Cannabidiolic acid in Hemp Seed Oil Table Spoon and Beyond
Abstract
:1. Introduction
2. Results and Discussion
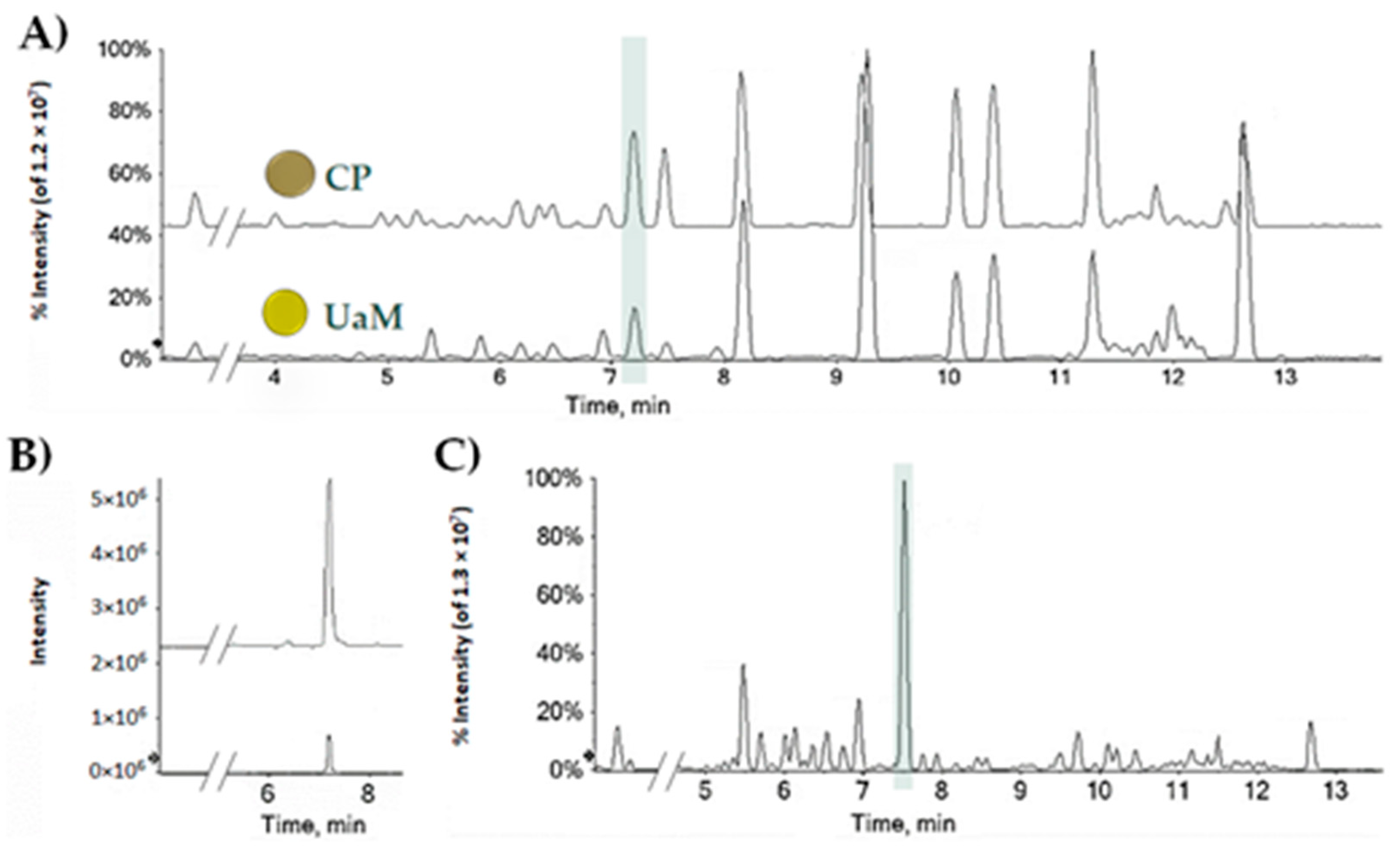
2.1. CBDA, a Nutraceutical Value in the Hemp Seed Oil Table Spoon and Hemp Wastes
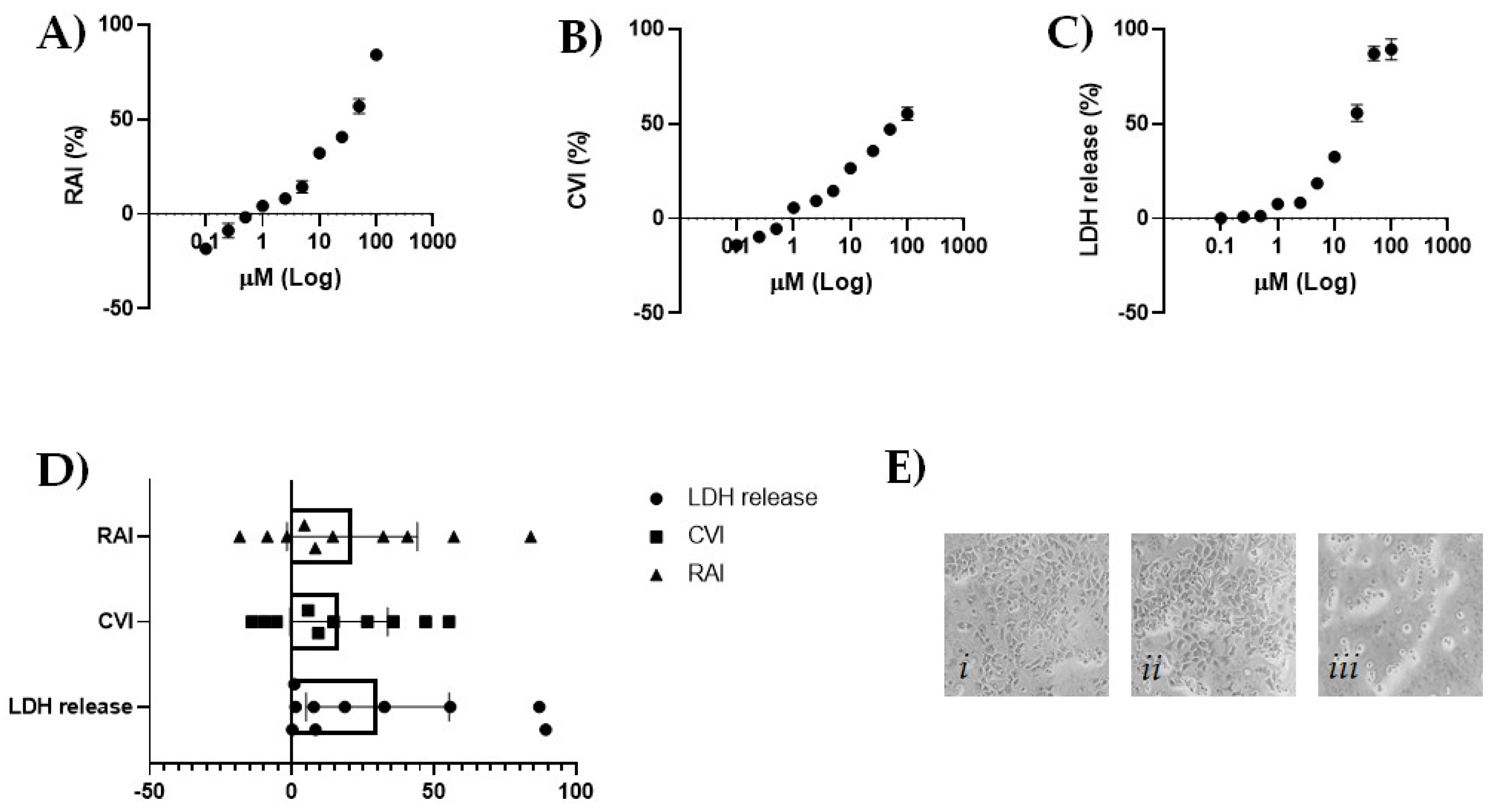
2.2. CBDA Cytotoxicity in HaCaT Cells
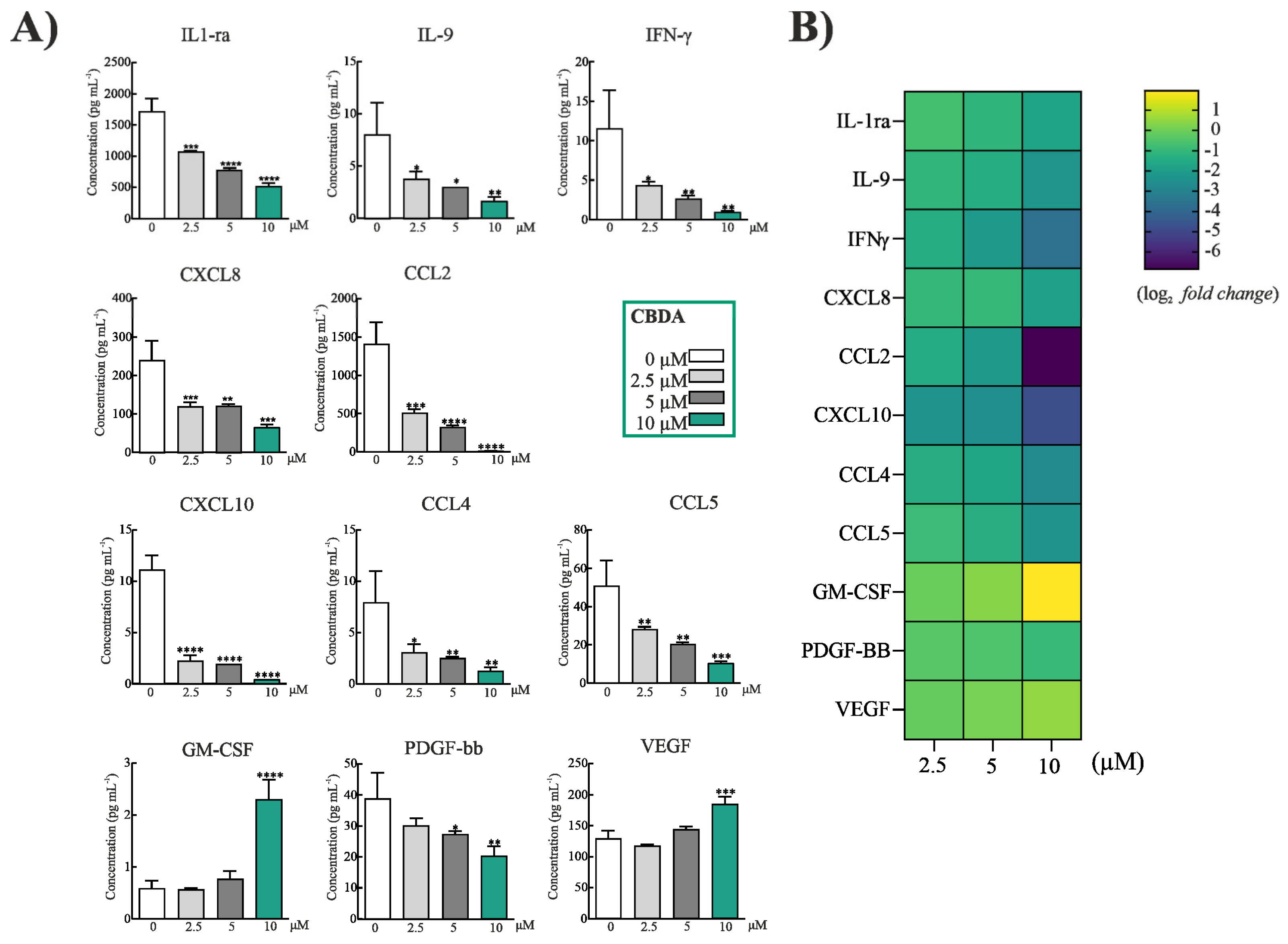
2.3. CBDA Modulated Cytokines and Chemokines in HaCaT Cells
2.4. Antibacterial Activity of CBDA: MIC and MBC Determination
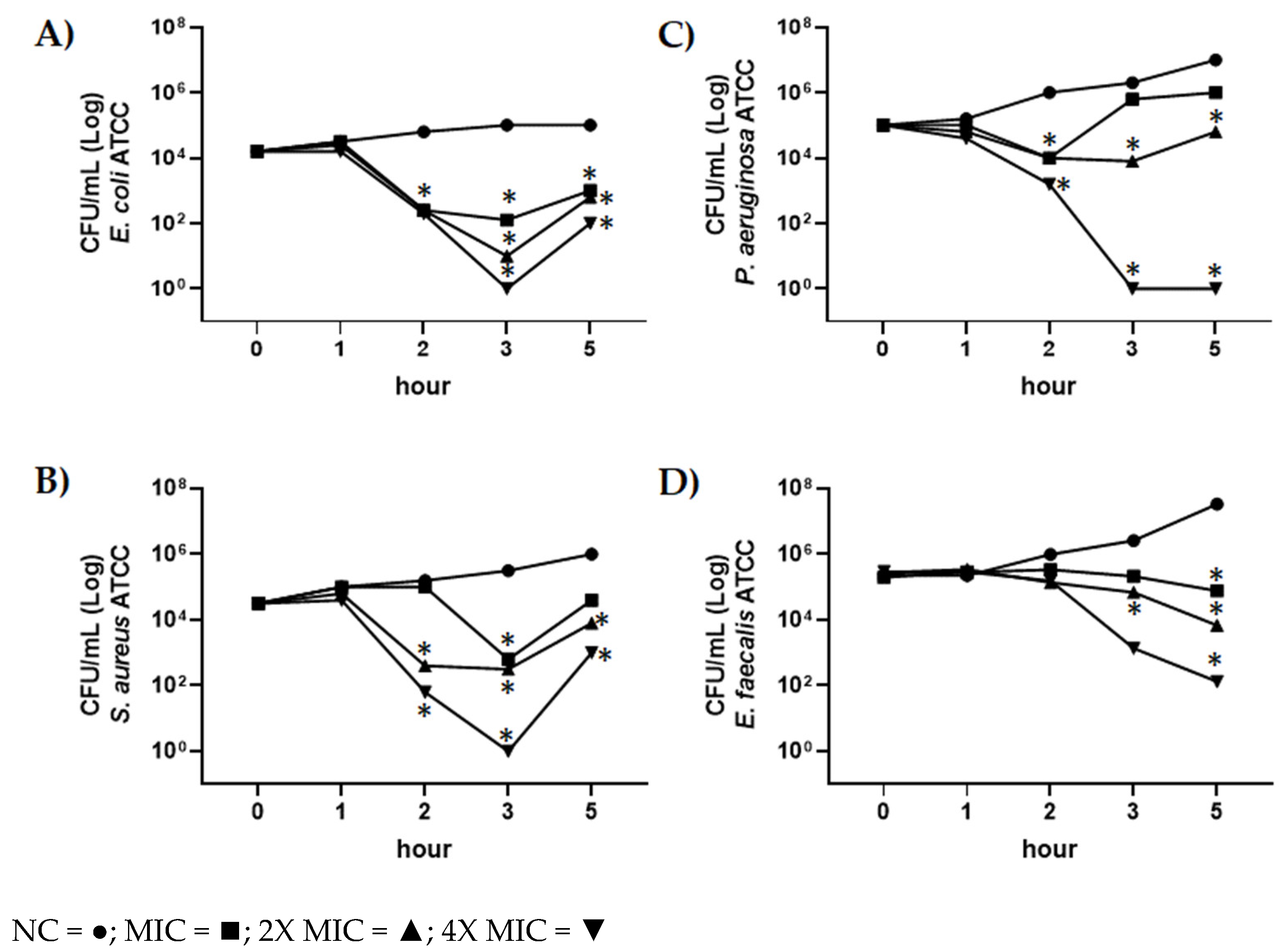
2.5. Evaluation of Antimicrobial Activity of CBDA by Time-Killing Assay
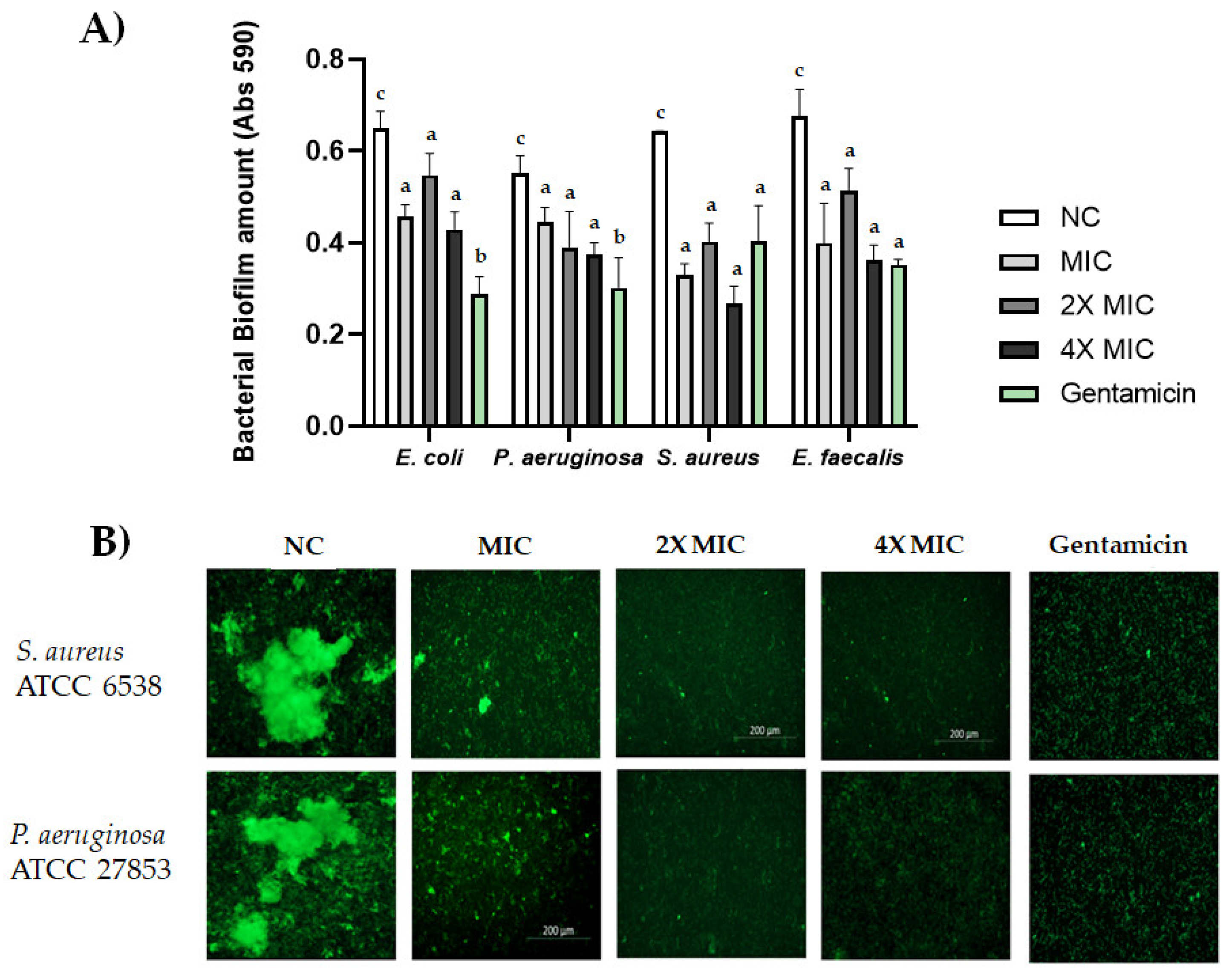
2.6. Biofilm Formation
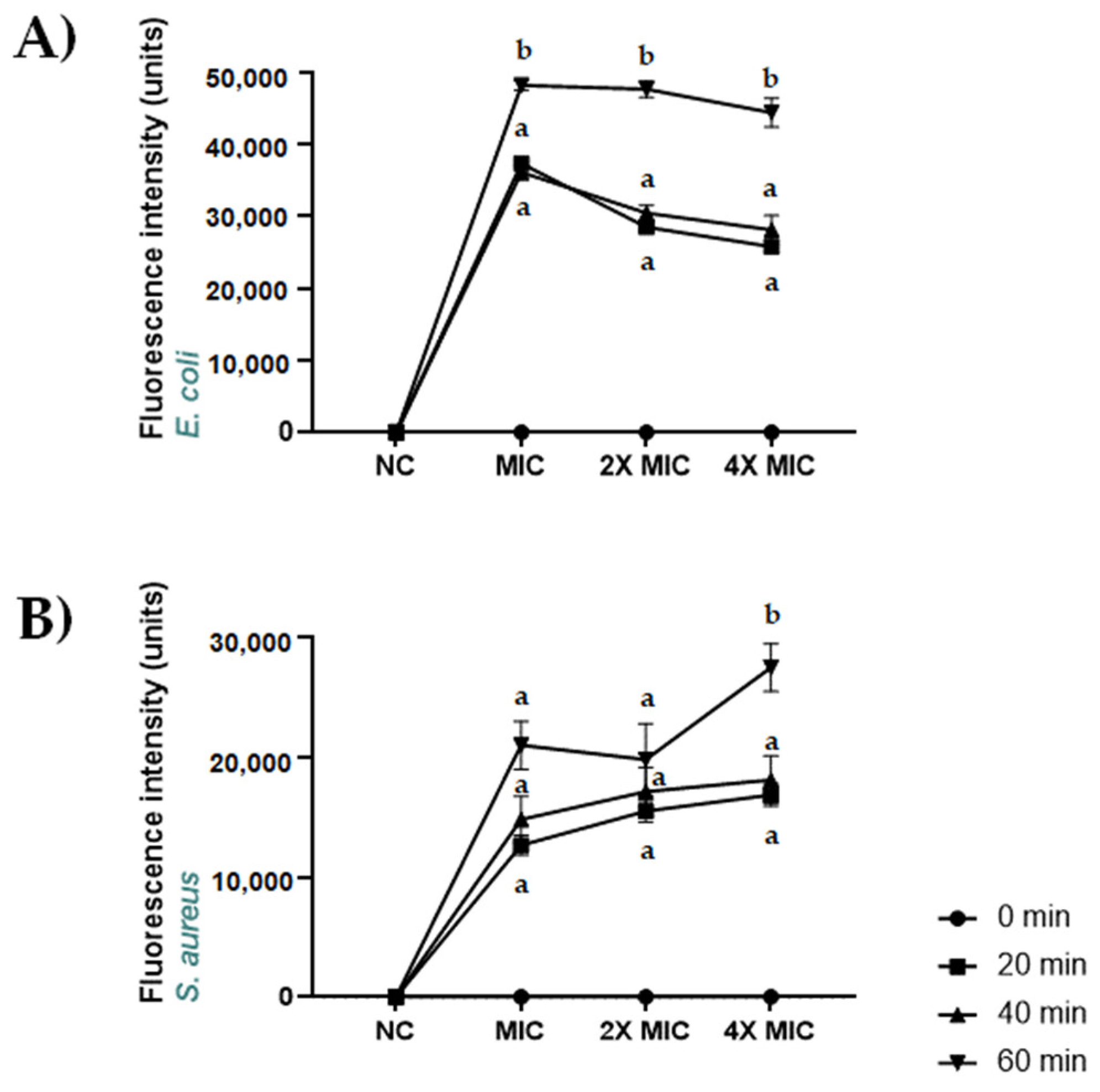
2.7. Permeabilization of S. aureus ATCC 6538 and E. coli ATCC 13762 Membranes Induced by CBDA
3. Materials and Methods
3.1. CBDA Quantitation in Hemp Seed Oil and Leaf Wastes
3.2. Cell Culture
3.3. MTT Cell Viability Assay
3.4. SRB Cell Viability Assay
3.5. LDH Cytotoxicity Assay
3.6. Cytokine Analysis
3.7. Bacterial Strains and Growth Conditions
3.7.1. Antibacterial Activity Assay—MIC and MBC Determination
3.7.2. In Vitro Time-Killing Assay
3.8. Biofilm Formation Assay
3.9. SYTOX Green Uptake Assay Using Fluorescent Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging-from the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Laneri, S. Nutricosmetics: A brief overview. Phytother. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.F.; Cervantes, E.L.; Luna-Vital, D.A.; Mojica, L. Food-derived bioactive compounds with anti-aging potential for nutricosmetic and cosmeceutical products. Crit. Rev. Food Sci. Nutr. 2021, 61, 3740–3755. [Google Scholar] [CrossRef]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef]
- Lakdawala, N.; Babalola, O., 3rd; Fedeles, F.; McCusker, M.; Ricketts, J.; Whitaker-Worth, D.; Grant-Kels, J.M. The role of nutrition in dermatologic diseases: Facts and controversies. Clin. Dermatol. 2013, 31, 677–700. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, A.; Ooyama, K.; Kojima, K.; Kachi, H.; Abe, T.; Amano, K.; Aoyama, T. Dietary supplementation of gamma-linolenic acid improves skin parameters in subjects with dry skin and mild atopic dermatitis. J. Oleo Sci. 2011, 60, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Miura, K.; Way, M.; Jiyad, Z.; Marquart, L.; Plasmeijer, E.I.; Campbell, S.; Isbel, N.; Fawcett, J.; Ferguson, L.E.; Davis, M.; et al. Omega-3 fatty acid intake and decreased risk of skin cancer in organ transplant recipients. Eur. J. Nutr. 2021, 60, 1897–1905. [Google Scholar] [CrossRef]
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, Characterization and Properties of Hemp Seed Oil and Its Emulsions. Molecules 2017, 22, 700. [Google Scholar] [CrossRef]
- Martinez, J.R.; la Paz, S.M.-D.; De la Puerta, R.; Garcia-Gimenez, M.D.; Fernández-Arche, M. Characterization of bioactive compounds in defatted hempseed (Cannabis sativa L.) by UHPLC-HRMS/MS and anti-inflammatory activity in primary human monocytes. Food Funct. 2020, 11, 4057–4066. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Crescente, G.; Formato, M.; Pecoraro, M.T.; Mallardo, M.; Piccolella, S.; Daniele, A.; Pacifico, S. Hempseed Lignanamides Rich-Fraction: Chemical Investigation and Cytotoxicity towards U-87 Glioblastoma Cells. Molecules 2020, 25, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Formato, M.; Pecoraro, M.T.; Crescente, G.; Pacifico, S. Discrimination of CBD-, THC- and CBC-type acid cannabinoids through diagnostic ions by UHPLC-HR-MS/MS in negative ion mode. J. Pharm. Biomed. Anal. 2021, 201, 114125. [Google Scholar] [CrossRef]
- Kanabus, J.; Bryła, M.; Roszko, M.; Modrzewska, M.; Pierzgalski, A. Cannabinoids-Characteristics and Potential for Use in Food Production. Molecules 2021, 26, 6723. [Google Scholar] [CrossRef]
- Gülck, T.; Møller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef]
- Christinat, N.; Savoy, M.C.; Mottier, P. Development, validation and application of a LC-MS/MS method for quantification of 15 cannabinoids in food. Food Chem. 2020, 318, 126469. [Google Scholar] [CrossRef]
- Scheau, C.; Badarau, I.A.; Mihai, L.G.; Scheau, A.E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef] [Green Version]
- Eagleston, L.R.M.; Kalani, N.K.; Patel, R.R.; Flaten, H.K.; Dunnick, C.A.; Dellavalle, R.P. Cannabinoids in dermatology: A scoping review. Dermatol. Online J. 2018, 24. [Google Scholar] [CrossRef]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The potential role of cannabinoids in dermatology. J. Dermatol. Treat. 2020, 31, 839–845. [Google Scholar] [CrossRef]
- Sunda, F.; Arowolo, A. A molecular basis for the anti-inflammatory and anti-fibrosis properties of cannabidiol. FASEB J. 2020, 34, 14083–14092. [Google Scholar] [CrossRef] [PubMed]
- Crescente, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Marín-Aguilar, F.; García-Giménez, M.D.; Fernández-Arche, M.A. Hemp (Cannabis sativa L.) seed oil: Analytical and phytochemical characterization of the unsaponifiable fraction. J. Agric. Food Chem. 2014, 62, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Izzo, L.; Pacifico, S.; Piccolella, S.; Castaldo, L.; Narváez, A.; Grosso, M.; Ritieni, A. Chemical Analysis of Minor Bioactive Components and Cannabidiolic acid in Commercial Hemp Seed Oil. Molecules 2020, 25, 3710. [Google Scholar] [CrossRef]
- Formato, M.; Crescente, G.; Scognamiglio, M.; Fiorentino, A.; Pecoraro, M.T.; Piccolella, S.; Catauro, M.; Pacifico, S. (−)-Cannabidiolic acid, a Still Overlooked Bioactive Compound: An Introductory Review and Preliminary Research. Molecules 2020, 25, 2638. [Google Scholar] [CrossRef]
- Giantulli, S.; Tortorella, E.; Brasili, F.; Scarpa, S.; Cerroni, B.; Paradossi, G.; Bedini, A.; Morrone, S.; Silvestri, I.; Domenici, F. Effect of 1-MHz ultrasound on the proinflammatory interleukin-6 secretion in human keratinocytes. Sci. Rep. 2021, 11, 19033. [Google Scholar] [CrossRef]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic acid and Cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef]
- Shangguan, F.; Zhou, H.; Ma, N.; Wu, S.; Huang, H.; Jin, G.; Wu, S.; Hong, W.; Zhuang, W.; Xia, H.; et al. A Novel Mechanism of Cannabidiol in Suppressing Hepatocellular Carcinoma by Inducing GSDME Dependent Pyroptosis. Front. Cell Dev. Biol. 2021, 9, 697832. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules 2021, 26, 802. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory Properties of Cannabidiol, a Nonpsychotropic Cannabinoid, in Experimental Allergic Contact Dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef] [PubMed]
- Pagano, S.; Coniglio, M.; Valenti, C.; Federici, M.I.; Lombardo, G.; Cianetti, S.; Marinucci, L. Biological effects of Cannabidiol on normal human healthy cell populations: Systematic review of the literature. Biomed Pharmacother. 2020, 132, 110728. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Lee, M.Y. The ameliorative effect of hemp seed hexane extracts on the Propionibacterium acnes-induced inflammation and lipogenesis in sebocytes. PLoS ONE 2018, 13, e0202933. [Google Scholar] [CrossRef]
- Park, C.; Zuo, J.; Somayaji, V.; Lee, B.J.; Löbenberg, R. Development of a novel cannabinoid-loaded microemulsion towards an improved stability and transdermal delivery. Int. J. Pharm. 2021, 604, 120766. [Google Scholar] [CrossRef]
- Corsini, E.; Galli, C.L. Cytokines and irritant contact dermatitis. Toxicol. Lett. 1998, 102–103, 277–282. [Google Scholar] [CrossRef]
- Coondoo, A. Cytokines in dermatology—A basic overview. Indian J. Dermatol. 2011, 56, 368–374. [Google Scholar] [CrossRef]
- Niklander, S.E.; Crane, H.L.; Darda, L.; Lambert, D.W.; Hunter, K.D. The role of icIL-1RA in keratinocyte senescence and development of the senescence-associated secretory phenotype. J. Cell Sci. 2021, 134, jcs252080. [Google Scholar] [CrossRef]
- Wan, J.; Wu, Y.; Ji, X.; Huang, L.; Cai, W.; Su, Z.; Wang, S.; Xu, H. IL-9 and IL-9-producing cells in tumor immunity. Cell Commun. Signal. 2020, 18, 50. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Xue, H.B.; Guan, X.H.; Shu, C.M.; Zhang, J.H.; Yu, J. Possible pathogenic role of T helper type 9 cells and interleukin (IL)-9 in atopic dermatitis. Clin. Exp. Immunol. 2014, 175, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Rebane, A.; Zimmermann, M.; Aab, A.; Baurecht, H.; Koreck, A.; Karelson, M.; Abram, K.; Metsalu, T.; Pihlap, M.; Meyer, N.; et al. Mechanisms of IFN-γ-induced apoptosis of human skin keratinocytes in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2012, 129, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- de Groot, M.; Teunissen, M.B.; Ortonne, J.P.; Lambert, J.R.; Naeyaert, J.M.; Picavet, D.I.; Arreaza, M.G.; Simon, J.S.; Kraan, M.; Bos, J.D.; et al. Expression of the chemokine receptor CCR5 in psoriasis and results of a randomized placebo controlled trial with a CCR5 inhibitor. Arch. Dermatol. Res. 2007, 299, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhu, L.; Feng, Y.; Yang, Y.; Liu, L.; Ran, Y. Effects of acitretin on proliferative inhibition and RANTES production of HaCaT cells. Arch. Dermatol. Res. 2008, 300, 575–581. [Google Scholar] [CrossRef]
- Lemster, B.H.; Carroll, P.B.; Rilo, H.R.; Johnson, N.; Nikaein, A.; Thomson, A.W. IL-8/IL-8 receptor expression in psoriasis and the response to systemic tacrolimus (FK506) therapy. Clin. Exp. Immunol. 1995, 99, 148–154. [Google Scholar] [CrossRef]
- Yang, C.C.; Hung, Y.L.; Ko, W.C.; Tsai, Y.J.; Chang, J.F.; Liang, C.W.; Chang, D.C.; Hung, C.F. Effect of Neferine on DNCB-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Int. J. Mol. Sci. 2021, 22, 8237. [Google Scholar] [CrossRef]
- Mai, W.; Lu, D.; Liu, X.; Chen, L. MCP-1 produced by keratinocytes is associated with leucocyte recruitment during elicitation of nickel-induced occupational allergic contact dermatitis. Toxicol. Ind. Health 2018, 34, 36–43. [Google Scholar] [CrossRef]
- Yang, L.; Yang, S.; Lei, J.; Hu, W.; Chen, R.; Lin, F.; Xu, A.E. Role of chemokines and the corresponding receptors in vitiligo: A pilot study. J. Dermatol. 2018, 45, 31–38. [Google Scholar] [CrossRef]
- Zou, A.; Jian, Q. CXCL10 and its related key genes as potential biomarkers for psoriasis: Evidence from bioinformatics and real-time quantitative polymerase chain reaction. Medicine 2021, 100, e27365. [Google Scholar] [CrossRef]
- Li, C.; Man, X.; Li, W.; Zhou, J.; Chen, J.; Cai, S.; Zheng, M. Regulation of adhesion by vascular endothelial growth factor in HaCaT cells. Mol. Cell Biochem. 2011, 346, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Lederle, W.; Stark, H.J.; Skobe, M.; Fusenig, N.E.; Mueller, M.M. Platelet-derived growth factor-BB controls epithelial tumor phenotype by differential growth factor regulation in stromal cells. Am. J. Pathol. 2006, 169, 1767–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachayon, M.; Jirakran, K.; Sodsai, P.; Klinchanhom, S.; Sughondhabirom, A.; Plaimas, K.; Suratanee, A.; Maes, M. Effects of cannabidiol on activated immune-inflammatory pathways in major depressive patients and healthy controls. medRxiv, 2022; in press. [Google Scholar] [CrossRef]
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.I.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Devel. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef] [PubMed]
- Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The Antimicrobial Activity of Cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Sionov, R.V.; Mechoulam, R.; Steinberg, D. Anti-Biofilm Activity of Cannabidiol against Candida albicans. Microorganisms 2021, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Pacifico, S.; Gallicchio, M.; Lorenz, P.; Duckstein, S.M.; Potenza, N.; Galasso, S.; Marciano, S.; Fiorentino, A.; Stintzing, F.C.; Monaco, P. Neuroprotective potential of Laurus nobilis antioxidant polyphenol-enriched leaf extracts. Chem. Res. Toxicol. 2014, 27, 611–626. [Google Scholar] [CrossRef]
- Miceli, M.; Dell’Aversana, C.; Russo, R.; Rega, C.; Cupelli, L.; Ruvo, M.; Altucci, L.; Chambery, A. Secretome profiling of cytokines and growth factors reveals that neuro-glial differentiation is associated with the down-regulation of Chemokine Ligand 2 (MCP-1/CCL2) in amniotic fluid derived-mesenchymal progenitor cells. Proteomics 2016, 16, 674–688. [Google Scholar] [CrossRef]
- Colicchio, R.; Nigro, E.; Colavita, I.; Pagliuca, C.; Di Maro, S.; Tomassi, S.; Scaglione, E.; Carbone, F.; Carriero, M.V.; Matarese, G.; et al. A novel smaller β-defensin-derived peptide is active against multidrug-resistant bacterial strains. FASEB J. 2021, 35, e22026. [Google Scholar] [CrossRef]
| MIC a (µg/mL) | MBC b (µg/mL) | |
|---|---|---|
| E. coli ATCC 13762 | 0.5 | 8 |
| S. aureus ATCC 6538 | 4 | 16 |
| P. aeruginosa ATCC 27853 | 8 | 32 |
| E. faecalis ATCC 29212 | 4 | 32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigro, E.; Pecoraro, M.T.; Formato, M.; Piccolella, S.; Ragucci, S.; Mallardo, M.; Russo, R.; Di Maro, A.; Daniele, A.; Pacifico, S. Cannabidiolic acid in Hemp Seed Oil Table Spoon and Beyond. Molecules 2022, 27, 2566. https://doi.org/10.3390/molecules27082566
Nigro E, Pecoraro MT, Formato M, Piccolella S, Ragucci S, Mallardo M, Russo R, Di Maro A, Daniele A, Pacifico S. Cannabidiolic acid in Hemp Seed Oil Table Spoon and Beyond. Molecules. 2022; 27(8):2566. https://doi.org/10.3390/molecules27082566
Chicago/Turabian StyleNigro, Ersilia, Maria Tommasina Pecoraro, Marialuisa Formato, Simona Piccolella, Sara Ragucci, Marta Mallardo, Rosita Russo, Antimo Di Maro, Aurora Daniele, and Severina Pacifico. 2022. "Cannabidiolic acid in Hemp Seed Oil Table Spoon and Beyond" Molecules 27, no. 8: 2566. https://doi.org/10.3390/molecules27082566
APA StyleNigro, E., Pecoraro, M. T., Formato, M., Piccolella, S., Ragucci, S., Mallardo, M., Russo, R., Di Maro, A., Daniele, A., & Pacifico, S. (2022). Cannabidiolic acid in Hemp Seed Oil Table Spoon and Beyond. Molecules, 27(8), 2566. https://doi.org/10.3390/molecules27082566












