Beta-Amyloid (Aβ1-42) Increases the Expression of NKCC1 in the Mouse Hippocampus
Abstract
1. Introduction
2. Results
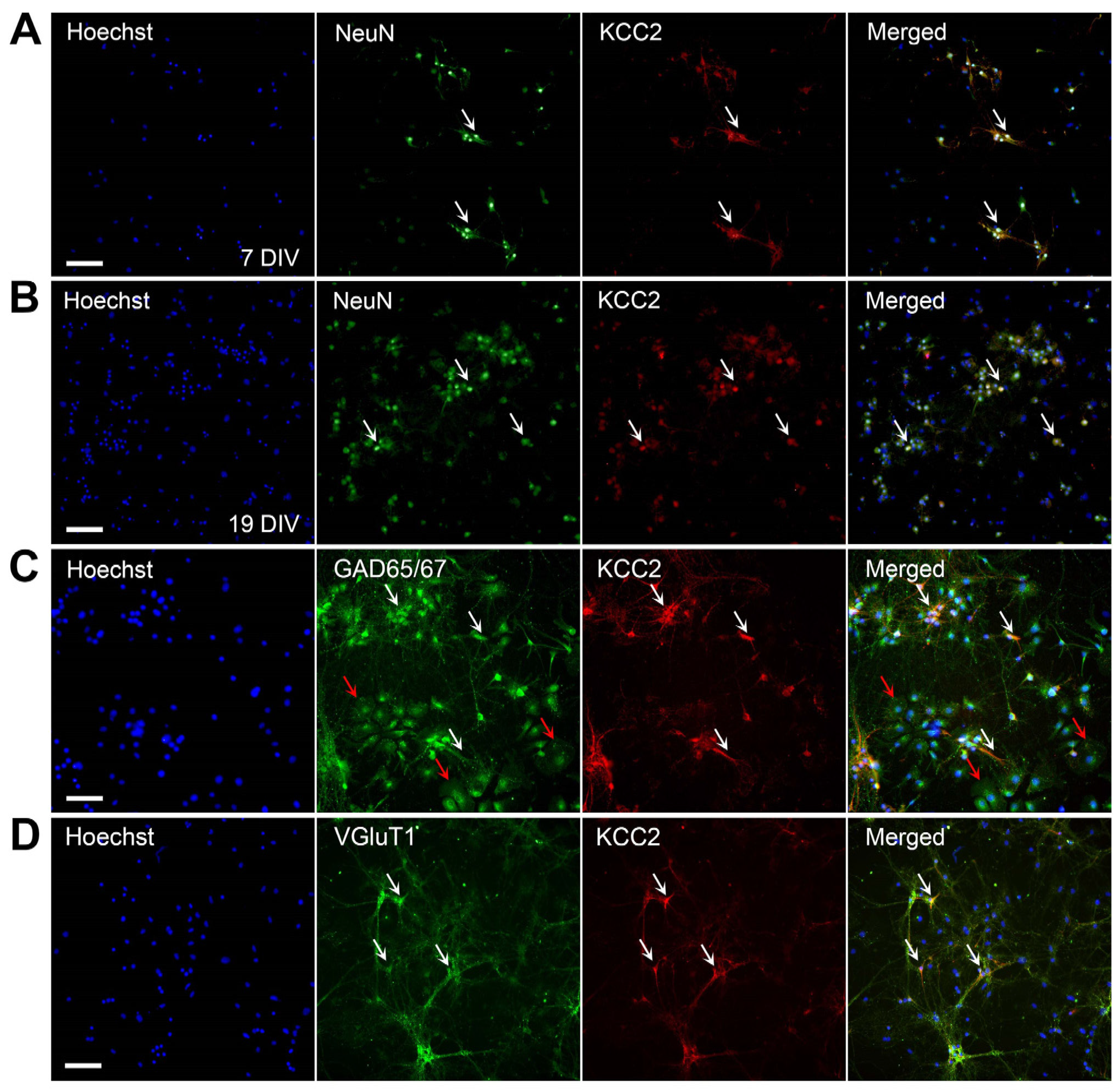
2.1. KCC2 Expression in Hippocampal Cultures
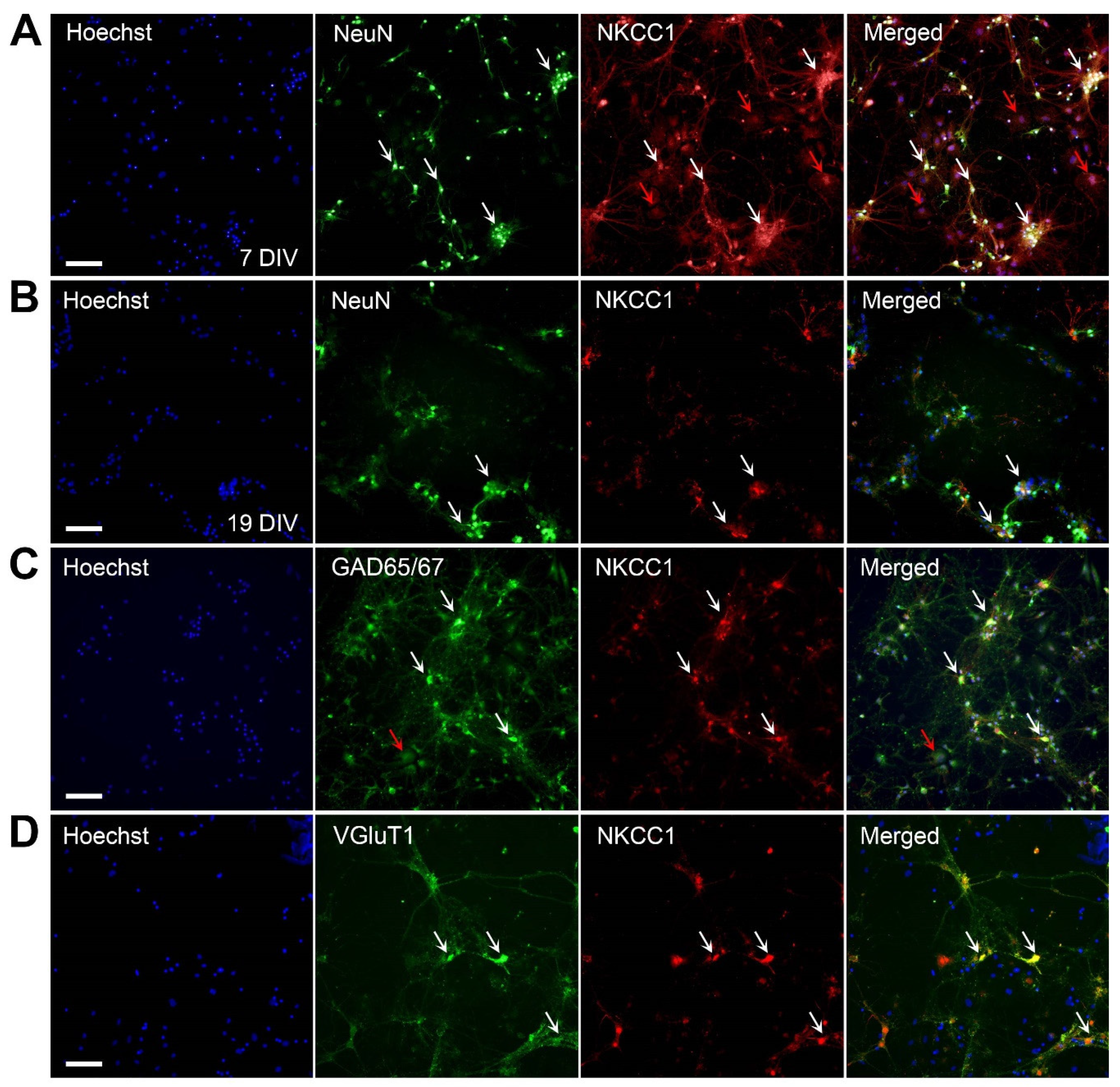
2.2. NKCC1 Expression in Hippocampal Cultures
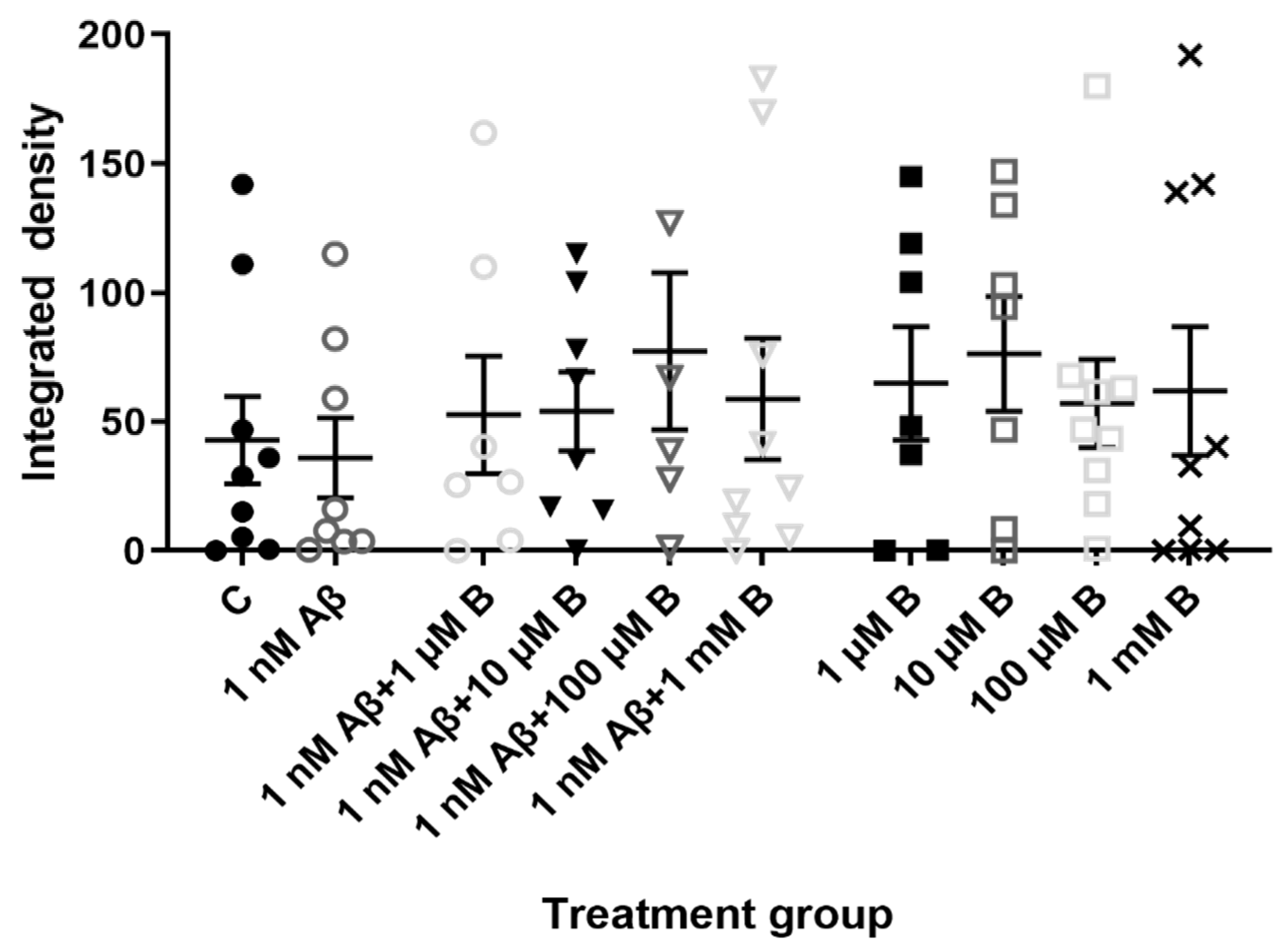
2.3. Effect of Aβ1-42 and Bumetanide on KCC2 and NKCC1 Expression in Hippocampal Cultures
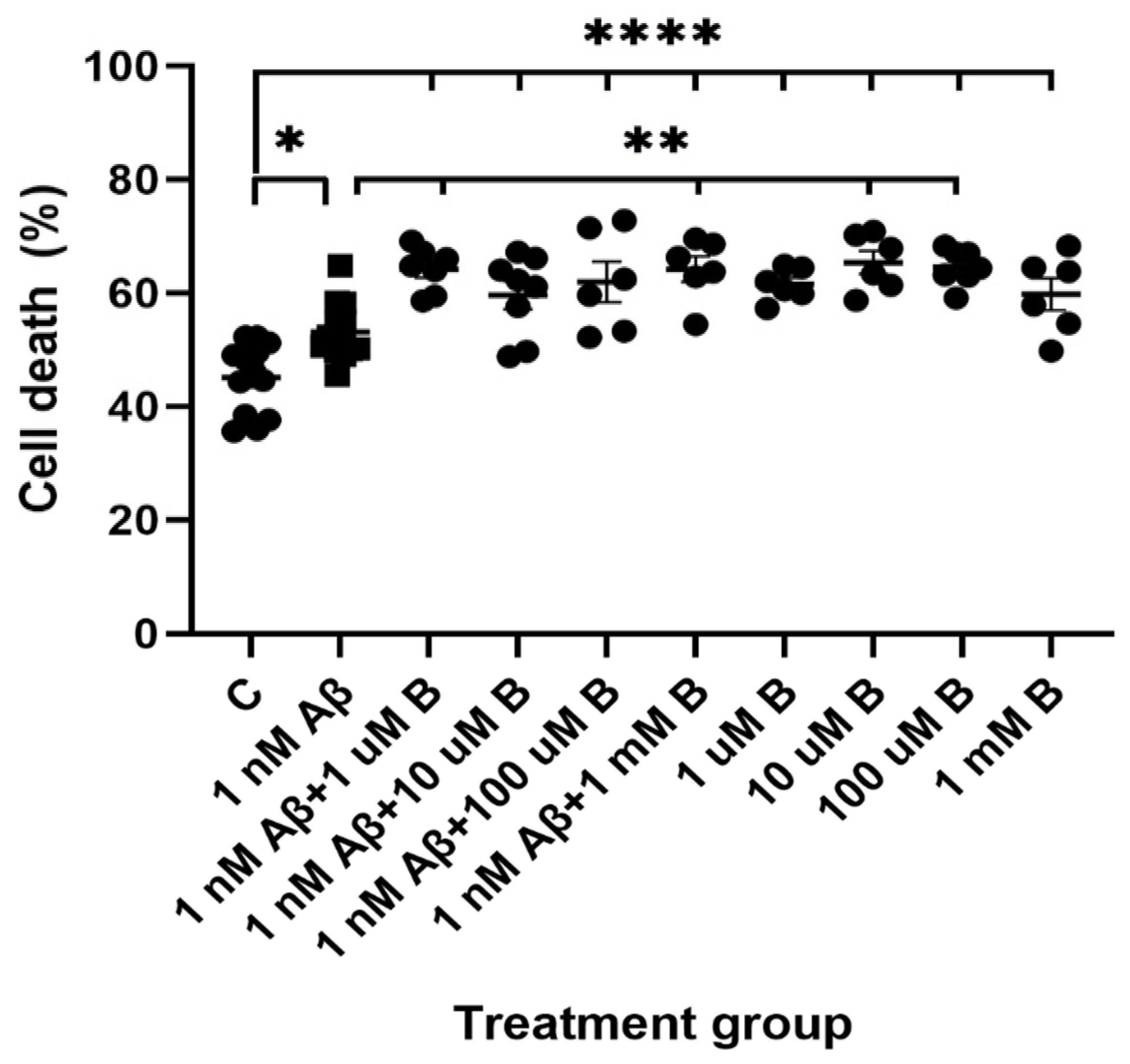
2.4. Effect of Aβ1-42 and Bumetanide on Cell Viability in Hippocampal Cultures
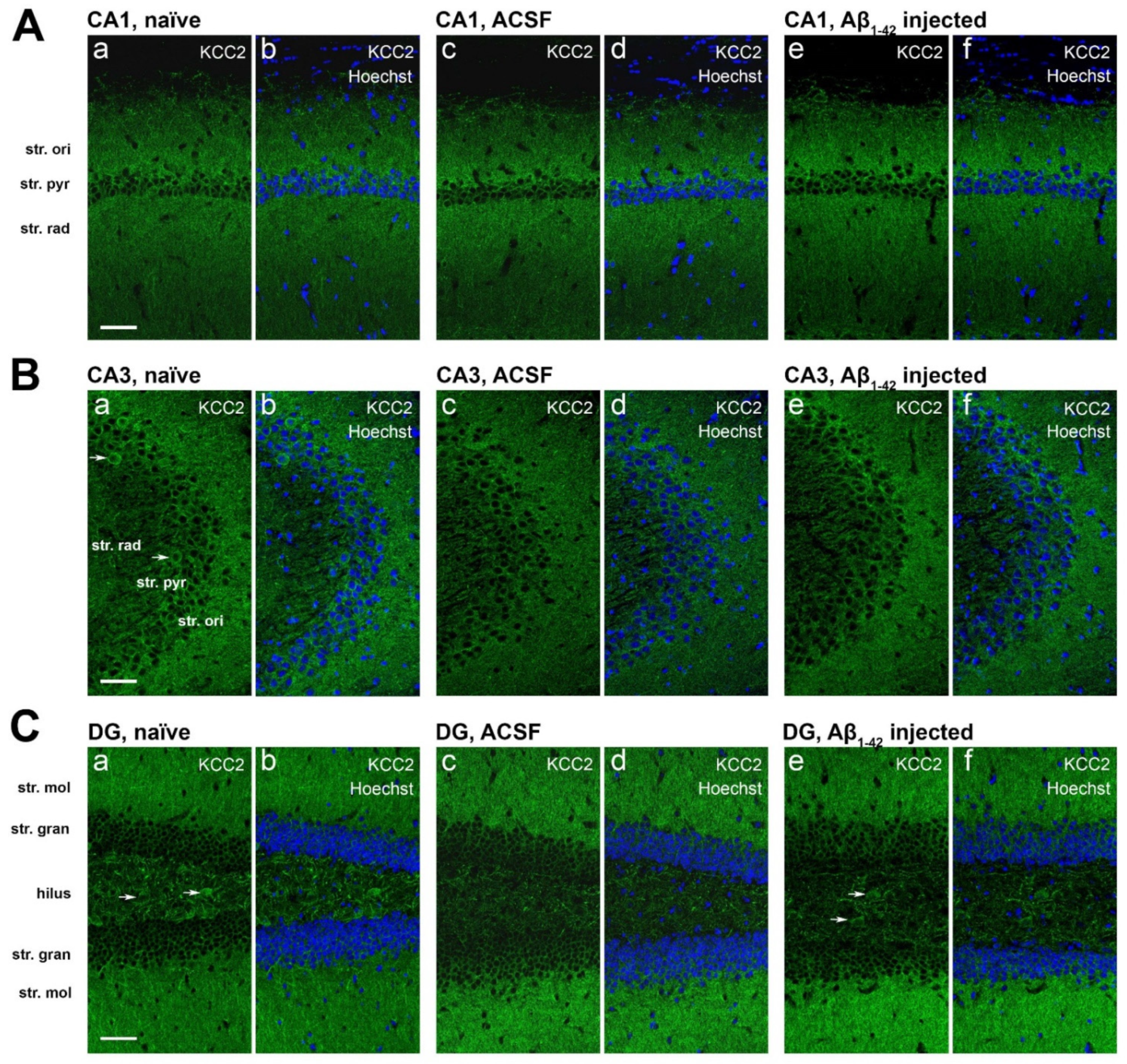
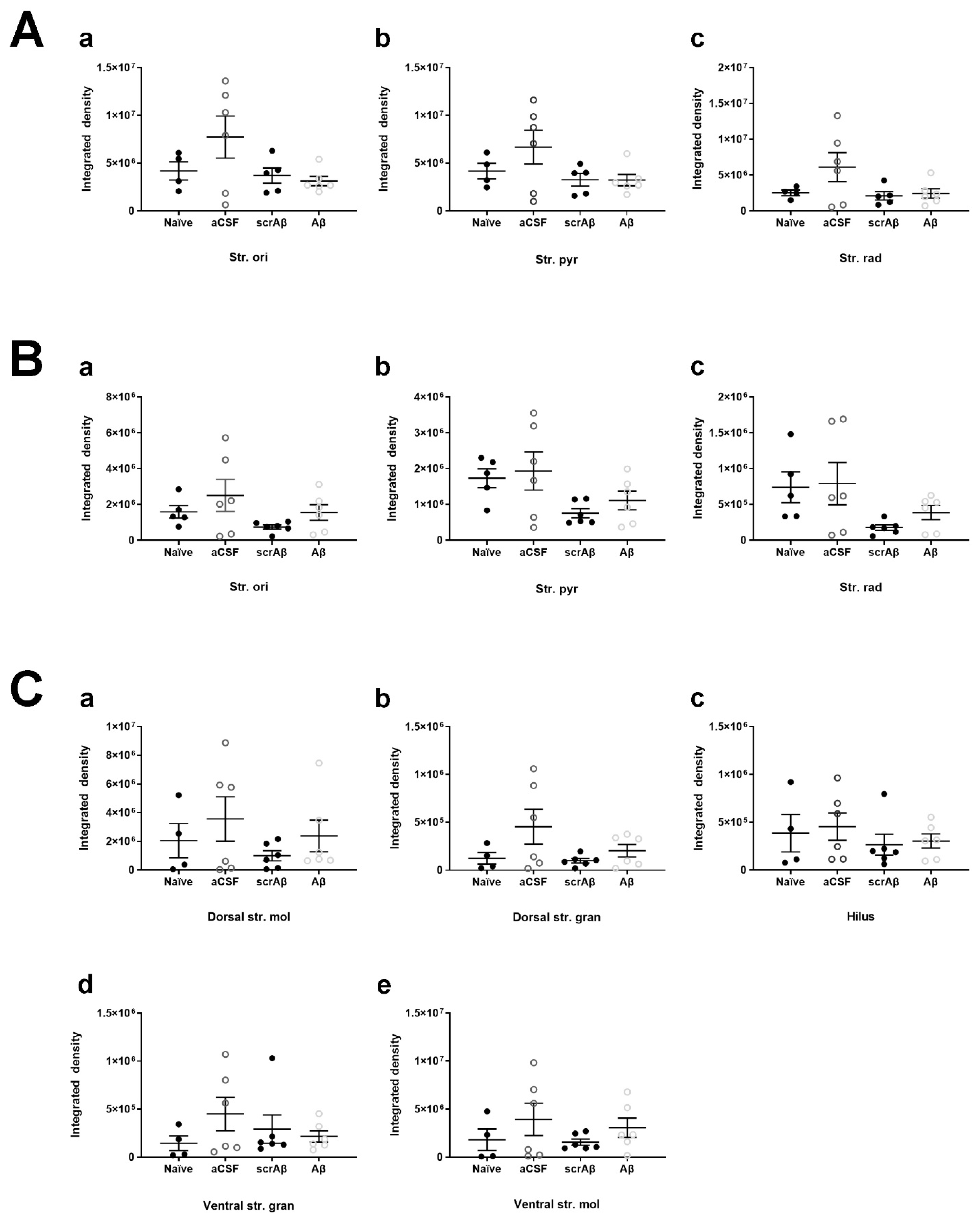
2.5. Effect of Aβ1-42 on KCC2 Expression in the Mouse Hippocampus
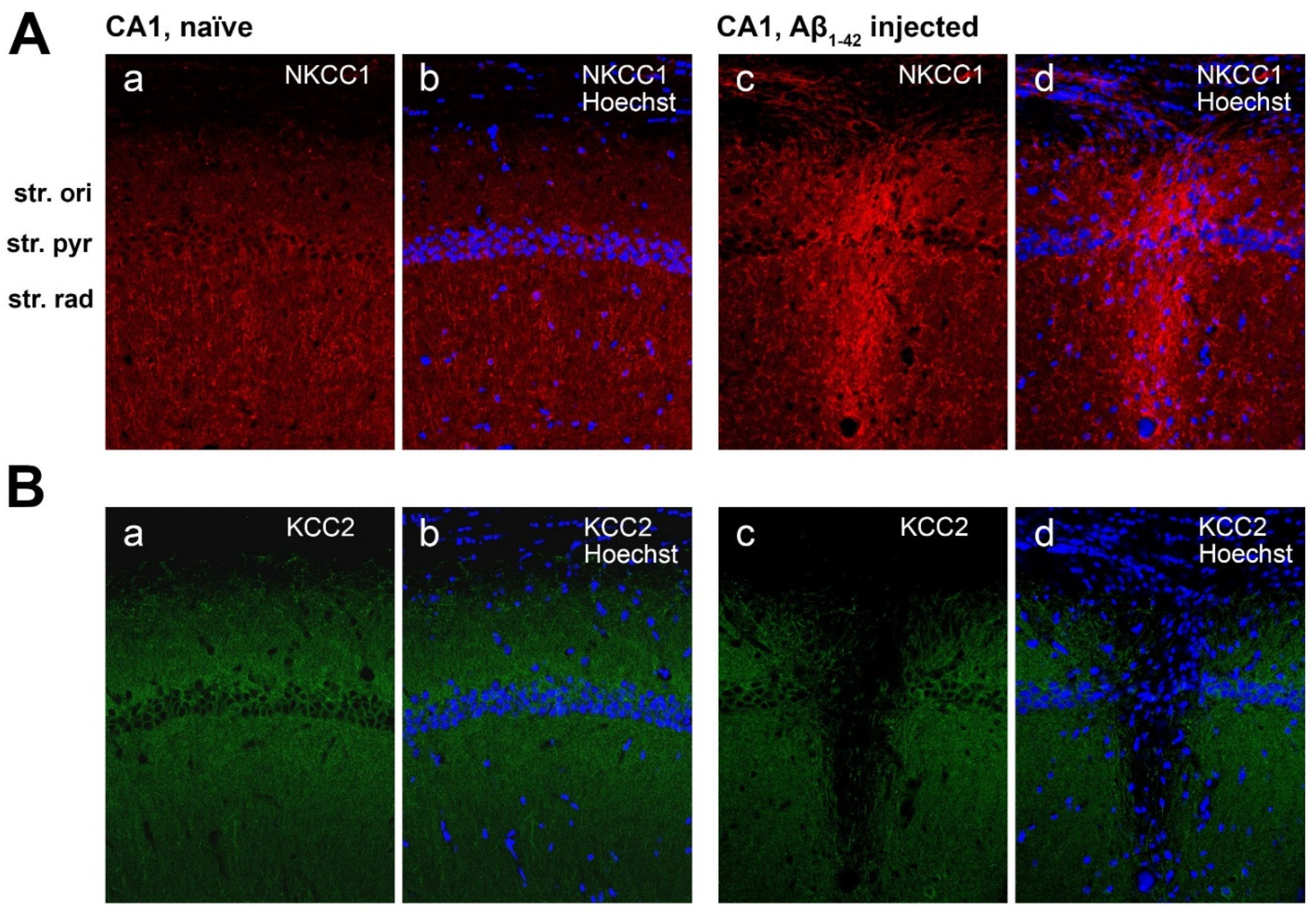
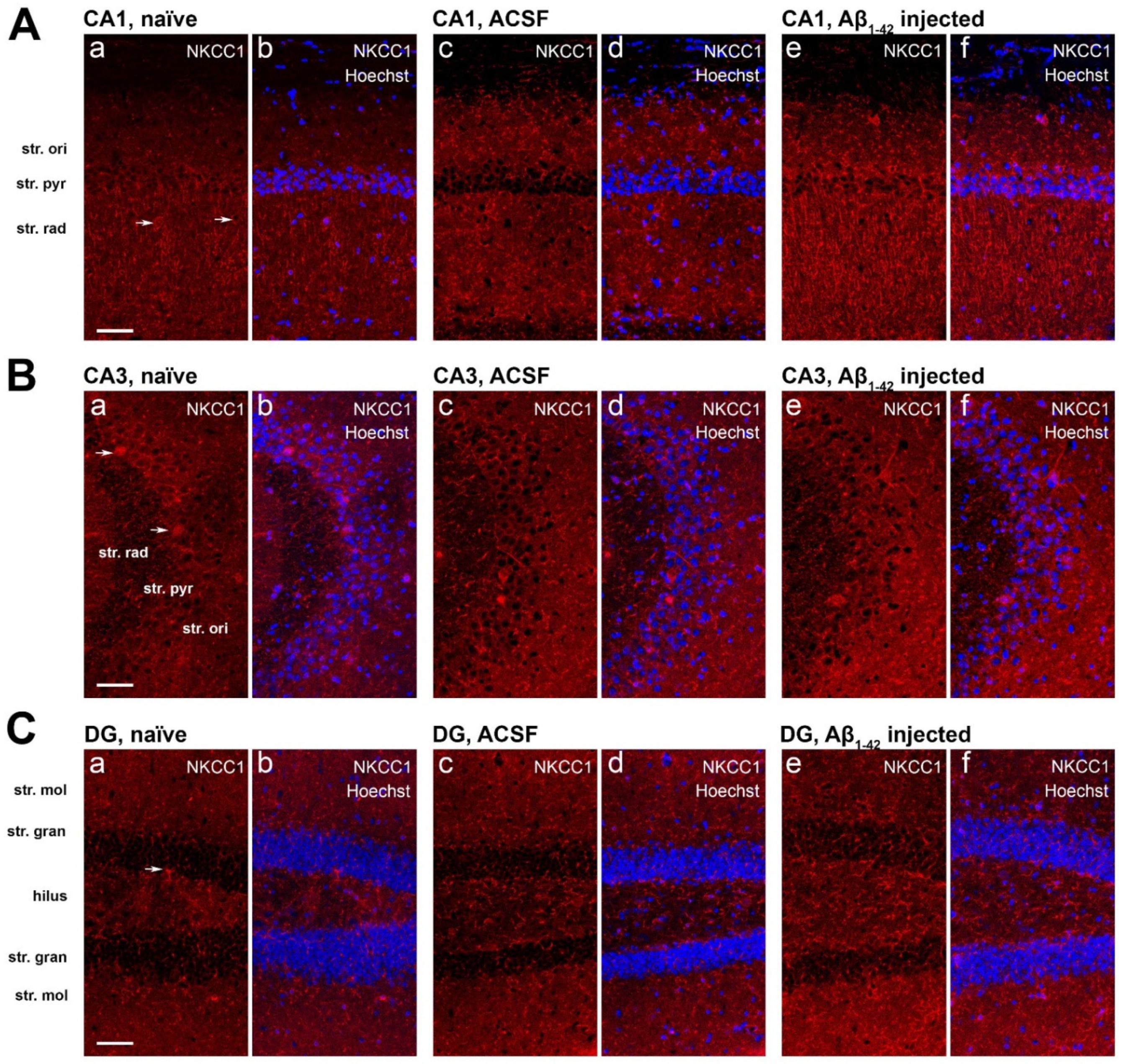
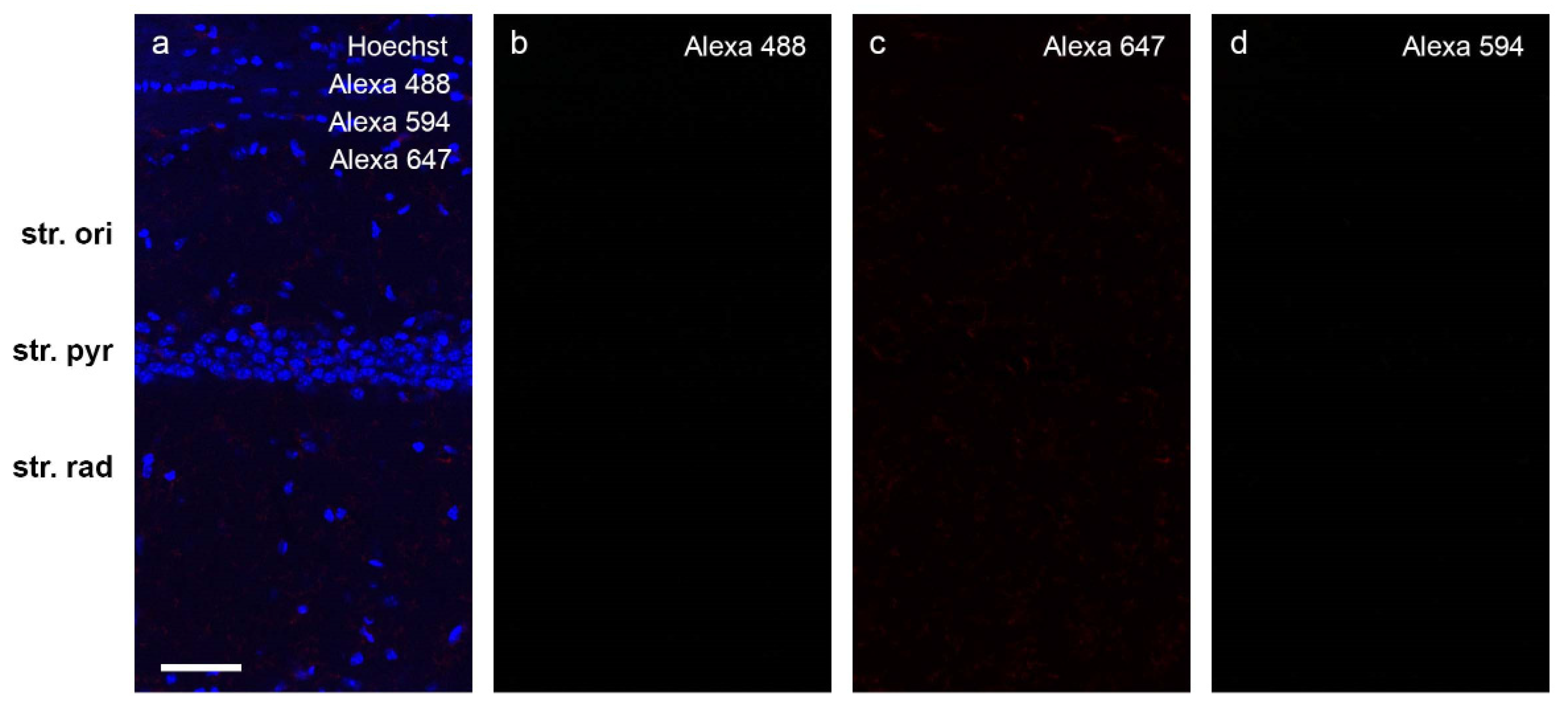
2.6. Effect of Aβ1-42 on NKCC1 Expression in the Mouse Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Primary Hippocampal Cell Culture
4.3. Aβ1-42 Preparation
4.4. Drug Treatments
4.5. Measuring Cell Viability Using the ReadyProbes Cell Viability Imaging Kit
4.6. Fluorescent Immunocytochemistry
4.7. Mouse In Vivo Experiments
4.8. Fluorescent Immunohistochemistry
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Braak, H.; Braak, E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Govindpani, K.; Guzmán, B.C.-F.; Vinnakota, C.; Waldvogel, H.J.; Faull, R.L.; Kwakowsky, A. Towards a better understanding of GABAergic remodeling in alzheimer’s disease. Int. J. Mol. Sci. 2017, 18, 1813. [Google Scholar] [CrossRef]
- Jensen, O.; Kaiser, J.; Lachaux, J.-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. (Regul. Ed.) 2007, 30, 317–324. [Google Scholar] [CrossRef]
- Mucke, L.; Palop, J.J. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef]
- Sohal, V.S.; Zhang, F.; Yizhar, O.; Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009, 459, 698–702. [Google Scholar] [CrossRef]
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef]
- Chen, C. β-amyloid increases dendritic Ca2+ influx by inhibiting the A-type K+ current in hippocampal CA1 pyramidal neurons. Biochem. Biophys. Res. Commun. 2005, 338, 1913–1919. [Google Scholar] [CrossRef]
- Kamenetz, F.; Tomita, T.; Hsieh, H.; Seabrook, G.; Borchelt, D.; Iwatsubo, T.; Sisodia, S.; Malinow, R. APP Processing and Synaptic Function. Neuron 2003, 37, 925–937. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Epilepsy and Cognitive Impairments in Alzheimer Disease. Arch. Neurol. 2009, 66, 435–440. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Calvo-Flores Guzmán, B.; Pandya, M.; Turner, C.; Waldvogel, H.J.; Faull, R.L. GABA A receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J. Neurochem. 2018, 145, 374–392. [Google Scholar] [CrossRef]
- Busche, M.A.; Eichhoff, G.; Adelsberger, H.; Abramowski, D.; Wiederhold, K.-H.; Haass, C.; Staufenbiel, M.; Konnerth, A.; Garaschuk, O. Clusters of Hyperactive Neurons near Amyloid Plaques in a Mouse Model of Alzheimer’s Disease. Sci. (Am. Assoc. Adv. Sci.) 2008, 321, 1686–1689. [Google Scholar] [CrossRef] [PubMed]
- Howell, O.; Atack, J.R.; Dewar, D.; McKernan, R.M.; Sur, C. Density and pharmacology of α5 subunit-containing GABA A receptors are preserved in hippocampus of Alzheimer’s disease patients. Neuroscience 2000, 98, 669–675. [Google Scholar] [CrossRef]
- Li, G.; Bien-Ly, N.; Andrews-Zwilling, Y.; Xu, Q.; Bernardo, A.; Ring, K.; Halabisky, B.; Deng, C.; Mahley, R.W.; Huang, Y. GABAergic Interneuron Dysfunction Impairs Hippocampal Neurogenesis in Adult Apolipoprotein E4 Knockin Mice. Cell Stem Cell 2009, 5, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Ramos, B.; Baglietto-Vargas, D.; del Rio, J.C.; Moreno-Gonzalez, I.; Santa-Maria, C.; Jimenez, S.; Caballero, C.; Lopez-Tellez, J.F.; Khan, Z.U.; Ruano, D.; et al. Early neuropathology of somatostatin/NPY GABAergic cells in the hippocampus of a PS1xAPP transgenic model of Alzheimer’s disease. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar]
- Fuhrer, T.E.; Palpagama, T.H.; Waldvogel, H.J.; Synek, B.J.L.; Turner, C.; Faull, R.L.; Kwakowsky, A. Impaired expression of GABA transporters in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. Neuroscience 2017, 351, 108–118. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Calvo-Flores Guzmán, B.; Govindpani, K.; Waldvogel, H.; Faull, R. Gamma-aminobutyric acid A receptors in Alzheimer’s disease: Highly localized remodeling of a complex and diverse signaling pathway. Neural Regen. Res. 2018, 13, 1362–1363. [Google Scholar] [CrossRef]
- Ben-Ari, Y. NKCC1Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci. (Regul. Ed.) 2017, 40, 536–554. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef]
- Cherubini, E.; Gaiarsa, J.L.; Ben-Ari, Y. GABA: An excitatory transmitter in early postnatal life. Trends Neurosci. (Regul. Ed.) 1991, 14, 515–519. [Google Scholar] [CrossRef]
- Kahle, K.T.; Staley, K.J.; Nahed, B.V.; Gamba, G.; Hebert, S.C.; Lifton, R.P.; Mount, D.B. Roles of the cation–chloride cotransporters in neurological disease. Nat. Clin. Pract. Neurol. 2008, 4, 490–503. [Google Scholar] [CrossRef]
- Deidda, G.; Parrini, M.; Naskar, S.; Bozarth, I.F.; Contestabile, A.; Cancedda, L. Reversing excitatory GABAAR signaling restores synaptic plasticity and memory in a mouse model of Down syndrome. Nat. Med. 2015, 21, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Merner, N.D.; Mercado, A.; Khanna, A.R.; Hodgkinson, A.; Bruat, V.; Awadalla, P.; Gamba, G.; Rouleau, G.A.; Kahle, K.T. Gain-of-function missense variant in SLC12A2, encoding the bumetanide-sensitive NKCC1 cotransporter, identified in human schizophrenia. J. Psychiatr. Res. 2016, 77, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Kriegstein, A.R. Blocking early GABA depolarization with bumetanide results in permanent alterations in cortical circuits and sensorimotor gating deficits. Cereb. Cortex 2011, 21, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Dargaei, Z.; Bang, J.Y.; Mahadevan, V.; Khademullah, C.S.; Bedard, S.; Parfitt, G.M.; Kim, J.C.; Woodin, M.A. Restoring GABAergic inhibition rescues memory deficits in a Huntington’s disease mouse model. Proc. Natl. Acad. Sci. USA 2018, 115, E1618–E1626. [Google Scholar] [CrossRef]
- Lee, H.H.C.; Deeb, T.Z.; Walker, J.A.; Davies, P.A.; Moss, S.J. NMDA receptor activity downregulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat. Neurosci. 2011, 14, 736–743. [Google Scholar] [CrossRef]
- Kaila, K.; Price, T.J.; Payne, J.A.; Puskarjov, M.; Voipio, J. Cation–chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 2014, 15, 637–654. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Thomas-Crusells, J.; Li, H.; Emri, Z.; Sipila, S.; Payne, J.A.; Minichiello, L.; Saarma, M.; Kaila, K. Mechanism of Activity-Dependent Downregulation of the Neuron-Specific K-Cl Cotransporter KCC2. J. Neurosci. 2004, 24, 4683–4691. [Google Scholar] [CrossRef]
- Kanaka, C.; Ohno, K.; Okabe, A.; Kuriyama, K.; Itoh, T.; Fukuda, A.; Sato, K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience 2001, 104, 933–946. [Google Scholar] [CrossRef]
- Payne, J.A.; Stevenson, T.J.; Donaldson, L.F. Molecular characterization of a putative K-Cl cotransporter in rat brain: A neuronal-specific isoform. J. Biol. Chem. 1996, 271, 16245–16252. [Google Scholar] [CrossRef]
- Vu, T.Q.; Payne, J.A.; Copenhagen, D.R. Localization and Developmental Expression Patterns of the Neuronal K-Cl Cotransporter (KCC2) in the Rat Retina. J. Neurosci. 2000, 20, 1414–1423. [Google Scholar] [CrossRef]
- Williams, J.R.; Sharp, J.W.; Kumari, V.G.; Wilson, M.; Payne, J.A. The neuron-specific K-Cl cotransporter, KCC2: Antibody development and initial characterization of the protein. J. Biol. Chem. 1999, 274, 12656–12664. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, A.I.; Sík, A.; Payne, J.A.; Kaila, K.; Freund, T.F. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur. J. Neurosci. 2001, 13, 2205–2217. [Google Scholar] [CrossRef] [PubMed]
- Staley, K.J.; Dzhala, V.I.; Talos, D.M.; Sdrulla, D.A.; Brumback, A.C.; Mathews, G.C.; Benke, T.A.; Delpire, E.; Jensen, F.E. NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 2005, 11, 1205–1213. [Google Scholar] [CrossRef]
- Plotkin, M.D.; Kaplan, M.R.; Peterson, L.N.; Gullans, S.R.; Hebert, S.C.; Delpire, E. Expression of the Na(+)-K(+)-2Cl- cotransporter BSC2 in the nervous system. Am. J. Physiol.-Cell Physiol. 1997, 272, 173–183. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.E.; Martinez, A.; Sun, D. Cerebral microvascular endothelial cell Na-K-Cl cotransport: Regulation by astrocyte-conditioned medium. Am. J. Physiol.-Cell Physiol. 1995, 268, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Hübner, C.A.; Lorke, D.E.; Hermans-Borgmeyer, I. Expression of the Na-K-2Cl-cotransporter NKCC1 during mouse development. Mech. Dev. 2001, 102, 267–269. [Google Scholar] [CrossRef]
- Yan, Y.; Dempsey, R.J.; Sun, D. Expression of Na+-K+-Cl− cotransporter in rat brain during development and its localization in mature astrocytes. Brain Res. 2001, 911, 43–55. [Google Scholar] [CrossRef]
- Taubes, A.; Nova, P.; Zalocusky, K.A.; Kosti, I.; Bicak, M.; Zilberter, M.Y.; Hao, Y.; Yoon, S.Y.; Oskotsky, T.; Pineda, S.; et al. Experimental and real-world evidence supporting the computational repurposing of bumetanide for APOE4-related Alzheimer’s disease. Nat. Aging 2021, 1, 932–947. [Google Scholar] [CrossRef]
- Khirug, S.; Huttu, K.; Ludwig, A.; Smirnov, S.; Voipio, J.; Rivera, C.; Kaila, K.; Khiroug, L. Distinct properties of functional KCC2 expression in immature mouse hippocampal neurons in culture and in acute slices. Eur. J. Neurosci. 2005, 21, 899–904. [Google Scholar] [CrossRef]
- Ludwig, A.; Li, H.; Saarma, M.; Kaila, K.; Rivera, C. Developmental up-regulation of KCC2 in the absence of GABAergic and glutamatergic transmission. Eur. J. Neurosci. 2003, 18, 3199–3206. [Google Scholar] [CrossRef]
- Swanwick, C.C.; Murthy, N.R.; Mtchedlishvili, Z.; Sieghart, W.; Kapur, J. Development of γ-aminobutyric acidergic synapses in cultured hippocampal neurons. J. Comp. Neurol. (1911) 2006, 495, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Potapov, K.; Kim, S.; Peppercorn, K.; Tate, W.P.; Ábrahám, I.M. Treatment of beta amyloid 1-42 (Aβ1-42)-induced basal forebrain cholinergic damage by a non-classical estrogen signaling activator in vivo. Sci. Rep. 2016, 6, 21101. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Kurz, J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J. Comp. Neurol. (1911) 1985, 240, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Frotscher, M.; Schlander, M.; Léránth, C. Cholinergic neurons in the hippocampus. A combined light- and electron-microscopic immunocytochemical study in the rat. Cell Tissue Res. 1986, 246, 293–301. [Google Scholar] [CrossRef]
- Wainer, B.H.; Levey, A.I.; Rye, D.B.; Mesulam, M.M.; Mufson, E.J. Cholinergic and non-cholinergic septohippocampal pathways. Neurosci. Lett. 1985, 54, 45–52. [Google Scholar] [CrossRef]
- Aguado, F.; Carmona, M.A.; Pozas, E.; Aguilo, A.; Martinez-Guijarro, F.J.; Alcantara, S.; Borrell, V.; Yuste, R.; Ibanez, C.F.; Soriano, E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl− co-transporter KCC2. Development 2003, 130, 1267–1280. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Jiang, J.; Zheng, X.; Justice, N.J.; Wang, K.; Ran, X.; Li, Y.; Huo, Q.; Zhang, J.; et al. APP modulates KCC2 expression and function in hippocampal GABAergic inhibition. eLife 2017, 6, e20142. [Google Scholar] [CrossRef]
- Lee, H.H.; Jurd, R.; Moss, S.J. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol. Cell. Neurosci. 2010, 45, 173–179. [Google Scholar] [CrossRef]
- Plotkin, M.D.; Snyder, E.Y.; Hebert, S.C.; Delpire, E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: A possible mechanism underlying GABA’s excitatory role in immature brain. J. Neurobiol. 1997, 33, 781–795. [Google Scholar] [CrossRef]
- Pfeffer, C.K.; Stein, V.; Keating, D.J.; Maier, H.; Rinke, I.; Rudhard, Y.; Hentschke, M.; Rune, G.M.; Jentsch, T.J.; Hubner, C.A. NKCC1-Dependent GABAergic Excitation Drives Synaptic Network Maturation during Early Hippocampal Development. J. Neurosci. 2009, 29, 3419–3430. [Google Scholar] [CrossRef]
- Wang, C.; Shimizu-Okabe, C.; Watanabe, K.; Okabe, A.; Matsuzaki, H.; Ogawa, T.; Mori, N.; Fukuda, A.; Sato, K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res. Dev. Brain Res. 2002, 139, 59–66. [Google Scholar] [CrossRef]
- Darman, R.B.; Forbush, B. A Regulatory Locus of Phosphorylation in the N Terminus of the Na-K-Cl Cotransporter, NKCC1. J. Biol. Chem. 2002, 277, 37542–37550. [Google Scholar] [CrossRef] [PubMed]
- Dowd, B.F.X.; Forbush, B. PASK (Proline-Alanine-rich STE20-related Kinase), a Regulatory Kinase of the Na-K-Cl Cotransporter (NKCC1). J. Biol. Chem. 2003, 278, 27347–27353. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; Mehrabi, S.; Soleimani, M.; Hassanzadeh, G.; Shahrokhi, A.; Mostafavi, H.; Hayat, P.; Barati, M.; Mehdizadeh, H.; Rahmanzadeh, R.; et al. BDNF modifies hippocampal KCC2 and NKCC1 expression in a temporal lobe epilepsy model. Acta Neurobiol. Exp. 2014, 74, 276–287. [Google Scholar]
- Pond, B.B.; Galeffi, F.; Ahrens, R.; Schwartz-Bloom, R.D. Chloride transport inhibitors influence recovery from oxygen–glucose deprivation-induced cellular injury in adult hippocampus. Neuropharmacology 2004, 47, 253–262. [Google Scholar] [CrossRef]
- Yan, Y.; Dempsey, R.J.; Flemmer, A.; Forbush, B.; Sun, D. Inhibition of Na+–K+–Cl− cotransporter during focal cerebral ischemia decreases edema and neuronal damage. Brain Res. 2003, 961, 22–31. [Google Scholar] [CrossRef]
- Flagella, M.; Clarke, L.L.; Miller, M.L.; Erway, L.C.; Giannella, R.A.; Andringa, A.; Gawenis, L.R.; Kramer, J.; Duffy, J.J.; Doetschman, T.; et al. Mice Lacking the Basolateral Na-K-2Cl Cotransporter Have Impaired Epithelial Chloride Secretion and Are Profoundly Deaf. J. Biol. Chem. 1999, 274, 26946–26955. [Google Scholar] [CrossRef]
- Kleschevnikov, A.M.; Belichenko, P.V.; Villar, A.J.; Epstein, C.J.; Malenka, R.C.; Mobley, W.C. Hippocampal Long-Term Potentiation Suppressed by Increased Inhibition in the Ts65Dn Mouse, a Genetic Model of Down Syndrome. J. Neurosci. 2004, 24, 8153–8160. [Google Scholar] [CrossRef]
- Schliess, F.; Schäfer, C.; vom Dahl, S.; Fischer, R.; Lordnejad, M.R.; Häussinger, D. Expression and regulation of the Na +/K +/2Cl − cotransporter NKCC1 in rat liver and human HuH-7 hepatoma cells. Arch. Biochem. Biophys. 2002, 401, 187–197. [Google Scholar] [CrossRef]
- Payne, J.A.; Rivera, C.; Voipio, J.; Kaila, K. Cation–chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. (Regul. Ed.) 2003, 26, 199–206. [Google Scholar] [CrossRef]
- Beaudoin Iii, G.M.J.; Lee, S.-H.; Singh, D.; Yuan, Y.; Ng, Y.-G.; Reichardt, L.F.; Arikkath, J. Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat. Protoc. 2012, 7, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Vinnakota, C.; Govindpani, K.; Tate, W.P.; Peppercorn, K.; Anekal, P.V.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. An 5 GABAA Receptor Inverse Agonist, 5IA, Attenuates Amyloid Beta-Induced Neuronal Death in Mouse Hippocampal Cultures. Int. J. Mol. Sci. 2020, 21, 3284. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.H.Y.; Palpagama, T.H.; Tate, W.P.; Peppercorn, K.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. The Acute Effects of Amyloid-Beta1–42 on Glutamatergic Receptor and Transporter Expression in the Mouse Hippocampus. Front. Neurosci. 2020, 13, 1427. [Google Scholar] [CrossRef]
- Yeung, J.H.Y.; Calvo-Flores Guzmán, B.; Palpagama, T.H.; Ethiraj, J.; Zhai, Y.; Tate, W.P.; Peppercorn, K.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Amyloid-beta1–42 induced glutamatergic receptor and transporter expression changes in the mouse hippocampus. J. Neurochem. 2020, 155, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores Guzmán, B.; Elizabeth Chaffey, T.; Hansika Palpagama, T.; Waters, S.; Boix, J.; Tate, W.P.; Peppercorn, K.; Dragunow, M.; Waldvogel, H.J.; Faull, R.L.M.; et al. The Interplay Between Beta-Amyloid 1–42 (Aβ1–42)-Induced Hippocampal Inflammatory Response, p-tau, Vascular Pathology, and Their Synergistic Contributions to Neuronal Death and Behavioral Deficits. Front. Mol. Neurosci. 2020, 13, 522073. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores Guzmán, B.; Kim, S.; Chawdhary, B.; Peppercorn, K.; Tate, W.P.; Waldvogel, H.J.; Faull, R.L.; Montgomery, J.; Kwakowsky, A. Amyloid-Beta1-42-Induced Increase in GABAergic Tonic Conductance in Mouse Hippocampal CA1 Pyramidal Cells. Molecules 2020, 25, 693. [Google Scholar] [CrossRef] [PubMed]
- Bragin, D.E.; Sanderson, J.L.; Peterson, S.; Connor, J.A.; Müller, W.S. Development of epileptiform excitability in the deep entorhinal cortex after status epilepticus. Eur. J. Neurosci. 2009, 30, 611–624. [Google Scholar] [CrossRef]
- Campbell, S.L.; Robel, S.; Cuddapah, V.A.; Robert, S.; Buckingham, S.C.; Kahle, K.T.; Sontheimer, H. GABAergic disinhibition and impaired KCC2 cotransporter activity underlie tumor-associated epilepsy. Glia 2015, 63, 23–36. [Google Scholar] [CrossRef]
- Côté, M.-P.; Gandhi, S.; Zambrotta, M.; Houlé, J.D. Exercise modulates chloride homeostasis after spinal cord injury. J. Neurosci. 2014, 34, 8976–8987. [Google Scholar] [CrossRef]
- He, Q.; Nomura, T.; Xu, J.; Contractor, A. The developmental switch in GABA polarity is delayed in fragile X mice. J. Neurosci. 2014, 34, 446–450. [Google Scholar] [CrossRef]
- Horn, Z.; Ringstedt, T.; Blaesse, P.; Kaila, K.; Herlenius, E. Premature expression of KCC2 in embryonic mice perturbs neural development by an ion transport-independent mechanism. Eur. J. Neurosci. 2010, 31, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Kwan, G.T.; Smith, T.R.; Tresguerres, M. Immunological characterization of two types of ionocytes in the inner ear epithelium of Pacific Chub Mackerel (Scomber japonicus). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2020, 190, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Lytle, C.; Jian-Chao, X.U.; Biemesderfer, D.; Forbush, B., III. Distribution and diversity of Na-K-Cl cotransport proteins: A study with monoclonal antibodies. Am. J. Physiol. Cell Physiol. 1995, 38, C1496–C1505. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, J.; Kintner, D.B.; Shull, G.E.; Sun, D. Na-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2005, 25, 54–66. [Google Scholar] [CrossRef]
- Inoue, Y.; Ishii, K.; Miyazaki, M.; Ueno, H. Purification of L-glutamate decarboxylase from monkey brain. Biosci. Biotechnol. Biochem. 2008, 72, 2269–2276. [Google Scholar] [CrossRef][Green Version]
- Yan, K.; Tang, Y.-Z.; Carr, C.E. Calcium-binding protein immunoreactivity characterizes the auditory system of Gekko gecko. J. Comp. Neurol. 2010, 518, 3409–3426. [Google Scholar] [CrossRef]
- Matsumoto, M.; Xie, W.; Inoue, M.; Ueda, H. Evidence for the Tonic Inhibition of Spinal Pain by Nicotinic Cholinergic Transmission through Primary Afferents. Mol. Pain 2007, 3, 41. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, P.; Vinnakota, C.; Guzmán, B.C.-F.; Newland, J.; Peppercorn, K.; Tate, W.P.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Beta-Amyloid (Aβ1-42) Increases the Expression of NKCC1 in the Mouse Hippocampus. Molecules 2022, 27, 2440. https://doi.org/10.3390/molecules27082440
Lam P, Vinnakota C, Guzmán BC-F, Newland J, Peppercorn K, Tate WP, Waldvogel HJ, Faull RLM, Kwakowsky A. Beta-Amyloid (Aβ1-42) Increases the Expression of NKCC1 in the Mouse Hippocampus. Molecules. 2022; 27(8):2440. https://doi.org/10.3390/molecules27082440
Chicago/Turabian StyleLam, Patricia, Chitra Vinnakota, Beatriz Calvo-Flores Guzmán, Julia Newland, Katie Peppercorn, Warren P. Tate, Henry J. Waldvogel, Richard L. M. Faull, and Andrea Kwakowsky. 2022. "Beta-Amyloid (Aβ1-42) Increases the Expression of NKCC1 in the Mouse Hippocampus" Molecules 27, no. 8: 2440. https://doi.org/10.3390/molecules27082440
APA StyleLam, P., Vinnakota, C., Guzmán, B. C.-F., Newland, J., Peppercorn, K., Tate, W. P., Waldvogel, H. J., Faull, R. L. M., & Kwakowsky, A. (2022). Beta-Amyloid (Aβ1-42) Increases the Expression of NKCC1 in the Mouse Hippocampus. Molecules, 27(8), 2440. https://doi.org/10.3390/molecules27082440






