.; Vignolini, P.; Mulinacci, N.; Romani, A. Characterization of Volatile and Flavonoid Composition of Different Cuts of Dried Onion (Allium Cepa L.) by HS-SPME-GC-MS, HS-SPME-GC× GC-TOF and HPLC-DAD. Molecules 2020, 25, 408. [Google Scholar] [CrossRef] [Green Version]
- Flora, S.J. Structural, Chemical and Biological Aspects of Antioxidants for Strategies against Metal and Metalloid Exposure. Oxid. Med. Cell. Longev. 2009, 2, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Ford-Lloyd, B.V.; Armstrong, S.J. Welsh onion: Allium fistulosum L. In Genetic Improvement of Vegetable Crops; Elsevier: Amsterdam, The Netherlands, 1993; pp. 51–58. [Google Scholar]
- Galano, A.; Alvarez-Idaboy, J.R. Glutathione: Mechanism and Kinetics of its Non-Enzymatic Defense Action against Free Radicals. RSC Adv. 2011, 1, 1763–1771. [Google Scholar] [CrossRef]
- Galano, A.; Francisco-Marquez, M. Reactions of OOH Radical with β-Carotene, Lycopene, and Torulene: Hydrogen Atom Transfer and Adduct Formation Mechanisms. J. Phys. Chem. B 2009, 113, 11338–11345. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, C.; Boudier, A.; Bonetti, J.; Clarot, I.; Leroy, P.; Parent, M. Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Antioxidants 2018, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- Gruhlke, M.C.; Slusarenko, A.J. The Biology of Reactive Sulfur Species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef]
- Gyawali, R.; Seo, H.; Lee, H.; Song, H.; Kim, D.; Byun, M.; Kim, K. Effect of γ-Irradiation on Volatile Compounds of Dried Welsh Onion (Allium fistulosum L.). Radiat. Phys. Chem. 2006, 75, 322–328. [Google Scholar] [CrossRef]
- Iranshahi, M. A Review of Volatile Sulfur-Containing Compounds from Terrestrial Plants: Biosynthesis, Distribution and Analytical Methods. J. Essent. Oil Res. 2012, 24, 393–434. [Google Scholar] [CrossRef]
- Jun, H.; Yang, J.; Choi, J.Y.; Lee, S.; Song, G.; Kim, K.S.; Kim, Y. Changes in Volatile Flavor Compounds in Steam-Dried Allium Hookeri Root. Food Sci. Biotechnol. 2016, 25, 1327–1331. [Google Scholar] [CrossRef]
- Kuo, M.C.; Ho, C.T. Volatile Constituents of the Distilled Oils of Welsh Onions (Allium fistulosum L. Variety Maichuon) and Scallions (Allium fistulosum L. Variety Caespitosum). J. Agric. Food Chem. 1992, 40, 111–117. [Google Scholar] [CrossRef]
- Lanzotti, V. The Analysis of Onion and Garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef] [Green Version]
- McGorrin, R.J. The significance of volatile sulfur compounds in food flavors: An overview. In Volatile Sulfur Compounds in Food; Anonymous, Ed.; ACS Publications: Washington, DC, USA, 2011; pp. 3–31. [Google Scholar]
- Mongpraneet, S.; Abe, T.; Tsurusaki, T. Accelerated Drying of Welsh Onion by Far Infrared Radiation Under Vacuum Conditions. J. Food Eng. 2002, 55, 147–156. [Google Scholar] [CrossRef]
- Nielsen, R.W.; Tachibana, C.; Hansen, N.E.; Winther, J.R. Trisulfides in Proteins. Antioxid. Redox Signal. 2011, 15, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Quintiliani, M.; Badiello, R.; Tamba, M.; Esfandi, A.; Gorin, G. Radiolysis of Glutathione in Oxygen-Containing Solutions of pH 7. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1977, 32, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008, 77, 1191–1205. [Google Scholar] [CrossRef]
- Lee, S.M.; Jo, Y.J.; Kim, Y.S. Investigation of the aroma-active compounds formed in the Maillard reaction between glutathione and reducing sugars. J. Agric. Food Chem. 2010, 58, 3116–3124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).