Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties
Abstract
1. Introduction
2. Results
2.1. Optimization of the Production of Chlorella vulgaris Hydrolysates
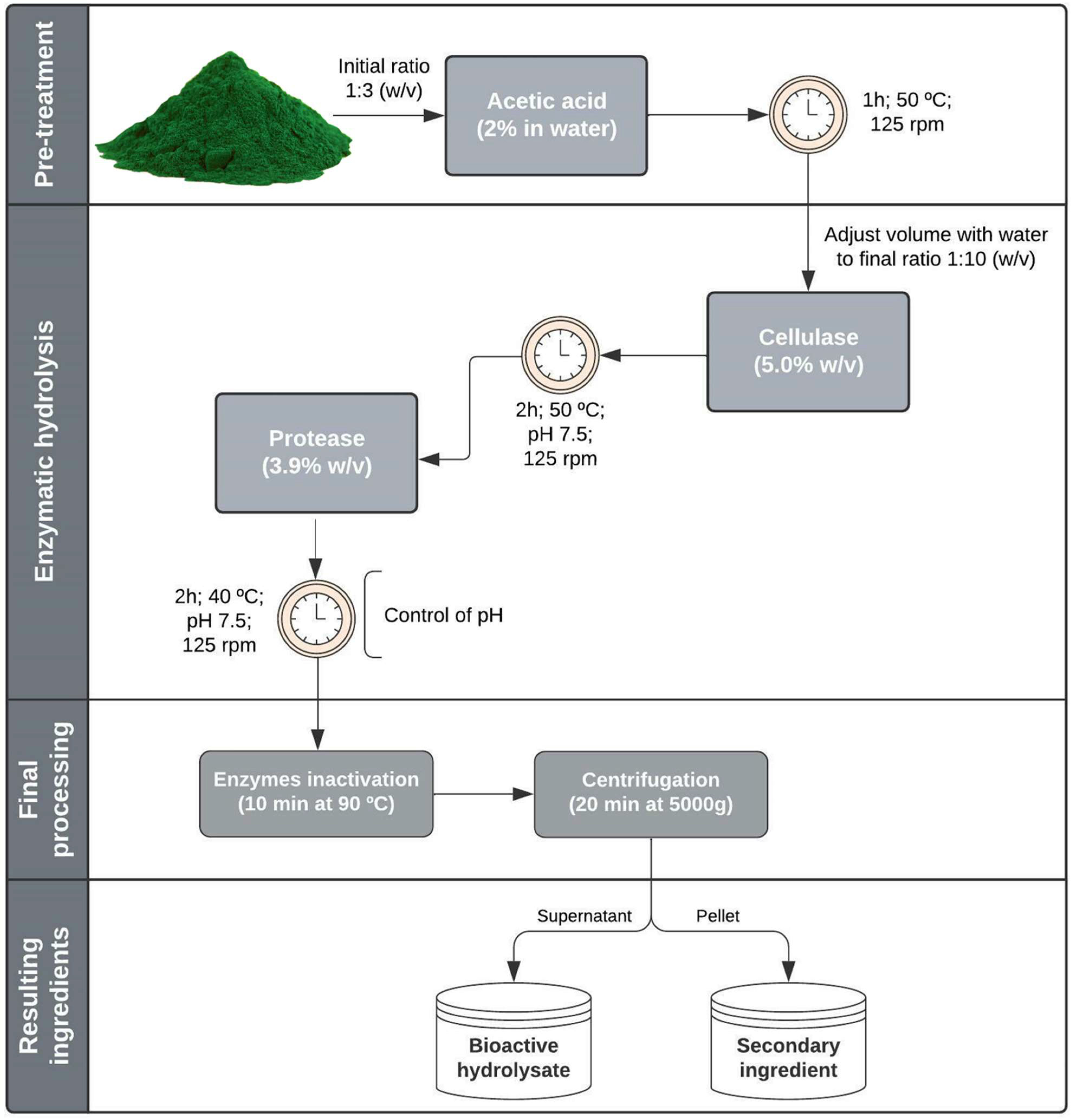
2.1.1. Acid Pretreatment and Enzymatic Hydrolysis
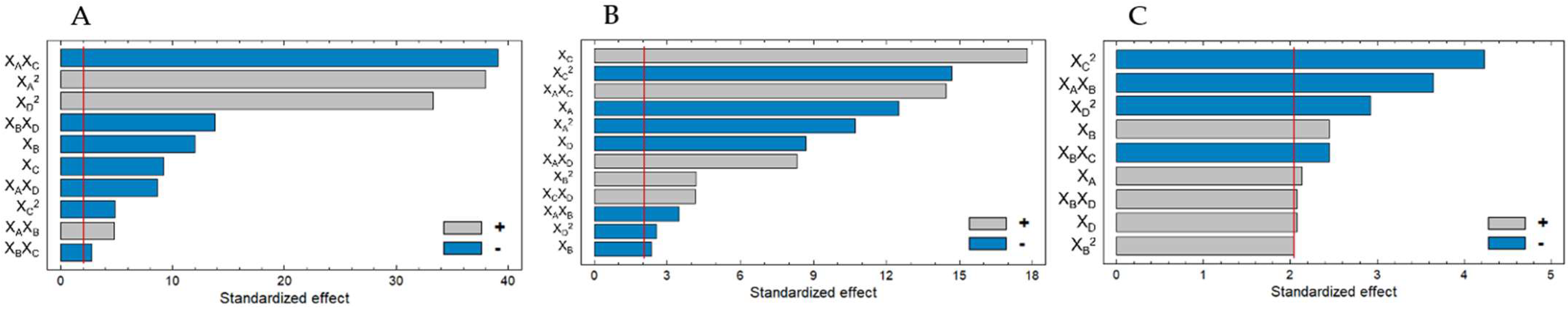
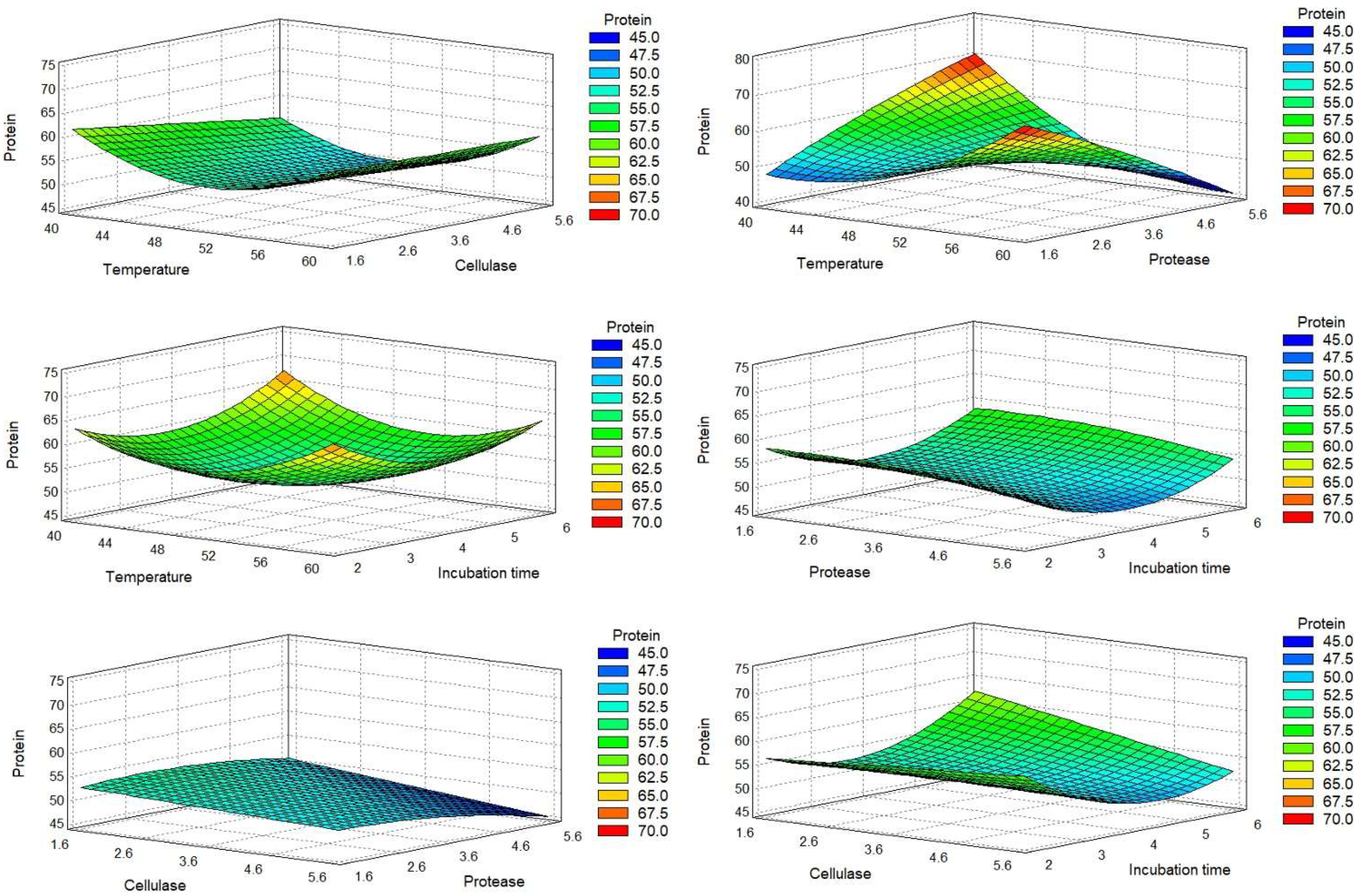
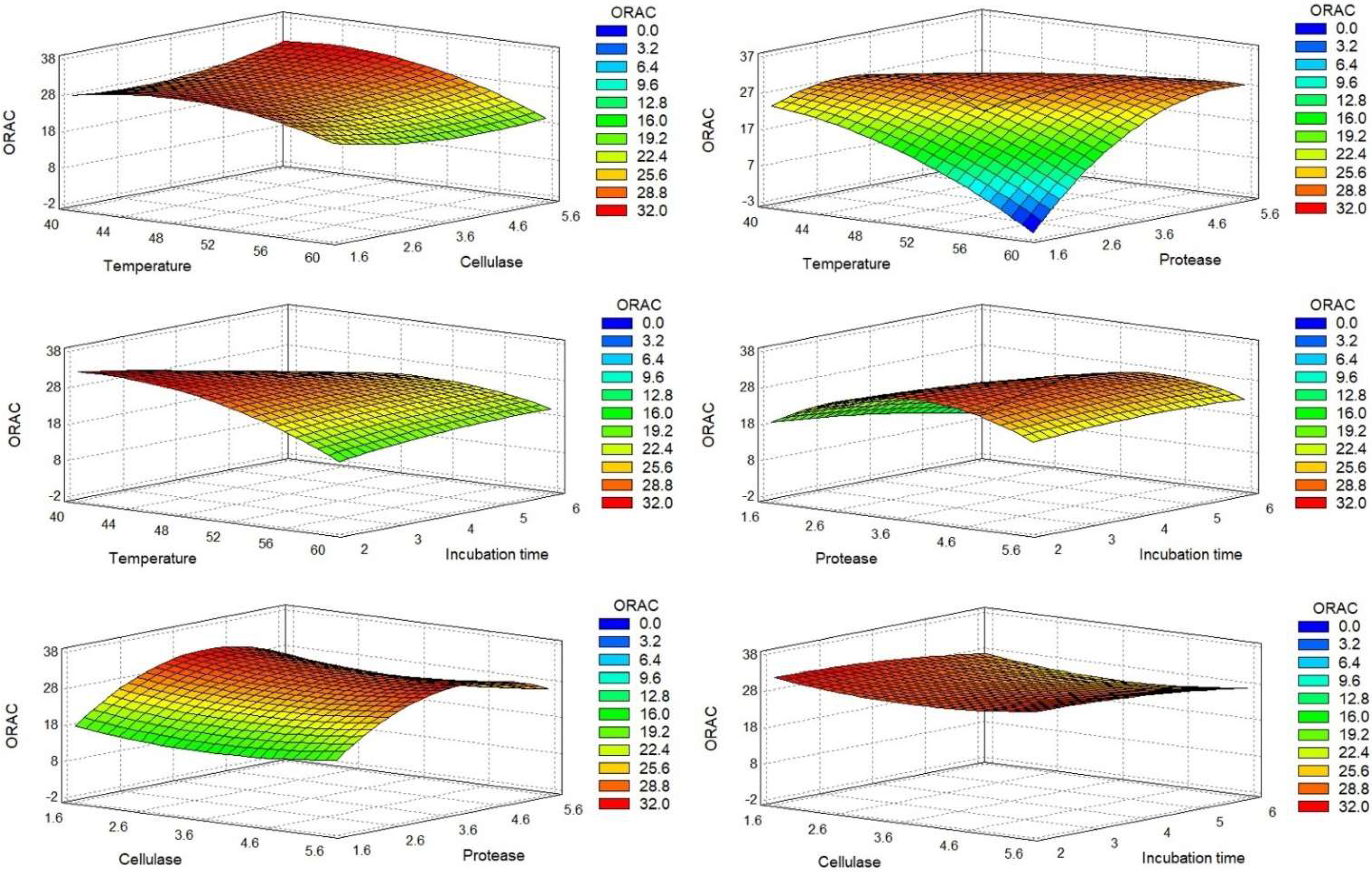
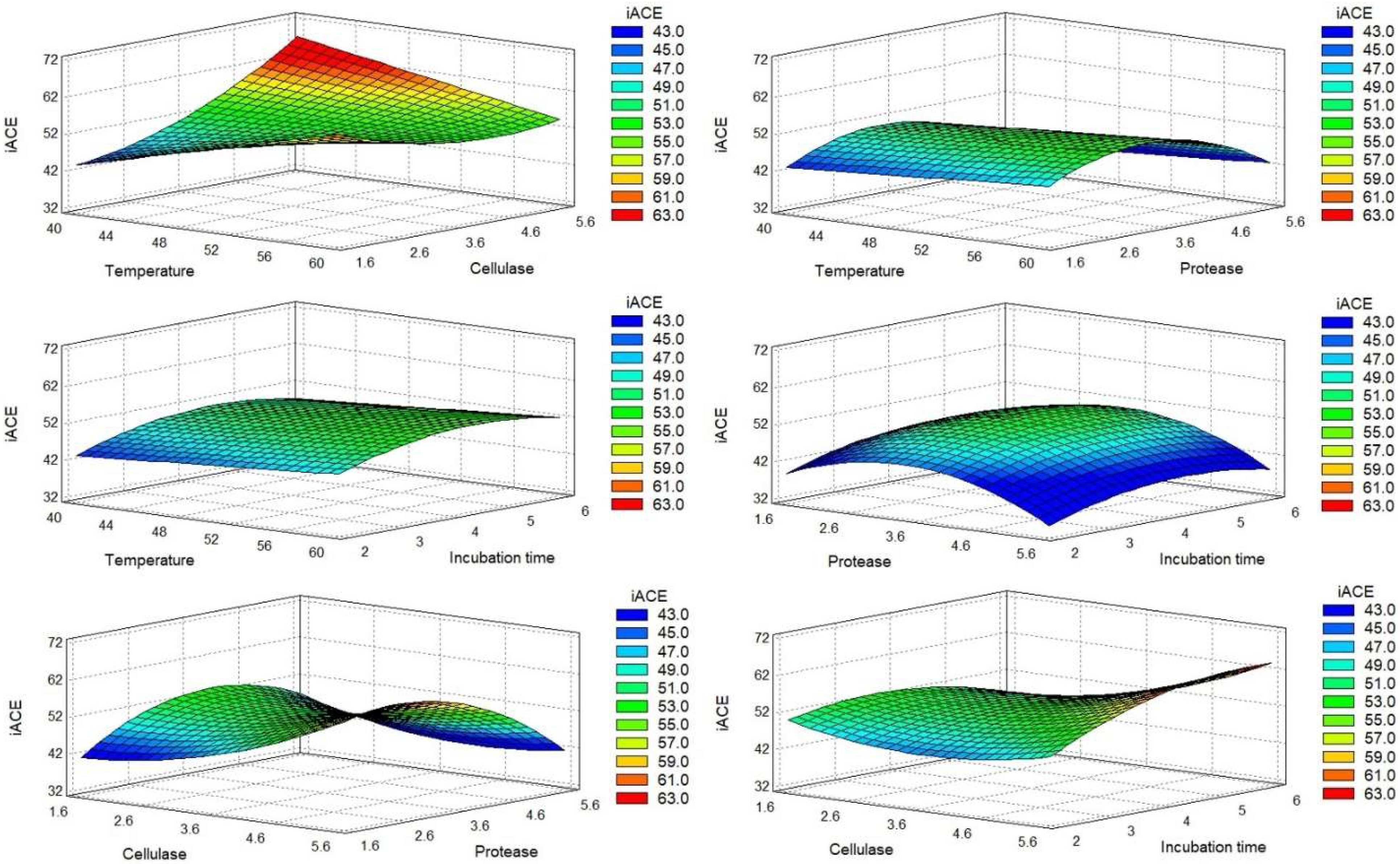
2.1.2. Optimal Conditions
0.074295XA2 + 0.0659997XAXB − 0.660452 XAXC − 0.0929XAXD − 0.201601XBXC −
0.824626XBXD − 0.339506XC2 + 1.54507XD2.
0.04632XA2 − 0.09945XAXB + 0.525791XAXC + 0.19837XAXD + 0.634165XB2 −
2.32134XC2 + 0.595499XCXD − 0.269216XD2.
0.38445XAXB + 1.07788XB2 − 1.54665XBXC + 1.09725XBXD − 2.23165XC2 −
1.06835XD2.
2.2. Scaled-Up Hydrolysates Bioactivities
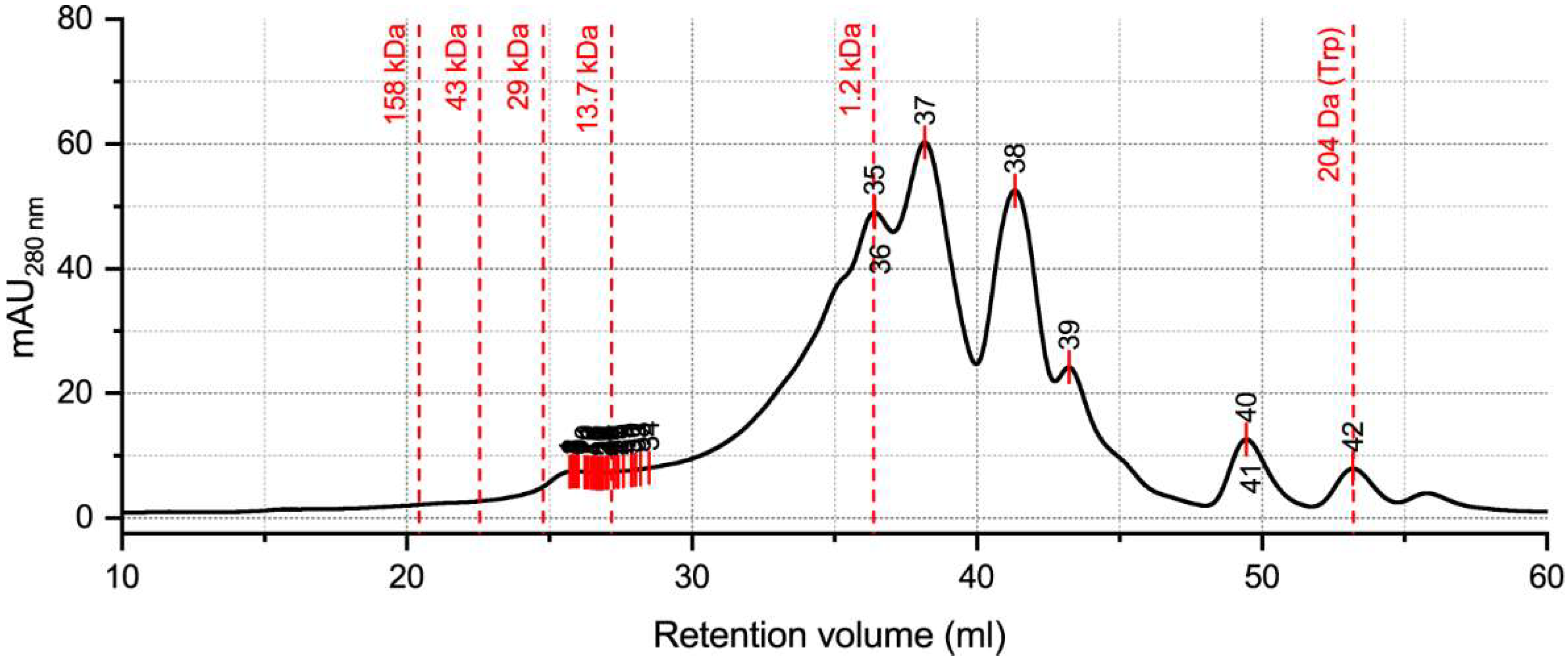
2.3. Hydrolysate Protein/Peptide Profile
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Hydrolysis Procedures
4.3. Experimental Design
Statistical Analysis and Statistical Model
βB,CXBXC + βB,DXBXD + βC,DXCXD + βA,AXA2 + βB,BXB2 + βC,CXC2 + βD,DXD2 + ε,
4.4. Protein Quantification
4.5. Analysis of Antioxidant Activity
4.6. Analysis of the Antihypertensive Potential
4.7. Analysis of the Antidiabetic Potential
4.8. Analysis by Size Exclusion Chromatography
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.M. Food and high value products from microalgae: Market opportunities and challenges. In Microalgae Biotechnology for Food, Health and High Value Products; Springer: Singapore, 2020; pp. 3–27. ISBN 9789811501692. [Google Scholar]
- Nova, P.; Martins, A.P.; Teixeira, C.; Abreu, H.; Silva, J.G.; Silva, A.M.; Freitas, A.C.; Gomes, A.M. Foods with microalgae and seaweeds fostering consumers health: A review on scientific and market innovations. J. Appl. Phycol. 2020, 32, 1789–1802. [Google Scholar] [CrossRef]
- Van der Voort, M.P.J.; Vulsteke, E.; de Visser, C.L.M. Macro-Economics of Algae Products: Output WP2A7.02; EnAlgae: Swansea, UK, 2015. [Google Scholar]
- Blockchain Market Worth $67.4 Billion by 2026—Report by MarketsandMarkets™. Available online: https://www.marketsandmarkets.com/PressReleases/algae-product.asp (accessed on 9 February 2022).
- De Freitas Coêlho, D.; Tundisi, L.L.; Cerqueira, K.S.; da Silva Rodrigues, J.R.; Mazzola, P.G.; Tambourgi, E.B.; de Souza, R.R. Microalgae: Cultivation aspects and bioactive compounds. Braz. Arch. Biol. Technol. 2019, 62, 19180343. [Google Scholar] [CrossRef]
- Enyidi, U.D. Chlorella vulgaris as Protein Source in the Diets of African Catfish Clarias gariepinus. Fishes 2017, 2, 17. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef]
- Blumreisinger, M.; Meindl, D.; Loos, E. Cell wall composition of chlorococcal algae. Phytochemistry 1983, 22, 1603–1604. [Google Scholar] [CrossRef]
- Callejo-López, J.A.; Ramírez, M.; Bolívar, J.; Cantero, D. Main variables affecting a chemical-enzymatic method to obtain protein and amino acids from resistant microalgae. J. Chem. 2019, 2019, 1390463. [Google Scholar] [CrossRef]
- Kose, A.; Ozen, M.O.; Elibol, M.; Oncel, S.S. Investigation of in vitro digestibility of dietary microalga Chlorella vulgaris and cyanobacterium Spirulina platensis as a nutritional supplement. 3 Biotech 2017, 7, 170. [Google Scholar] [CrossRef]
- Riordan, J.F. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003, 4, 225. [Google Scholar] [CrossRef][Green Version]
- Samarakoon, K.W.; O-Nam, K.; Ko, J.Y.; Lee, J.H.; Kang, M.C.; Kim, D.; Lee, J.B.; Lee, J.S.; Jeon, Y.J. Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochloropsis oculata) protein hydrolysate. J. Appl. Phycol. 2013, 25, 1595–1606. [Google Scholar] [CrossRef]
- Cao, D.; Lv, X.; Xu, X.; Yu, H.; Sun, X.; Xu, N. Purification and identification of a novel ACE inhibitory peptide from marine alga Gracilariopsis lemaneiformis protein hydrolysate. Eur. Food Res. Technol. 2017, 243, 1829–1837. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Elsner, P.; Fluhr, J.W.; Gehring, W.; Kerscher, M.J.; Krutmann, J.; Lademann, J.; Makrantonaki, E.; Wilhelm, K.-P.; Zouboulis, C.C. Anti-Aging Data and Support Claims—Consensus Statement. JDDG J. Dtsch. Dermatol. Ges. 2011, 9, S1–S32. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Suich, R.; Derringer, G.C. Is the regression equation adequate?—A further note. Technometrics 1980, 22, 125–126. [Google Scholar] [CrossRef]
- Safi, C.; Frances, C.; Ursu, A.V.; Laroche, C.; Pouzet, C.; Vaca-Garcia, C.; Pontalier, P.Y. Understanding the effect of cell disruption methods on the diffusion of Chlorella vulgaris proteins and pigments in the aqueous phase. Algal Res. 2015, 8, 61–68. [Google Scholar] [CrossRef]
- Office of Regulatory Affairs Compliance Policy Guides—CPG Sec. 562.100 Acetic Acid—Use in Foods—Labeling of Foods in Which Used; Food and Drug Administration: Silver Spring, MD, USA, 1989.
- Phwan, C.K.; Chew, K.W.; Sebayang, A.H.; Ong, H.C.; Ling, T.C.; Malek, M.A.; Ho, Y.C.; Show, P.L. Effects of acids pre-treatment on the microbial fermentation process for bioethanol production from microalgae. Biotechnol. Biofuels 2019, 12, 191. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Ursu, A.V.; Laroche, C.; Zebib, B.; Pontalier, P.Y.; Vaca-Garcia, C. Release of hydro-soluble microalgal proteins using mechanical and chemical treatments. Algal Res. 2014, 3, 55–60. [Google Scholar] [CrossRef]
- Phong, W.N.; Show, P.L.; Le, C.F.; Tao, Y.; Chang, J.S.; Ling, T.C. Improving cell disruption efficiency to facilitate protein release from microalgae using chemical and mechanical integrated method. Biochem. Eng. J. 2018, 135, 83–90. [Google Scholar] [CrossRef]
- Cunha, S.A.; de Castro, R.; Coscueta, E.R.; Pintado, M. Hydrolysate from mussel Mytilus galloprovincialis meat: Enzymatic hydrolysis, optimization and bioactive properties. Molecules 2021, 26, 5228. [Google Scholar] [CrossRef] [PubMed]
- Gerde, J.A.; Montalbo-Lomboy, M.; Yao, L.; Grewell, D.; Wang, T. Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour. Technol. 2012, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.; Borges, S.; Pintado, M. Enzymatic hydrolysis of insect: Alphitobius diaperinus towards the development of bioactive peptide hydrolysates. Food Funct. 2020, 11, 3539–3548. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Munekata, P.; Franco, D.; Carballo, J.; Barba, F.; Lorenzo, J. Antioxidant Potential of Extracts Obtained from Macro- (Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata) and Micro-Algae (Chlorella vulgaris and Spirulina platensis) Assisted by Ultrasound. Medicines 2018, 5, 33. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Amorim, M.M.; Voss, G.B.; Nerli, B.B.; Picó, G.A.; Pintado, M.E. Bioactive properties of peptides obtained from Argentinian defatted soy flour protein by Corolase PP hydrolysis. Food Chem. 2016, 198, 36–44. [Google Scholar] [CrossRef]
- Nova, P.; Pimenta-Martins, A.; Laranjeira Silva, J.; Silva, A.M.; Gomes, A.M.; Freitas, A.C. Health benefits and bioavailability of marine resources components that contribute to health—What’s new? Crit. Rev. Food Sci. Nutr. 2020, 60, 3680–3692. [Google Scholar] [CrossRef]
- Li, Y.; Lammi, C.; Boschin, G.; Arnoldi, A.; Aiello, G. Recent Advances in Microalgae Peptides: Cardiovascular Health Benefits and Analysis. J. Agric. Food Chem. 2019, 67, 11825–11838. [Google Scholar] [CrossRef]
- Josse, R.G.; Chiasson, J.L.; Ryan, E.A.; Lau, D.C.W.; Ross, S.A.; Yale, J.F.; Leiter, L.A.; Maheux, P.; Tessier, D.; Wolever, T.M.S.; et al. Acarbose in the treatment of elderly patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2003, 59, 37–42. [Google Scholar] [CrossRef]
- Sangeetha, R. Independent and synergistic activity of the flavonoids of Gracilaria corticata as promising antidiabetic agents. Braz. J. Pharm. Sci. 2021, 56, e18766. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, F. Evaluation of the green alga Chlorella pyrenoidosa for management of diabetes. J. Food Drug Anal. 2020, 20, 28. [Google Scholar] [CrossRef]
- Sheih, I.C.; Fang, T.J.; Wu, T.K. Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Ko, S.C.; Kang, N.; Kim, E.A.; Kang, M.C.; Lee, S.H.; Kang, S.M.; Lee, J.B.; Jeon, B.T.; Kim, S.K.; Park, S.J.; et al. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012, 47, 2005–2011. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibitory activity, antioxidant properties, phenolic content and amino acid profiles of Fucus spiralis L. Protein hydrolysate fractions. Mar. Drugs 2017, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; Fitzgerald, R.J. Peptide identification from a: Porphyra dioica protein hydrolysate with antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.J.; Carrillo, O.V.; Almarales, Á.; Bermúdez, R.C.; Alonso, M.E.; Borges, L.; Quintana, M.M.; Fontaine, R.; Llauradó, G.; Hernández, M. Protein hydrolysates from the alga Chlorella vulgaris 87/1 with the potentialities in immunonutrition. Biotecnol. Appl. 2009, 26, 162–165. [Google Scholar]
- Sreesai, S.; Pakpain, P. Nutrient Recycling by Chlorella vulgaris from Septage Effluent of the Bangkok City, Thailand. ScienceAsia 2007, 33, 293–299. [Google Scholar] [CrossRef]
- Ng, J.Y.; Chua, M.L.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Chlorella vulgaris Extract as a Serum Replacement That Enhances Mammalian Cell Growth and Protein Expression. Front. Bioeng. Biotechnol. 2020, 8, 1068. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Reis, C.A.; Pintado, M. Phenylethyl isothiocyanate extracted from watercress by-products with aqueous micellar systems: Development and optimisation. Antioxidants 2020, 9, 698. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic soy protein hydrolysis: A tool for biofunctional food ingredient production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef]
- Borges, S.; Piccirillo, C.; Scalera, F.; Martins, R.; Rosa, A.; Couto, J.A.; Almeida, A.; Pintado, M. Valorization of porcine by-products: A combined process for protein hydrolysates and hydroxyapatite production. Bioresour. Bioprocess. 2022, 9, 30. [Google Scholar] [CrossRef]
| Nutrients | Content (g/100 g) |
| Protein | 52.2 |
| Fat | 7.9 |
| Carbohydrates | 10.9 |
| Dietary fiber | 15.5 |
| Ash | 11.1 |
| Moisture | 2.4 |
| Pigments | Content (mg/100 g) |
| Chlorophyll | 1533 |
| Total carotenoids | 258 |
| Run | Factors | Response 1 | |||||
|---|---|---|---|---|---|---|---|
| Hydrolysis Temperature (°C) (XA) | % Cellulase (XB) | % Protease (XC) | Hydrolysis Time (h) (XD) | Protein Content (%) | ORAC (µmol TE/mL) | iACE (%) 2 | |
| 1 | 50 | 1.67 | 3.33 | 2 | 56.58 ± 0.001 | 31.95 ± 0.03 | 58.20 ± 0.20 |
| 2 | 40 | 3.33 | 1.67 | 4 | 48.42 ± 0.02 | 28.09 ± 0.05 | 45.85 ± 0.05 |
| 3 | 50 | 3.33 | 3.33 | 4 | 52.48 ± 0.16 | 24.43 ± 0.01 | 46.28 ± 0.18 |
| 4 | 50 | 5.00 | 3.33 | 2 | 60.31 ± 0.05 | 30.12 ± 0.003 | 58.08 ± 0.10 |
| 5 | 40 | 5.00 | 3.33 | 4 | 55.19 ± 0.32 | 29.44 ± 0.03 | 56.99 ± 0.01 |
| 6 | 50 | 3.33 | 1.67 | 6 | 84.46 ± 0.17 | 9.24 ± 0.004 | 51.29 ± 0.01 |
| 7 | 50 | 5.00 | 3.33 | 6 | 53.92 ± 0.11 | 28.38 ± 0.002 | 60.71 ± 0.01 |
| 8 | 60 | 3.33 | 1.67 | 4 | 70.44 ± 0.25 | 26.45 ± 0.01 | 44.46 ± 10.71 |
| 9 | 40 | 3.33 | 5.00 | 4 | 54.67 ± 0.08 | 19.04 ± 0.003 | 55.94 ± 0.01 |
| 10 | 60 | 1.67 | 3.33 | 4 | 61.64 ± 0.09 | 24.84 ± 0.04 | 62.21 ± 0.01 |
| 11 | 50 | 3.33 | 3.33 | 4 | 55.41 ± 0.02 | 31.41 ± 0.02 | 63.59 ± 0.01 |
| 12 | 60 | 5.00 | 3.33 | 4 | 58.56 ± 0.29 | 19.40 ± 0.01 | 52.74 ± 0.06 |
| 13 | 50 | 3.33 | 1.67 | 2 | 57.24 ± 0.10 | 20.81 ± 0.02 | 42.85 ± 0.01 |
| 14 | 50 | 5.00 | 1.67 | 4 | 51.68 ± 0.04 | 14.38 ± 0.02 | 50.94 ± 0.01 |
| 15 | 40 | 3.33 | 3.33 | 6 | 67.57 ± 0.19 | 17.71 ± 0.07 | 48.17 ± 2.44 |
| 16 | 50 | 3.33 | 3.33 | 4 | 50.23 ± 0.03 | 28.40 ± 0.003 | 41.22 ± 0.01 |
| 17 | 40 | 3.33 | 3.33 | 2 | 63.89 ± 0.01 | 29.34 ± 0.02 | 35.17 ± 0.01 |
| 18 | 50 | 3.33 | 5.00 | 6 | 56.00 ± 0.21 | 24.81 ± 0.02 | 32.80 ± 0.01 |
| 19 | 40 | 1.67 | 3.33 | 4 | 65.61 ± 4.22 | 28.25 ± 0.05 | 40.84 ± 0.01 |
| 20 | 50 | 3.33 | 5.00 | 2 | 54.94 ± 0.02 | 28.45 ± 0.01 | 32.64 ± 0.01 |
| 21 | 60 | 3.33 | 5.00 | 4 | 64.37 ± 0.05 | 24.90 ± 0.10 | 56.61 ± 0.01 |
| 22 | 50 | 1.67 | 3.33 | 6 | 61.19 ± 0.18 | 27.53 ± 0.02 | 46.20 ± 0.20 |
| 23 | 60 | 3.33 | 3.33 | 2 | 67.06 ± 0.54 | 17.10 ± 0.10 | 39.95 ± 0.05 |
| 24 | 50 | 1.67 | 5.00 | 4 | 51.56 ± 0.19 | 30.41 ± 0.05 | 48.26 ± 0.04 |
| 25 | 60 | 3.33 | 3.33 | 6 | 60.53 ± 2.63 | 21.34 ± 0.08 | 53.00 ± 0.01 |
| 26 | 50 | 5.00 | 5.00 | 4 | 47.62 ± 0.17 | 27.66 ± 0.06 | 44.03 ± 0.01 |
| 27 | 50 | 1.67 | 1.67 | 4 | 53.38 ± 0.13 | 15.73 ± 0.02 | 38.00 ± 0.004 |
| Model | Sum of Squares | DF | Mean Square | F-Value |
|---|---|---|---|---|
| XB (Cellulase) | 45.7915 | 1 | 45.7915 | 144.25 |
| XC (Protease) | 26.9145 | 1 | 26.9145 | 84.78 |
| XA2 | 458.333 | 1 | 458.333 | 1443.81 |
| XAXB | 7.25992 | 1 | 7.25992 | 22.87 |
| XAXC | 484.661 | 1 | 484.661 | 1526.75 |
| XAXD | 23.8453 | 1 | 23.8453 | 75.12 |
| XBXC | 2.5088 | 1 | 2.5088 | 7.90 |
| XBXD | 60.4451 | 1 | 60.4451 | 190.41 |
| XC2 | 7.49445 | 1 | 7.49445 | 23.61 |
| XD2 | 352.242 | 1 | 352.242 | 1109.61 |
| R2 = 98.50, Adj-R2 = 98.04, CV = 0.56 | ||||
| Model | Sum of Squares | DF | Mean Square | F-Value |
|---|---|---|---|---|
| XA (Temperature) | 285.086 | 1 | 285.086 | 156.48 |
| XB (Cellulase) | 9.98998 | 1 | 9.98998 | 5.48 |
| XC (Protease) | 576.474 | 1 | 576.474 | 316.41 |
| XD (Time) | 137.904 | 1 | 137.904 | 75.69 |
| XA2 | 209.962 | 1 | 209.962 | 115.24 |
| XAXB | 21.9784 | 1 | 21.9784 | 12.06 |
| XAXC | 380.681 | 1 | 380.681 | 208.95 |
| XAXD | 125.928 | 1 | 125.928 | 69.12 |
| XB2 | 31.8683 | 1 | 31.8683 | 17.49 |
| XC2 | 393.421 | 1 | 393.421 | 215.94 |
| XCXD | 31.5217 | 1 | 31.5217 | 17.30 |
| XD2 | 11.9879 | 1 | 11.9879 | 6.58 |
| R2 = 88.66, Adj-R2 = 85.08, CV = 1.35 | ||||
| Model | Sum of Squares | DF | Mean Square | F-Value |
|---|---|---|---|---|
| XA (Temperature) | 112.839 | 1 | 112.839 | 4.57 |
| XB (Cellulase) | 147.757 | 1 | 147.757 | 5.98 |
| XD (Time) | 106.64 | 1 | 106.64 | 4.31 |
| XA XB | 328.448 | 1 | 328.448 | 13.29 |
| XB2 | 103.358 | 1 | 103.358 | 4.18 |
| XB XC | 147.662 | 1 | 147.662 | 5.97 |
| XB XD | 107.018 | 1 | 107.018 | 4.33 |
| XC2 | 443.049 | 1 | 443.049 | 17.93 |
| XD2 | 210.548 | 1 | 210.548 | 8.52 |
| R2 = 42.86, Adj-R2 = 30.90, CV = 4.97 | ||||
| Factors | Response | Multiple Responses | ||
|---|---|---|---|---|
| Protein | ORAC | iACE | ||
| Temperature (°C) | 59.9 | 40.0 | 40.0 | 40.0 |
| Cellulase (%) | 5.0 | 5.0 | 5.0 | 5.0 |
| Protease (%) | 1.7 | 2.9 | 2.7 | 3.9 |
| Time (h) | 2.0 | 2.0 | 5.3 | 2.3 |
| Evaluated Characteristics | Obtained Results |
|---|---|
| Bioactive hydrolysate yield (%) | 61 ± 0.5 |
| Protein (%) | 45 ± 1.7 |
| ORAC (µmol TE/g hydrolysate) | 463 ± 39.9 |
| ORAC (µmol TE/g protein) | 1035 ± 68.7 |
| iACE (IC50 µg of protein/mL) | 286 ± 55.0 |
| α-Glucosidase inhibition (%) (30 mg hydrolysate/mL) | 31 ± 3.9 |
| Specie | Hydrolysate/Extract Production | Antioxidant Activity | iACE (IC50) | α-Glucosidase Inhibition | Ref. |
|---|---|---|---|---|---|
| Bifurcaria bifurcata | Ultrasound-assisted extraction using water/ethanol | 556.20 µmol TE/g DW | [28] | ||
| Chlorella vulgaris | Ultrasound-assisted extraction using water/ethanol | 31.21 µmol TE/g DW | [28] | ||
| Enzymatic hydrolysis (pepsin) | 29.6 µM | [35] | |||
| Chlorella ellipsoidea | Enzymatic hydrolysis (alcalase) | 128.4 µM | [36] | ||
| Fucus spiralis | Enzymatic hydrolysis (cellulase and bromelain) | 0.5–2.0 mg/mL | [37] | ||
| Chlorella pyrenoidosa | 68.28% (1 mg/mL) | [34] | |||
| Porphyra dioica | Alcalase and Flavourzyme | 3.14 µM TE/µM peptide | 163.6 µM | [38] |
| Factors | Levels | ||
|---|---|---|---|
| Low (−1) | Central (0) | High (+1) | |
| Temperature (XA) | 1.67 | 3.33 | 5.00 |
| % Cellulase (XB) | 1.67 | 3.33 | 5.00 |
| % Protease (XC) | 40 | 50 | 60 |
| Time (h) (XD) | 2 | 4 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties. Molecules 2022, 27, 2505. https://doi.org/10.3390/molecules27082505
Cunha SA, Coscueta ER, Nova P, Silva JL, Pintado MM. Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties. Molecules. 2022; 27(8):2505. https://doi.org/10.3390/molecules27082505
Chicago/Turabian StyleCunha, Sara A., Ezequiel R. Coscueta, Paulo Nova, Joana Laranjeira Silva, and Maria Manuela Pintado. 2022. "Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties" Molecules 27, no. 8: 2505. https://doi.org/10.3390/molecules27082505
APA StyleCunha, S. A., Coscueta, E. R., Nova, P., Silva, J. L., & Pintado, M. M. (2022). Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties. Molecules, 27(8), 2505. https://doi.org/10.3390/molecules27082505