Effect of the Integrated Addition of a Red Tara Pods (Caesalpinia spinosa) Extract and NaCl over the Neo-Formed Contaminants Content and Sensory Properties of Crackers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Analytic Reagents
2.2. Red Tara Pod Extract
2.3. Preparation of Crackers
2.4. Chemical Analysis
2.4.1. Total Polyphenol Content (TPC)
2.4.2. Antioxidant Capacity (AOC)
2.4.3. Determination of the Acrylamide (AA) Content
2.4.4. Determination of the 5-Hydroxymethylfurrfural (HMF) Content
2.5. Sensory Evaluation
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Red Tara Pods Extract
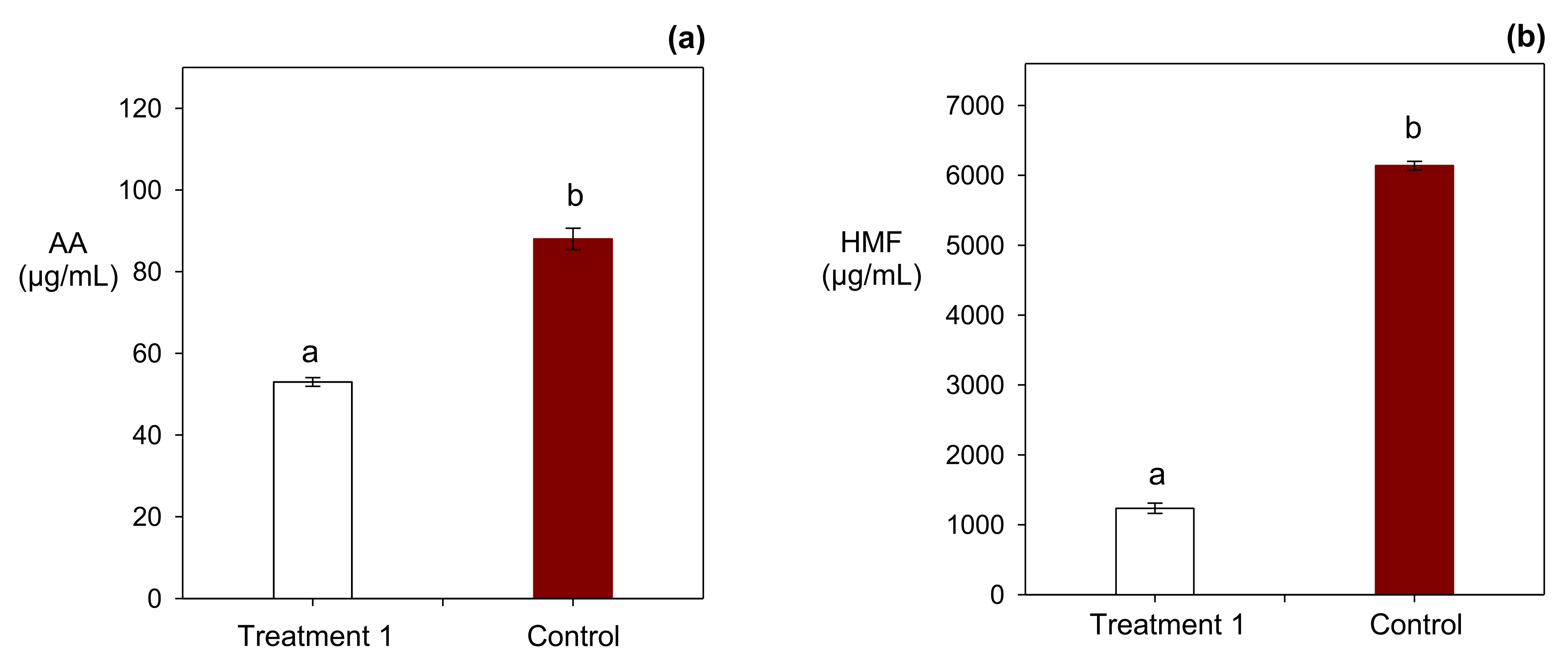
3.2. Effect of the Integrated Addition of the Red Tara Pods Extract and NaCl on the Acrylamide and HMF Content of Crackers
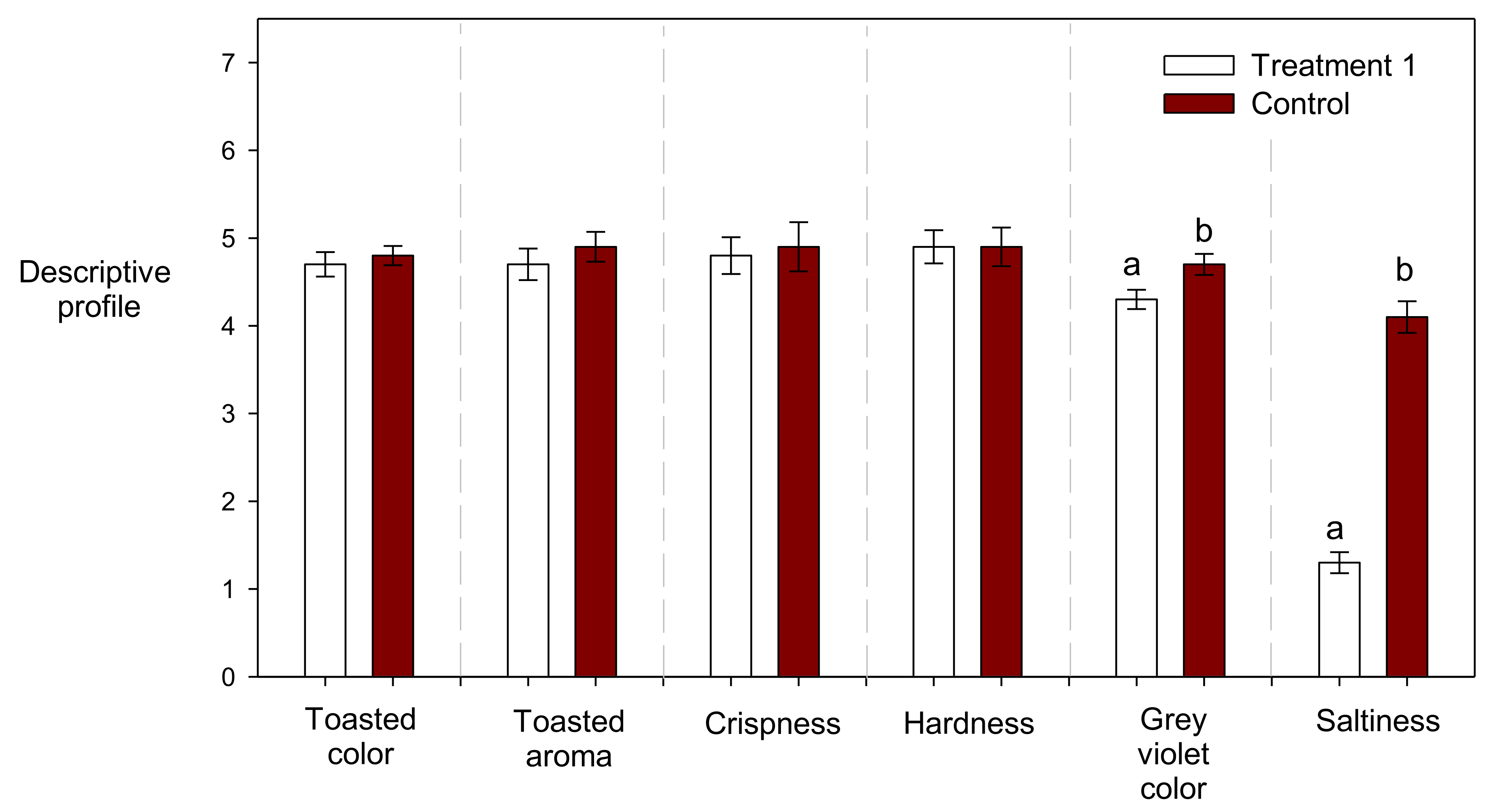
3.3. Effect of the Addition of the Red Tara Pods Extract and NaCl on the Descriptive Profile of Crackers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- La Tercera (LT). Chile Ocupa el Cuarto Lugar en Ranking Internacional de Consumo de Calorías. Available online: http://www.latercera.com/noticia/tendencias/2015/02/ (accessed on 7 May 2021).
- Yaylayan, V.A. Classification of the Maillard reaction: A conceptual approach. Trends Food Sci. Technol. 1997, 8, 13–18. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, B.; Ran, R.; Liu, Y.; Chen, H.; Kai, G.; Shi, J. Risk assessment, formation, and mitigation of dietary acrylamide: Current status and future prospects. Food Chem. Toxicol. 2014, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Der Fels-Klerx, H.J.; Capuano, E.; Nguyen, H.T.; Ataç Mogol, B.; Kocadaǧli, T.; Göncüoǧlu Taş, N.; Hamzalioǧlu, A.; Van Boekel, M.A.J.S.; Gökmen, V. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: NaCl and temperature-time profile effects and kinetics. Food Res. Int. 2014, 57, 210–217. [Google Scholar] [CrossRef]
- Pedreschi, F.; Saavedra, I.; Bunger, A.; Zuñiga, R.N.; Pedreschi, R.; Chirinos, R.; Campos, D.; Mariotti-Celis, M.S. Tara pod (Caesalpinia spinosa) extract mitigates neo-contaminant formation in Chilean bread preserving their sensory attributes. LWT 2018, 95, 116–122. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Ameur, L.A.; Trystram, G.; Birlouez-Aragon, I. Accumulation of 5-hydroxymethyl-2-furfural in cookies during the backing process: Validation of an extraction method. Food Chem. 2006, 98, 790–796. [Google Scholar] [CrossRef]
- Birlouez-Aragon, I.; Morales, F.; Fogliano, V.; Pain, J.P. Les enjeux santé et technologiques d’une meilleure maîtrise des contaminants néoformés par les industriels du secteur alimentaire. Pathol. Biol. 2010, 58, 232–238. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mariotti, M.S.; Granby, K. Current issues in dietary acrylamide: Formation, mitigation and risk assessment. J. Sci. Food Agric. 2014, 94, 9–20. [Google Scholar] [CrossRef]
- Mariotti, S.; Pedreschi, F.; Carrasco, J.A.; Granby, K. Patented techniques for acrylamide mitigation in high-temperature processed foods. Recent Pat. Food. Nutr. Agric. 2011, 3, 158–171. [Google Scholar] [CrossRef]
- Barrios-Rodríguez, Y.F.; Pedreschi, F.; Rosowski, J.; Gómez, J.P.; Figari, N.; Castillo, O.; Mariotti Celis, M.S. Is the dietary acrylamide exposure in Chile a public health problem? Food Addit. Contam. Part A 2021, 38, 1126–1135. [Google Scholar] [CrossRef]
- Zhu, F.; Cai, Y.Z.; Ke, J.; Corke, H. Dietary plant materials reduce acrylamide formation in cookie and starch-based model systems. J. Sci. Food Agric. 2011, 91, 2477–2483. [Google Scholar] [CrossRef]
- Jin, C.; Wu, X.; Zhang, Y. Relationship between antioxidants and acrylamide formation: A review. Food Res. Int. 2013, 51, 611–620. [Google Scholar] [CrossRef]
- Ou, S.; Shi, J.; Huang, C.; Zhang, G.; Teng, J.; Jiang, Y.; Yang, B. Effect of antioxidants on elimination and formation of acrylamide in model reaction systems. J. Hazard. Mater. 2010, 182, 863–868. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Zhang, Y. New Research Developments on Acrylamide: Analytical Chemistry, Formation Mechanism, and Mitigation Recipes. Chem. Rev. 2009, 109, 4375–4397. [Google Scholar] [CrossRef]
- Kolek, E.; Šimko, P.; Simon, P. Inhibition of acrylamide formation in asparagine/d-glucose model system by NaCl addition. Eur. Food Res. Technol. 2006, 224, 283–284. [Google Scholar] [CrossRef]
- Pedreschi, F.; Bustos, O.; Mery, D.; Moyano, P.; Kaack, K.; Granby, K. Color kinetics and acrylamide formation in NaCl soaked potato chips. J. Food Eng. 2007, 79, 989–997. [Google Scholar] [CrossRef]
- Gökmen, V.; Şenyuva, H.Z. Acrylamide formation is prevented by divalent cations during the Maillard reaction. Food Chem. 2007, 103, 196–203. [Google Scholar] [CrossRef]
- Levine, R.A.; Ryan, S.M. Determining the effect of calcium cations on acrylamide formation in cooked wheat products using a model system. J. Agric. Food Chem. 2009, 57, 6823–6829. [Google Scholar] [CrossRef]
- Noor Aziah, A.; Komathi, C.A. Acceptability attributes of crackers made from different types of composite flour. Int. Food Res. J. 2009, 16, 479–482. [Google Scholar]
- Congreso Nacional de Chile. Ley sobre composición nutricional de los alimentos y su publicidad. Available online: https://www.bcn.cl/leychile/navegar?idNorma=1041570 (accessed on 20 August 2021).
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- ISO 8586:2012. Sensory analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. Available online: https://www.iso.org/standard/45352.html (accessed on 7 August 2021).
- ISO 11132:2012 Sensory Analysis—Methodology—Guidelines for Monitoring the Performance of a Quantitative Sensory Panel. Available online: https://www.iso.org/standard/50124.html (accessed on 10 August 2021).
- Chambi, F.; Chirinos, R.; Pedreschi, R.; Betalleluz-Pallardel, I.; Debaste, F.; Campos, D. Antioxidant potential of hydrolyzed polyphenolic extracts from tara (Caesalpinia spinosa) pods. Ind. Crops Prod. 2013, 47, 168–175. [Google Scholar] [CrossRef]
- Avilés, R.; Carrión, J.; Huamán, J.; Bravo, M.; Rivera, D.; Rojas, N. Actividad antioxidante, polifenoles totales y contenido de taninos de extractos de tara (Caesalpinia spinosa). Rev. Peru. Química Ing. Química 2010, 13, 5–11. [Google Scholar]
- Nuñez, W.; Quispe, R. Evaluación antioxidante y antienzimática in vitro y antiinflamatoria in vivo del extracto hidroalcohólico de la Caesalpinia spinosa “tara”. Tesis de pregrado, Universidad Nacional Mayor de San Marcos, Lima, Perú, 2015. [Google Scholar]
- Doroteo, V.H.; Terry, C.; Díaz, C. Compuestos fenólicos y actividades antioxidante, antielastasa, anticolagenasa y fotoprotectora in vitro de myrciaria dubia (camu camu) y Caesalpinia spinosa (tara). Rev. Soc. Química Perú 2012, 78, 254–263. [Google Scholar]
- Bravo, N. Estudio de la Extracción de Taninos a partir de la Tara (Caesalpinia Spinosa (Molina) Kuntze) aplicando los métodos Taguchi y Superficie de Respuesta. Tesis postgrado, Universidad Nacional Agraria La Molina, Lima, Perú, 2010. [Google Scholar]
- López, S.A.; Oré, R.; Miranda, V.C.; Trabucco, J.; Orihuela, D.; Linares, G.J.; Villafani, B.Y.; Ríos, S.; Siles, M. Capacidad antioxidante de poblaciones silvestres de “tara” (Caesalpinia spinosa) de las localidades de Picoy y Santa Fe (Provincia de Tarma, departamento de Junín). Sci. Agropecu. 2011, 2, 25–29. [Google Scholar] [CrossRef][Green Version]
- Constantinou, C.; Koutsidis, G. Investigations on the effect of antioxidant type and concentration and model system matrix on acrylamide formation in model Maillard reaction systems. Food Chem. 2016, 197, 769–775. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, C. Relationship between Antioxidants and Acrylamide Formation. Food Res. Int. 2013, 51, 611–620. [Google Scholar]
- Kahkeshani, N.; Saeidnia, S.; Abdollahi, M. Role of antioxidants and phytochemicals on acrylamide mitigation from food and reducing its toxicity. J. Food Sci. Technol. 2015, 52, 3169–3186. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Chen, F.; Yuan, Y.; Zhu, Y.; Yan, H.; Hu, X. Role of plant polyphenols in acrylamide formation and elimination. Food Chem. 2015, 186, 46–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, C. Relationship between antioxidants and acrylamide formation. In Acrylamide in Food: Analysis, Content and Potential Health Effects; Gökmen, V., Ed.; Elsevier Inc.: San Diego, CA, USA, 2016; pp. 325–353. [Google Scholar]
- Kotsiou, K.; Tasioula-Margari, M.; Capuano, E.; Fogliano, V. Effect of standard phenolic compounds and olive oil phenolic extracts on acrylamide formation in an emulsion system. Food Chem. 2011, 124, 242–247. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Z.; Jiang, S.; Yu, M.; Huang, C.; Qiu, R.; Zou, Y.; Zhang, Q.; Ou, S.; Zhou, H.; et al. Chlorogenic acid increased acrylamide formation through promotion of HMF formation and 3-aminopropionamide deamination. J. Hazard. Mater. 2014, 268, 1–5. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Wu, G.; Zhang, H.; Wang, L.; Qian, H.; Qi, X. Reduction of 5-hydroxymethylfurfural formation by flavan-3-ols in Maillard reaction models and fried potato chips. J. Sci. Food Agric. 2018, 98, 5294–5301. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Różańska, M.; Piechowska, P.; Waśkiewicz, A.; Zawirska-Wojtasiak, R. Effects of polyphenols on volatile profile and acrylamide formation in a model wheat bread system. Food Chem. 2019, 297, 125008. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Garro Galvez, J.M.; Riedl, B.; Conner, A.H. Analytical Studies on Tara Tannins. Holzforschung 1997, 51, 235–243. [Google Scholar] [CrossRef]
- Claus, A.; Mongili, M.; Weisz, G.; Schieber, A.; Carle, R. Impact of formulation and technological factors on the acrylamide content of wheat bread and bread rolls. J. Cereal Sci. 2008, 47, 546–554. [Google Scholar] [CrossRef]
- Fiore, A.; Troise, A.D.; Ataç Mogol, B.; Roullier, V.; Gourdon, A.; El Mafadi Jian, S.; Hamzalioǧlu, B.A.; Gökmen, V.; Fogliano, V. Controlling the maillard reaction by reactant encapsulation: Sodium chloride in cookies. J. Agric. Food Chem. 2012, 60, 10808–10814. [Google Scholar] [CrossRef]
- Locas, C.P.; Yaylayan, V.A. Isotope labeling studies on the formation of 5-(hydroxymethyl)-2- furaldehyde (HMF) from sucrose by pyrolysis-GC/MS. J. Agric. Food Chem. 2008, 56, 6717–6723. [Google Scholar] [CrossRef]
- Morgado, C.; Bernardo, P.; Henriques, I.; Jesus, S.; Rego, A.; Delgado, I.; Coelho, I.; Castanheira, I.; Félix, N.; Fernandes, A. Effect of Rocha Pear Peel extracts added to wheat and rye bread formulations on acrylamide reduction and sensory quality Maintenance. In Proceedings of the International Congress on Engineering and Sustainability in the XXI Century, Faro, Portugal, 9–11 October 2019; Monteiro, J., Silva, A., Mortal, A., Aníbal, J., da Silva, M., Oliveira, M., Sousa, N., Eds.; Springer Nature: Cham, Switzerland, 2021. [Google Scholar]
| Ingredient | Quantity (g) |
|---|---|
| Flour | 100 |
| Salt | 0–2 * |
| Lard | 7 |
| Yeast | 3.5 |
| Water | 45 |
| Treatments | Red Tara Pod Extract (µg/mL) * | NaCl (g/100 g) ** |
|---|---|---|
| 1 | 2560 | 0.3 |
| 2 | 440 | 0.3 |
| 3 | 2560 | 1.7 |
| 4 | 440 | 1.7 |
| 5 | 1500 | 1 |
| 6 | 1500 | 1 |
| 7 | 1500 | 1 |
| Treatment | Polyphenolic Extract (µg/mL) * | NaCl (g/100 g) ** | HMF (µg/kg) *** | Acrylamide (µg/kg) *** | ||
|---|---|---|---|---|---|---|
| Mean | CV | Mean | CV | |||
| 1 | 2560 | 0.3 | 1236 a | 0.06 | 53.00 a | 0.02 |
| 2 | 440 | 0.3 | 2544 b | 0.05 | 72.33 b | 0.02 |
| 3 | 2560 | 1.7 | 4239 d | 0.01 | 50.33 a | 0.08 |
| 4 | 440 | 1.7 | 4184 d | 0.01 | 71.00 b | 0.05 |
| 5 | 1500 | 1 | 3902 c | 0.02 | 69.67 b | 0.02 |
| 6 | 1500 | 1 | 3928 c | 0.02 | 66.67 b | 0.02 |
| 7 | 1500 | 1 | 3920 c | 0.06 | 68.00 b | 0.01 |
| Treatments | Polyphenolic Extract (µg/mL) * | NaCl (g/100 g) ** | Toasted Color | Grey Violet Color | Toasted Aroma | Crispness | Hardness | Toasted Flavor | Saltiness |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2560 | 0.3 | 4.7 | 4.3 b | 4.7 | 4.8 | 4.9 | 4.9 | 1.3 a |
| 2 | 440 | 0.3 | 4.8 | 2.9 a | 4.7 | 4.8 | 4.8 | 4.9 | 1.4 a |
| 3 | 2560 | 1.7 | 4.9 | 4.8 c | 4.9 | 5.2 | 5.1 | 4.9 | 5.0 c |
| 4 | 440 | 1.7 | 4.9 | 2.9 a | 4.9 | 5.0 | 5.0 | 5.0 | 4.9 c |
| 5 | 1500 | 1 | 4.9 | 4.3 b | 4.7 | 4.9 | 4.8 | 4.9 | 4.2 b |
| 6 | 1500 | 1 | 4.9 | 4.2 b | 4.7 | 4.9 | 4.8 | 4.9 | 4.2 b |
| 7 | 1500 | 1 | 4.9 | 4.2 b | 4.7 | 4.8 | 4.9 | 4.9 | 4.1 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedreschi, F.; Matus, J.; Bunger, A.; Pedreschi, R.; Huamán-Castilla, N.L.; Mariotti-Celis, M.S. Effect of the Integrated Addition of a Red Tara Pods (Caesalpinia spinosa) Extract and NaCl over the Neo-Formed Contaminants Content and Sensory Properties of Crackers. Molecules 2022, 27, 1020. https://doi.org/10.3390/molecules27031020
Pedreschi F, Matus J, Bunger A, Pedreschi R, Huamán-Castilla NL, Mariotti-Celis MS. Effect of the Integrated Addition of a Red Tara Pods (Caesalpinia spinosa) Extract and NaCl over the Neo-Formed Contaminants Content and Sensory Properties of Crackers. Molecules. 2022; 27(3):1020. https://doi.org/10.3390/molecules27031020
Chicago/Turabian StylePedreschi, Franco, Joans Matus, Andrea Bunger, Romina Pedreschi, Nils Leander Huamán-Castilla, and María Salomé Mariotti-Celis. 2022. "Effect of the Integrated Addition of a Red Tara Pods (Caesalpinia spinosa) Extract and NaCl over the Neo-Formed Contaminants Content and Sensory Properties of Crackers" Molecules 27, no. 3: 1020. https://doi.org/10.3390/molecules27031020
APA StylePedreschi, F., Matus, J., Bunger, A., Pedreschi, R., Huamán-Castilla, N. L., & Mariotti-Celis, M. S. (2022). Effect of the Integrated Addition of a Red Tara Pods (Caesalpinia spinosa) Extract and NaCl over the Neo-Formed Contaminants Content and Sensory Properties of Crackers. Molecules, 27(3), 1020. https://doi.org/10.3390/molecules27031020