Self-Assembled Alkylated Polyamine Analogs as Supramolecular Anticancer Agents
Abstract
1. Introduction
2. Results and Discussion
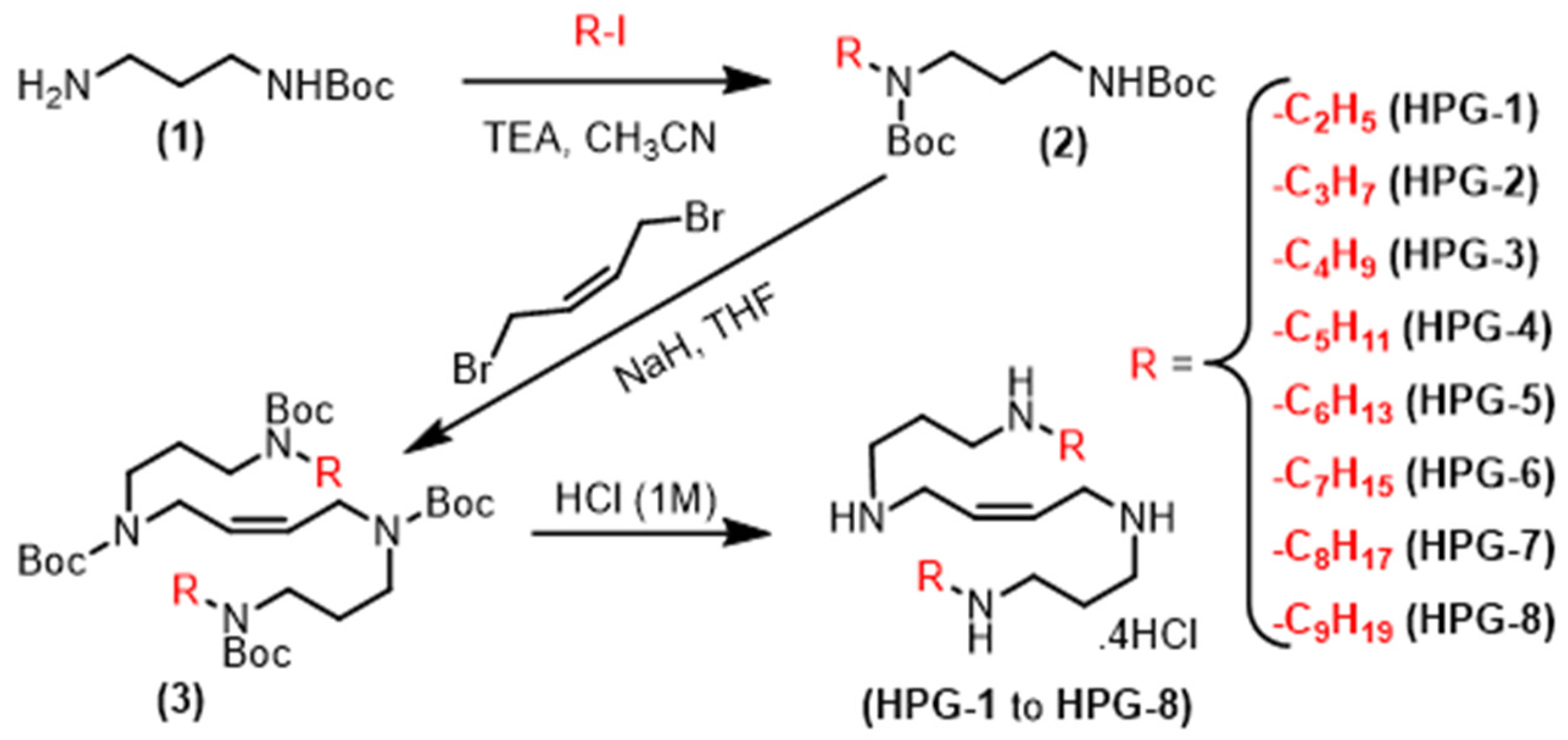
2.1. Synthesis of Alkylated Analogs of PG11047
2.2. Self-Assembly
2.3. Inhibition of the Cancer Cell Growth and SMOX by the HPG Nanoparticles
3. Materials and Methods
3.1. Materials
3.2. General Procedure for the Synthesis and Characterization of (Z)-N1,N1′-(But-2-Ene-1,4-Diyl)Bis(N3-Ethylpropane-1,3-Diamine, (PG11047)
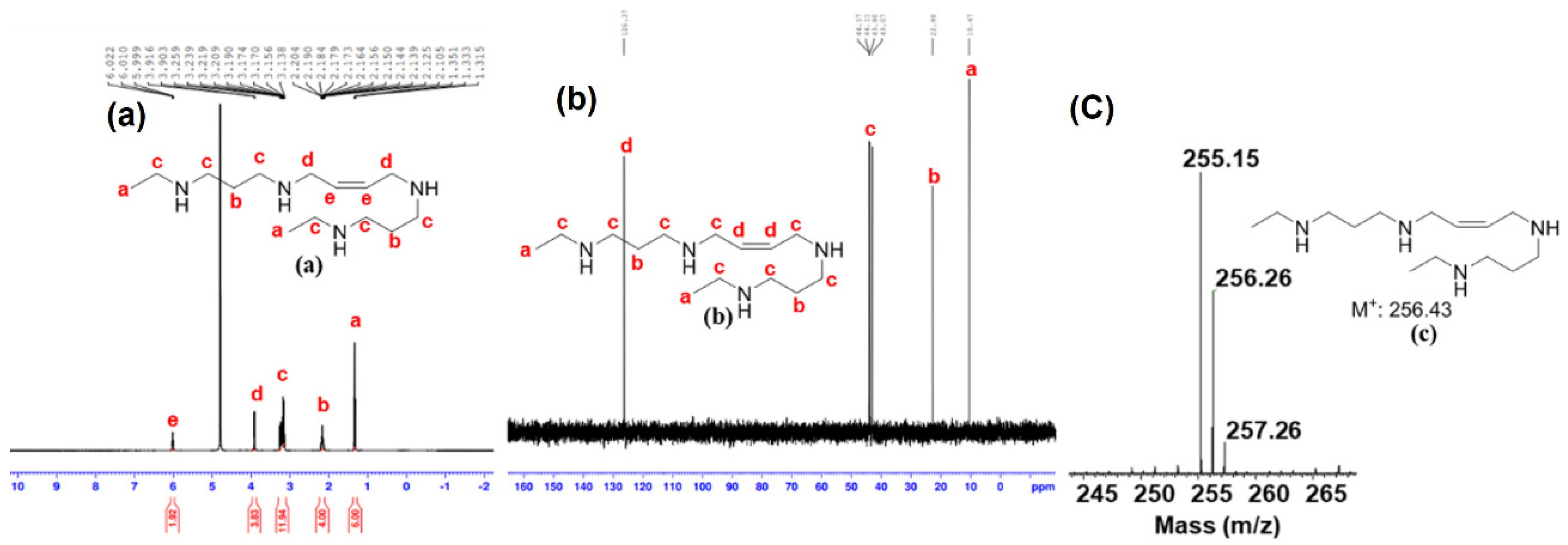
- Synthesis of (Z)-N1,N1′-(but-2-ene-1,4-diyl)bis(N3-propylpropane-1,3-diamine) (HPG-2): (yield—52%). 1H NMR (400 MHz, D2O): δ 5.99–5.98 (m, 2 H), 3.89–3.88 (m, 4 H), 3.23–3.15 (m, 8 H), 3.07–3.04 (m, 4 H), 2.19–2.11 (m, 4 H), 1.77–1.68 (m, 4 H), and 1.01–0.98 (t, J = 7.2 Hz, 6 H) ppm. 13C NMR (400 MHz, D2O): δ 126.35, 49.38, 44.22, 44.16, 44.11, 22.75, 19.13, and 10.11 ppm. MS (Orbitrap) calculated for C16H36N4, m/z 284.29, found 284.23 [M+].
- Synthesis of (Z)-N1,N1′-(but-2-ene-1,4-diyl)bis(N3-butylpropane-1,3-diamine) (HPG-3): (yield—50%). 1H NMR (400 MHz, D2O): δ 6.03–5.95 (m, 2 H), 3.89–3.88 (m, 4 H), 3.26–3.15 (m, 8 H), 3.11–3.04 (m, 4 H), 2.19–2.11 (m, 4 H), 1.72–1.65 (m, 4 H), 1.46–1.37 (m, 4 H), and 0.97–0.93 (t, J = 7.2 Hz, 6 H) ppm. 13C NMR (400 MHz, D2O): δ 126.35, 47.61, 44.23, 44.16, 44.11, 27.50, 22.76, 19.10, and 12.70 ppm. MS (Orbitrap) calculated for C18H40N4, m/z 312.32, found 313.25 [M + H]+.
- Synthesis of (Z)-N1,N1′-(but-2-ene-1,4-diyl)bis(N3-pentylpropane-1,3-diamine) (HPG-4): (yield—50%). 1H NMR (400 MHz, D2O): δ 6.00–5.98 (t, J = 4.4 Hz, 2 H), 3.89–3.80 (m, 4 H), 3.23–3.15 (m, 8 H), 3.11–3.07 (m, 4 H), 2.19–2.11 (m, 4 H), 1.75–1.67 (m, 4 H), 1.39–1.35 (m, 8 H), and 0.93–0.89 (m, 6 H) ppm. 13C NMR (400 MHz, D2O): δ 126.36, 47.85, 44.21, 44.17, 44.11, 27.78, 25.14, 22.75, 21.43, and 12.98 ppm. MS (Orbitrap) calculated for C20H44N4, m/z 340.35, found m/z 341.36 [M + H]+ and 171.18 [C10H23N2]+.
- Synthesis of (Z)-N1,N1′-(but-2-ene-1,4-diyl)bis(N3-hexylpropane-1,3-diamine) (HPG-5): (yield—53%). 1H NMR (400 MHz, D2O): δ 5.99–5.98 (m, 2 H), 3.89–3.88 (m, 4 H), 3.23–3.15 (m, 8 H), 3.10–3.07 (m, 4 H), 2.19–2.11 (m, 4 H), 1.74–1.67 (m, 4 H), 1.40–1.32 (m, 12 H), and 0.91–0.88 (m, 6 H). 13C NMR (400 MHz, D2O): δ 126.36, 47.88, 44.21, 44.17, 44.12, 30.40, 25.41, 25.30, 22.75, 21.68, and 13.18 ppm. MS (Orbitrap) calculated for C22H48N4, m/z 368.38, found m/z 369.39 [M + H]+ and 185.20 [C11H25N2]+.
- Synthesis of (Z)-N1,N1′-(but-2-ene-1,4-diyl)bis(N3-heptylpropane-1,3-diamine) (HPG-6): (yield—50%). 1H NMR (400 MHz, D2O): δ 5.99 (s, 2 H), 3.89–3.81 (m, 4 H), 3.23–3.15 (m, 8 H), 3.10–3.07 (m, 4 H), 2.19–2.11 (m, 4 H), 1.72–1.67 (m, 4 H), 1.37–1.31 (m, 16 H), and 0.90–0.87 (m, 6 H) ppm. 13C NMR (400 MHz, D2O): δ 126.36, 47.88, 44.21, 44.17, 44.12, 30.75, 27.83, 25.58, 25.46, 22.76, 21.86, and 13.30 ppm. MS (Orbitrap) calculated for C24H52N4, m/z 396.41, found m/z 397.43 [M + H]+ and 199.21 [C12H27N2]+.
- Synthesis of (Z)-N1,N1′-(but-2-ene-1,4-diyl)bis(N3-octylpropane-1,3-diamine) (HPG-7): (yield—51%). 1H NMR (400 MHz, D2O): δ 5.98–5.96 (t, J = 3.6 Hz, 2 H), 3.87–3.86 (m, 4 H), 3.21–3.13 (m, 8 H), 3.08–3.05 (m, 4 H), 2.16–2.09 (m, 4 H), 1.72–1.66 (m, 4 H), 1.39–1.28 (m, 20 H), and 0.88–0.85 (t, J = 5.6 Hz, 6 H) ppm. 13C NMR (400 MHz, D2O): δ 126.36, 47.88, 44.21, 44.17, 44.11, 30.97, 28.12, 28.10, 25.62, 25.45, 22.76, 21.95, and 13.35 ppm. MS (Orbitrap) calculated for C26H56N4, m/z 424.45, found m/z 425.45 [M + H]+ and 213.23 [C13H29N2]+.
- Synthesis of (Z)-N1,N1′-(but-2-ene-1,4-diyl)bis(N3-nonylpropane-1,3-diamine) (HPG-8): (yield—51%). 1H NMR (400 MHz, D2O): δ 5.98 (s, 2 H), 3.89–3.80 (m, 4 H), 3.23–3.15 (m, 8 H), 3.10–3.06 (m, 4 H), 2.18–2.11 (m, 4 H), 1.72–1.67 (m, 4 H), 1.36–1.30 (m, 24 H), and 0.88–0.87 (m, 6 H) ppm. 13C NMR (400 MHz, D2O): δ 126.36, 47.89, 44.21, 44.17, 44.11, 31.08, 28.40, 28.31, 28.14, 25.61, 25.46, 22.76, 22.00, and 13.38 ppm. MS (MALDI) calculated for C28H60N4, m/z 452.48, found 453.41 [M + H]+.
3.3. Measurement of Size and Zeta Potential of the Self-Assembled Nanoparticles
3.4. TEM Imaging
3.5. Cell Lines and Culture Conditions
3.6. SMOX Activity Assay and Enzyme Inhibition Studies
3.7. Cytotoxicity of the PG Analogs In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Casero, R.A.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.V.; Woster, P.M.; Wallace, H.M. Induction of apoptosis in human leukaemic cells by IPENSpm, a novel polyamine analogue and anti-metabolite. Biochem. J. 2002, 367, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ikeguchi, Y.; Mackintosh, C.A.; McCLOSKEY, D.E.; Pegg, A.E. Effect of spermine synthase on the sensitivity of cells to anti-tumour agents. Biochem. J. 2003, 373, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Pledgie, A.; Rubin, E.; Marton, L.J.; Woster, P.M.; Sukumar, S.; Casero, R.A., Jr.; Davidson, N.E. Role of p53/p21Waf1/Cip1 in the regulation of polyamine analogue-induced growth inhibition and cell death in human breast cancer cells. Cancer Biol. Ther. 2005, 4, 1006–1013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seiler, N. Pharmacological aspects of cytotoxic polyamine analogs and derivatives for cancer therapy. Pharmacol. Ther. 2005, 107, 99–119. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Zhang, S.; Wang, B.; Zhao, Y.; Yang, B.; Yu, S. Transfection efficiency of cationic lipids with different hydrophobic domains in gene delivery. Bioconjugate Chem. 2010, 21, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, B.G.; Pattabiraman, N.; Marton, L.J. Molecular mechanics of the interactions of spermine with DNA: DNA bending as a result of ligand binding. Nucleic Acids Res. 1990, 18, 1271–1282. [Google Scholar] [CrossRef]
- Reddy, V.K.; Valasinas, A.; Sarkar, A.; Basu, H.S.; Marton, L.J.; Frydman, B. Conformationally restricted analogues of 1N,12N-bisethylspermine: Synthesis and growth inhibitory effects on human tumor cell lines. J. Med. Chem. 1998, 41, 4723–4732. [Google Scholar] [CrossRef]
- Hacker, A.; Marton, L.J.; Sobolewski, M.; Casero, R.A. In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells. Cancer Chemother. Pharmacol. 2008, 63, 45–53. [Google Scholar] [CrossRef]
- Ignatenko, N.A.; Yerushalmi, H.F.; Pandey, R.; Kachel, K.L.; Stringer, D.E.; Marton, L.J.; Gerner, E.W. Gene expression analysis of HCT116 colon tumor-derived cells treated with the polyamine analog PG-11047. Cancer Genom. Proteomacol. 2009, 6, 161–175. [Google Scholar]
- Streiff, R.R.; Bender, J.F. Phase 1 study of N1-N11-diethylnorspermine (DENSPM) administered TID for 6 days in patients with advanced malignancies. Investig. New Drugs 2001, 19, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Bowling, M.; DeClue, C.; Armstrong, D.; Fetting, J.; Casero, R.; Davidson, N. A phase I study of diethylnorspermine (DENSPM) in previously treated patients with metastatic breast cancer (MBC). Breast Cancer Res. Treat. 2001, 69, 286. [Google Scholar]
- Dong, Y.; Zhu, Y.; Li, J.; Zhou, Q.-H.; Wu, C.; Oupickyý, D. Synthesis of bisethylnorspermine lipid prodrug as gene delivery vector targeting polyamine metabolism in breast cancer. Mol. Pharm. 2012, 9, 1654–1664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, Y.; Li, J.; Kanvinde, S.; Lin, Z.; Hazeldine, S.; Singh, R.K.; Oupicky, D. Self-immolative polycations as gene delivery vectors and prodrugs targeting polyamine metabolism in cancer. Mol. Pharm. 2015, 12, 332–341. [Google Scholar] [CrossRef]
- Laskar, P.; Dufes, C. Emergence of cationic polyamine dendrimersomes: Design, stimuli sensitivity and potential biomedical applications. Nanoscale Adv. 2021, 3, 6007–6026. [Google Scholar] [CrossRef]
- Puchkov, P.A.; Maslov, M.A. Lipophilic Polyamines as Promising Components of Liposomal Gene Delivery Systems. Pharmaceutics 2021, 13, 920. [Google Scholar] [CrossRef]
- Valasinas, A.; Sarkar, A.; Reddy, V.K.; Marton, L.J.; Basu, H.S.; Frydman, B. Conformationally restricted analogues of 1N,14N-bisethylhomospermine (BE-4-4-4): Synthesis and growth inhibitory effects on human prostate cancer cells. J. Med. Chem. 2001, 44, 390–403. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Magazù, S.; Calandra, P. Amphiphiles self-assembly: Basic concepts and future perspectives of supramolecular approaches. Adv. Condens. Matter Phys. 2015, 2015, 1–22. [Google Scholar] [CrossRef]
- Chaturvedi, R.; de Sablet, T.; Asim, M.; Piazuelo, M.B.; Barry, D.P.; Verriere, T.G.; Sierra, J.C.; Hardbower, D.M.; Delgado, A.G.; Schneider, B.G.; et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene 2015, 34, 3429–3440. [Google Scholar] [CrossRef]
- Sierra, J.C.; Piazuelo, M.B.; Luis, P.B.; Barry, D.P.; Allaman, M.M.; Asim, M.; Sebrell, T.A.; Finley, J.L.; Rose, K.L.; Hill, S.; et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene 2020, 39, 4465–4474. [Google Scholar] [CrossRef]
- Dunston, T.T.; Khomutov, M.A.; Gabelli, S.B.; Stewart, T.M.; Foley, J.R.; Kochetkov, S.N.; Khomutov, A.R.; Casero, R.A., Jr. Identification of a Novel Substrate-Derived Spermine Oxidase Inhibitor. Acta Naturae 2020, 12, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Hang, Y.; Tang, S.; Tang, W.; Vetvicka, D.; Zhang, C.; Xie, Y.; Yu, F.; Yu, A.; Sil, D.; Li, J.; et al. Polycation fluorination improves intraperitoneal siRNA delivery in metastatic pancreatic cancer. J. Control. Release 2021, 333, 139–150. [Google Scholar] [CrossRef] [PubMed]

| Sample | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| HPG-2 | 173 ± 7 | 0.21 | 27 |
| HPG-3 | 186 ± 3 | 0.064 | 24 |
| HPG-4 | 174 ± 3 | 0.15 | 15 |
| HPG-5 | 158 ± 4 | 0.23 | 16 |
| HPG-6 | 225 ± 6 | 0.17 | 22 |
| HPG-7 | 318 ± 8 | 0.13 | 11 |
| HPG-8 | 626 ± 83 | 0.17 | 17 |
| Sample Code | IC50 HCT116 | IC50 A549 | IC50 SMOX * |
|---|---|---|---|
| PG11047 | 8 µM | >10 µM | ND |
| HPG-2 | <1.0 µM | <1.0 µM | ND |
| HPG-3 | 8 µM | >10 µM | ND |
| HPG-4 | >10 µM | 10 µM | ND |
| HPG-5 | 8 µM | 7 µM | 90 µM |
| HPG-6 | 3 µM | 3 µM | 30 µM |
| HPG-7 | 1.0 µM | <1.0 µM | <10 µM |
| HPG-8 | <1.0 µM | 7 µM | <10 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sil, D.; Panja, S.; Jogdeo, C.M.; Kumar, R.; Yu, A.; Holbert, C.E.; Ding, L.; Foley, J.R.; Stewart, T.M.; Casero, R.A., Jr.; et al. Self-Assembled Alkylated Polyamine Analogs as Supramolecular Anticancer Agents. Molecules 2022, 27, 2441. https://doi.org/10.3390/molecules27082441
Sil D, Panja S, Jogdeo CM, Kumar R, Yu A, Holbert CE, Ding L, Foley JR, Stewart TM, Casero RA Jr., et al. Self-Assembled Alkylated Polyamine Analogs as Supramolecular Anticancer Agents. Molecules. 2022; 27(8):2441. https://doi.org/10.3390/molecules27082441
Chicago/Turabian StyleSil, Diptesh, Sudipta Panja, Chinmay M. Jogdeo, Raj Kumar, Ao Yu, Cassandra E. Holbert, Ling Ding, Jackson R. Foley, Tracy Murray Stewart, Robert A. Casero, Jr., and et al. 2022. "Self-Assembled Alkylated Polyamine Analogs as Supramolecular Anticancer Agents" Molecules 27, no. 8: 2441. https://doi.org/10.3390/molecules27082441
APA StyleSil, D., Panja, S., Jogdeo, C. M., Kumar, R., Yu, A., Holbert, C. E., Ding, L., Foley, J. R., Stewart, T. M., Casero, R. A., Jr., & Oupický, D. (2022). Self-Assembled Alkylated Polyamine Analogs as Supramolecular Anticancer Agents. Molecules, 27(8), 2441. https://doi.org/10.3390/molecules27082441










