New Calix[4]arene—Fluoresceine Conjugate by Click Approach—Synthesis and Preparation of Photocatalytically Active Solid Lipid Nanoparticles
Abstract
1. Introduction
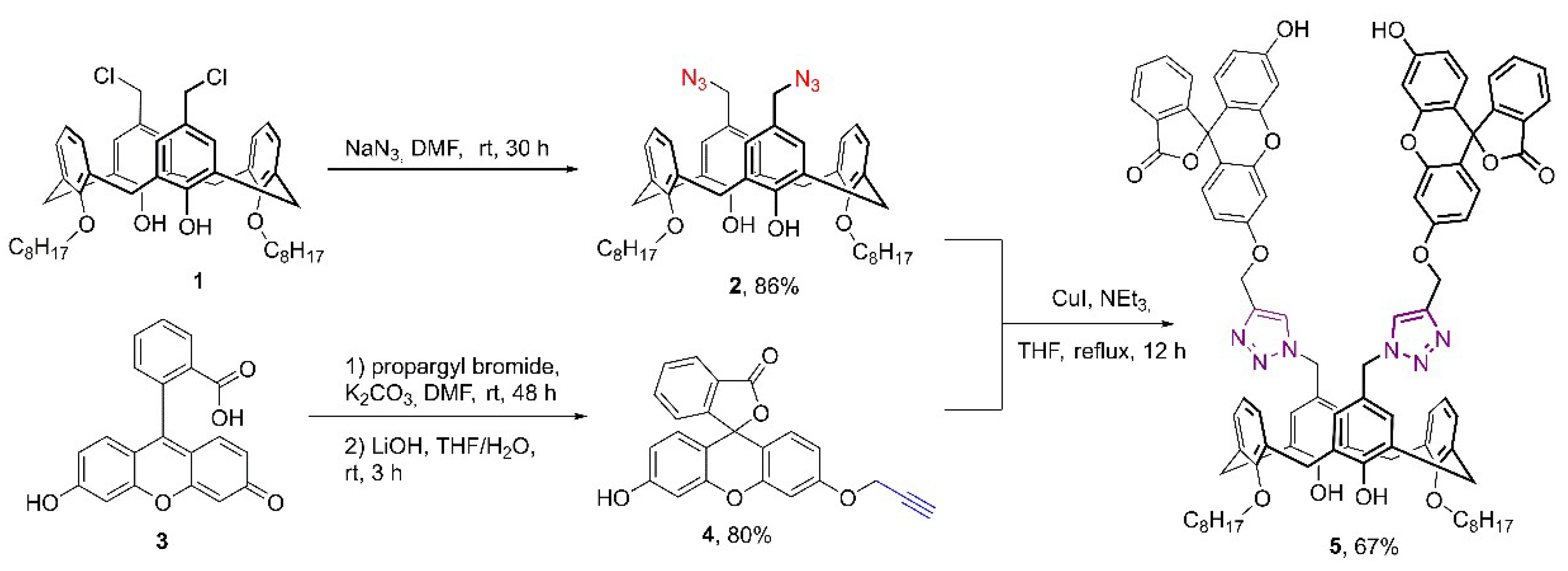
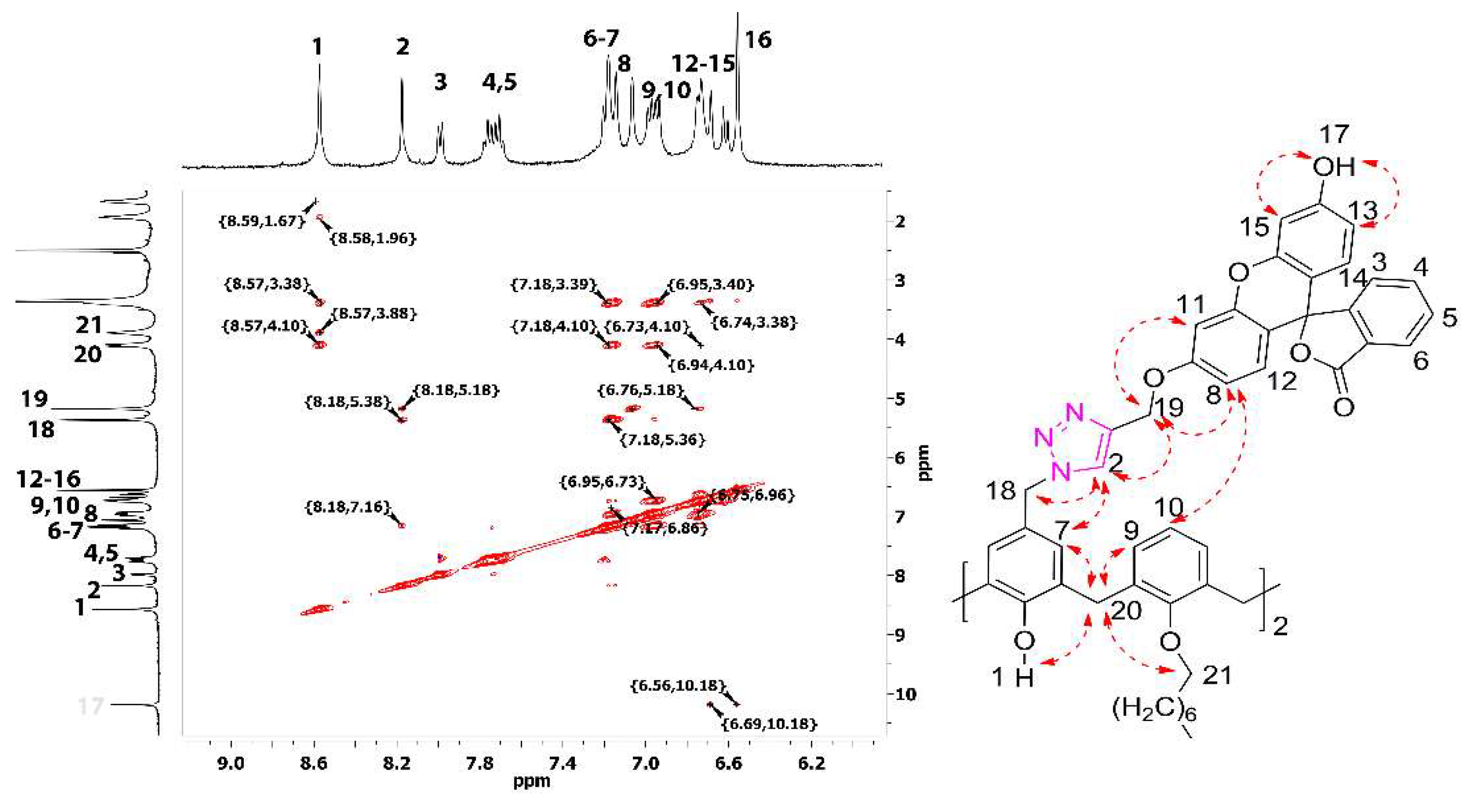
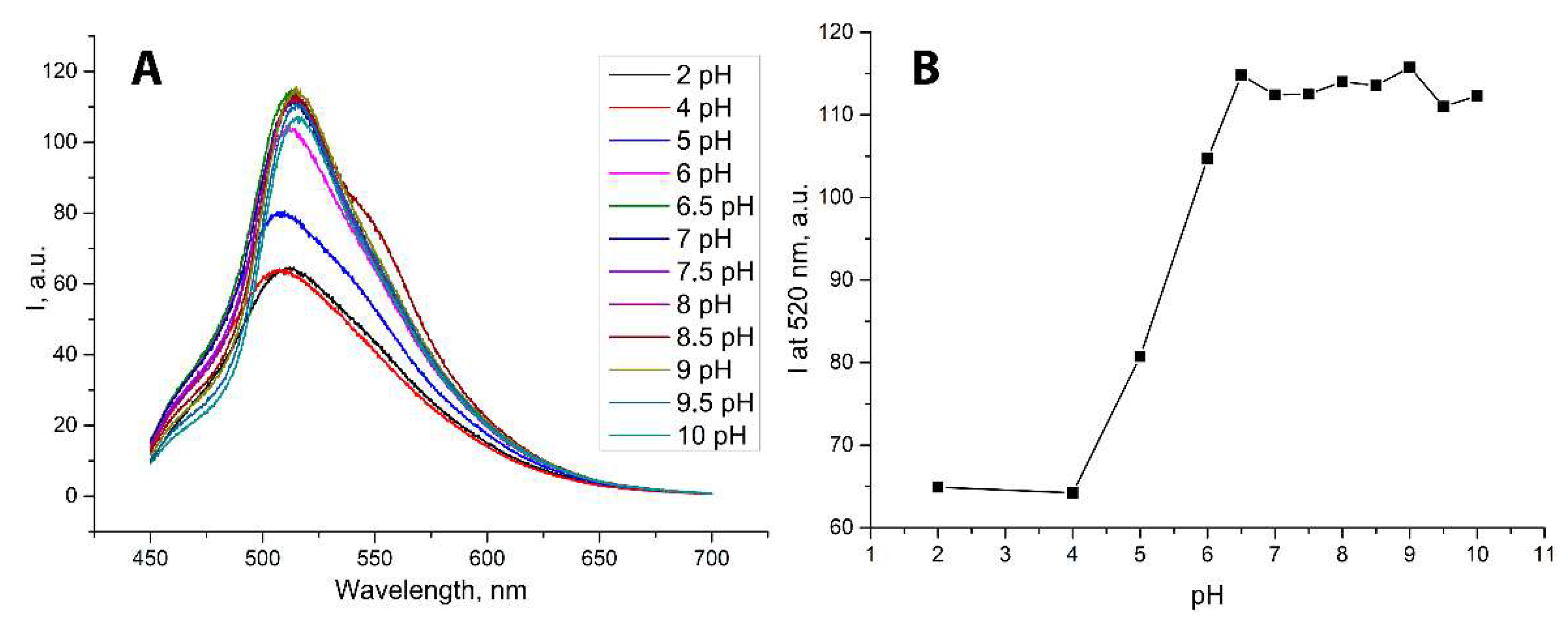
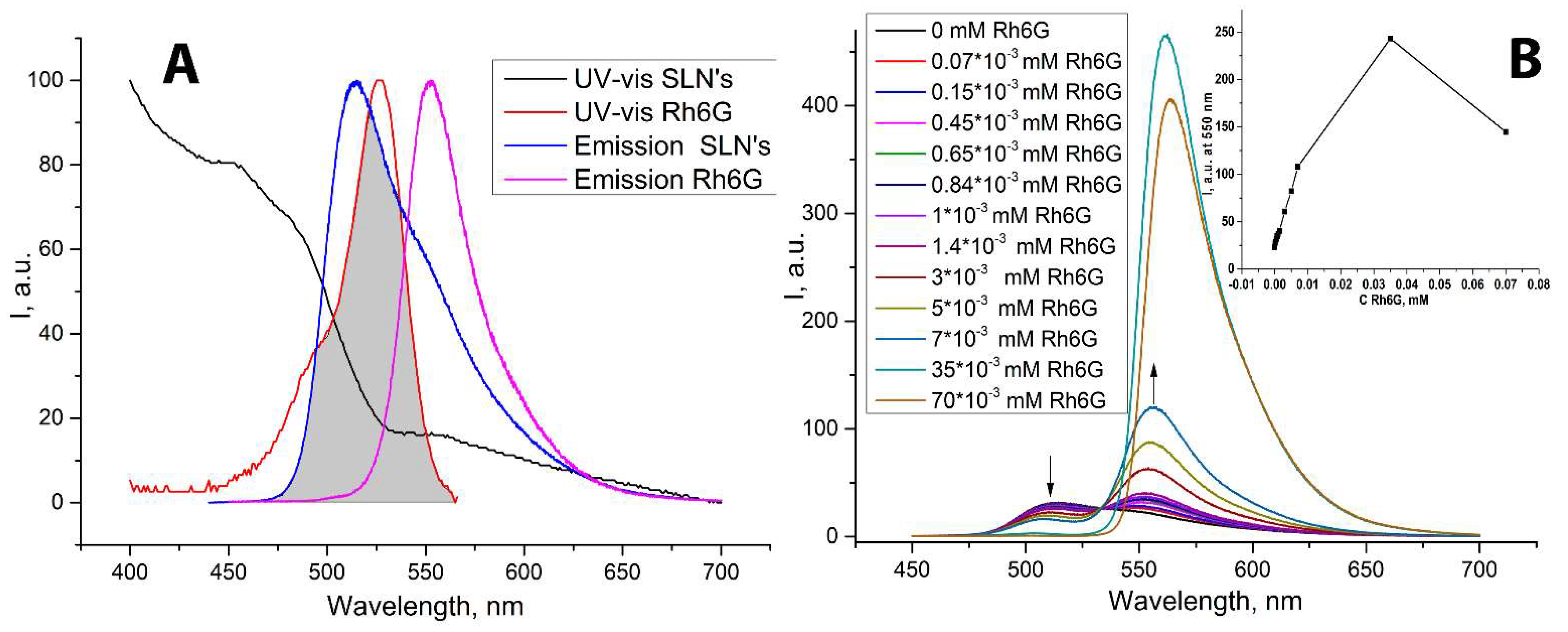
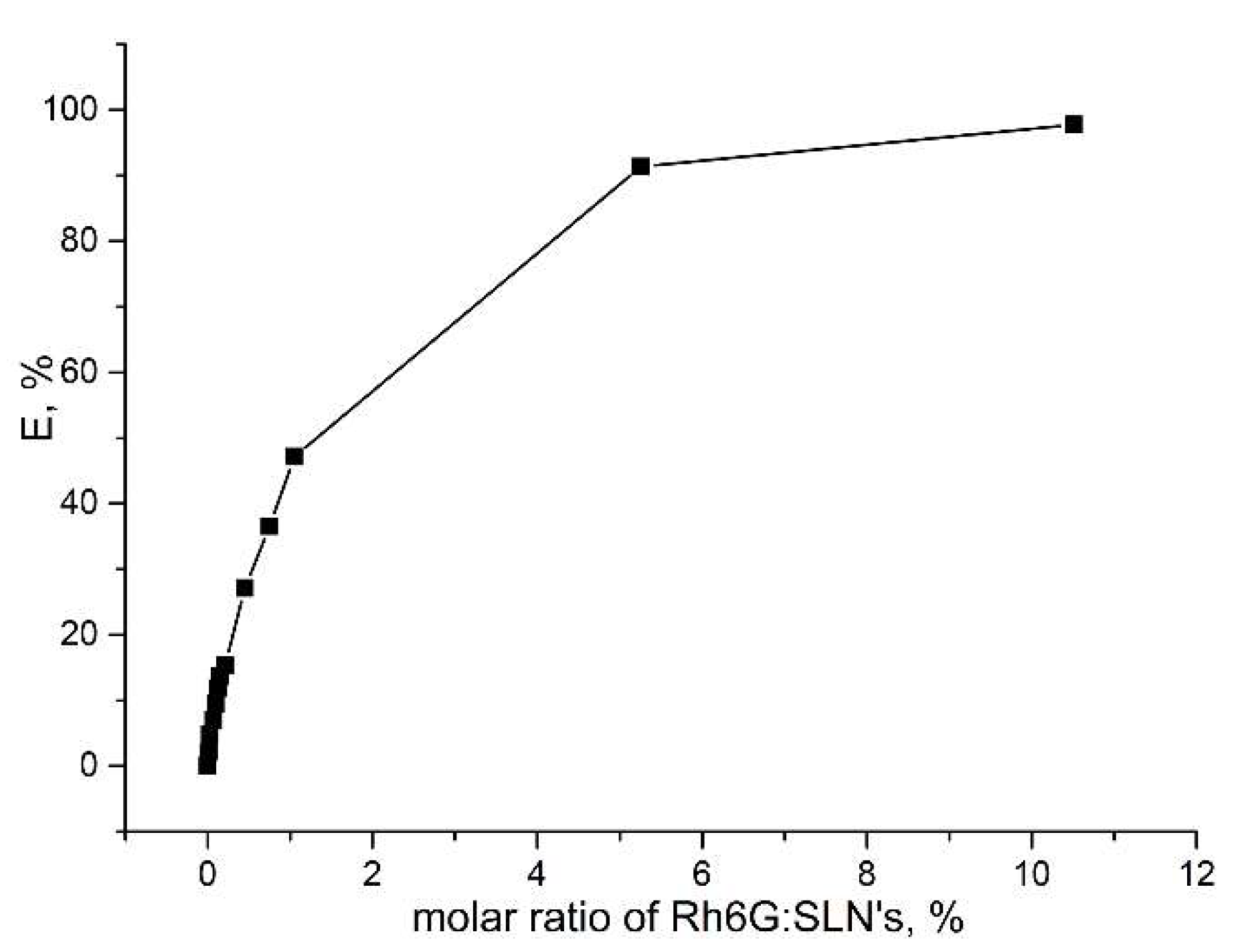
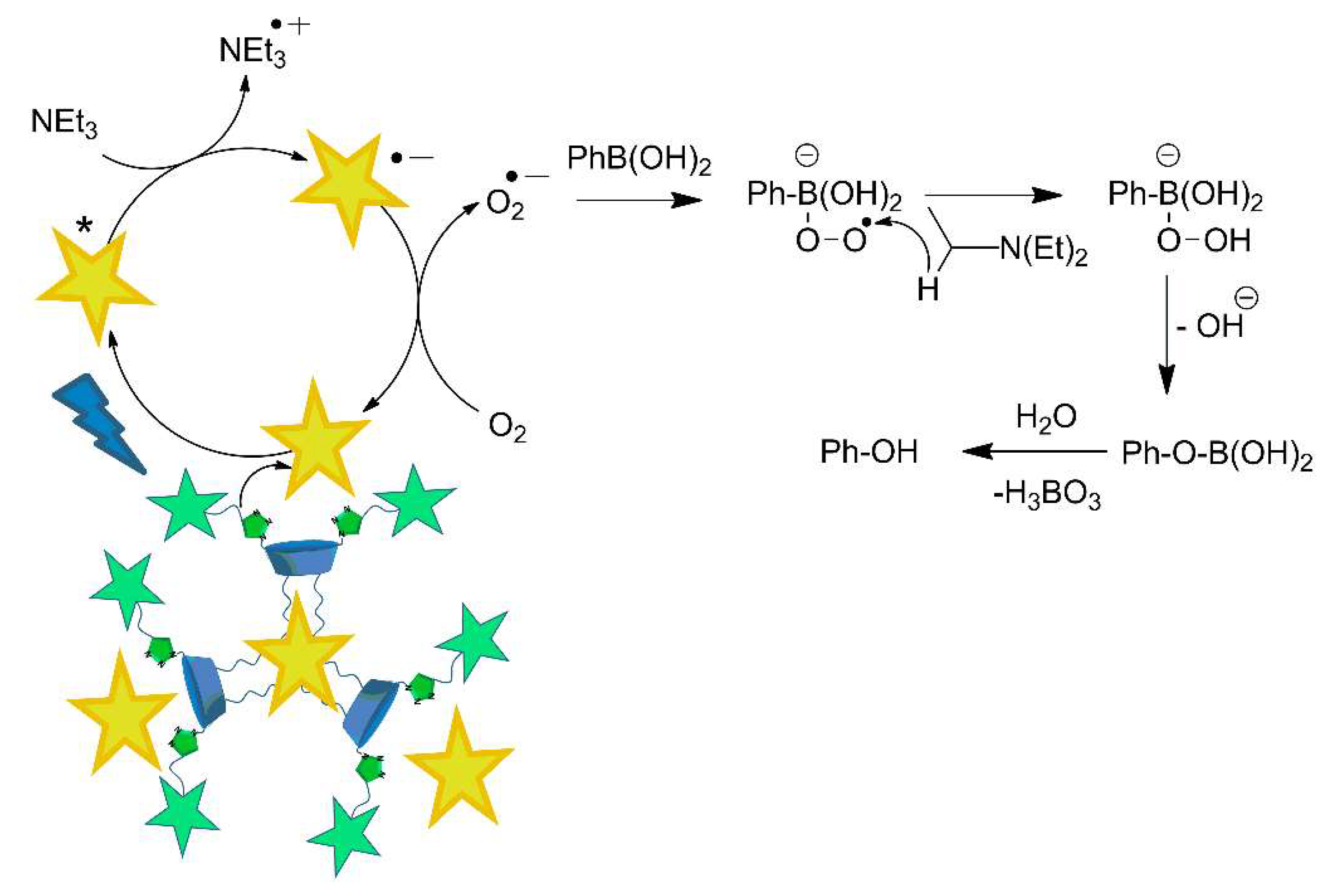
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of SLNs
3.2. Photocatalytic Oxidation of Phenylboronic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lehn, J.-M. Perspectives in Supramolecular Chemistry-From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew. Chem. Int.Ed. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, W. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y. Biomedical Application of Supramolecular Systems Based on Host-Guest Interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sedgwick, A.C.; Hirao, T.; Sessler, J.L. Supramolecular fluorescent sensors: An historical overview and update. Coord. Chem. Rev. 2021, 427, 213560. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gao, A.; Hou, J.-T.; Yi, T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coord. Chem. Rev. 2021, 434, 213792. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kubota, R.; Minami, T. Molecular self-assembled chemosensors and their arrays. Coord. Chem. Rev. 2021, 429, 213607. [Google Scholar] [CrossRef]
- Zou, Q.; Bao, J.; Yan, X. Functional Nanomaterials Based on Self-Assembly of Endogenic NIR-Absorbing Pigments for Diagnostic and Therapeutic Applications. Small Methods 2022, 2101359. [Google Scholar] [CrossRef]
- Han, J.; Li, H.; Yoon, J. Activated supramolecular nano-agents: From diagnosis to imaging-guided tumor treatment. Nano Today 2022, 43, 101392. [Google Scholar] [CrossRef]
- Zuo, M.; Velmurugan, K.; Wang, K.; Tian, X.; Hu, X.-Y. Insight into functionalized-macrocycles-guided supramolecular photocatalysis. Beilstein J. Org. Chem. 2021, 17, 139–155. [Google Scholar] [CrossRef]
- Fan, X.; Guo, X. Development of calixarene-based drug nanocarriers. J. Mol. Liq. 2021, 325, 115246. [Google Scholar] [CrossRef]
- Baldini, L.; Casnati, A.; Sansone, F. Multivalent and Multifunctional Calixarenes in Bionanotechnology. Eur. J. Org. Chem. 2020, 2020, 5056–5069. [Google Scholar] [CrossRef]
- Wang, J.; Ding, X.; Guo, X. Assembly behaviors of calixarene-based amphiphile and supra-amphiphile and the applications in drug delivery and protein recognition. Adv. Colloid Interface Sci. 2019, 269, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikov, A.; Sollovieva, S.; Antipin, I.; Ferlay, S. Coordination Polymers based on calixarene derivatives: Structures and properties. Coord. Chem. Rev. 2017, 352, 151–186. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef]
- Heina, J.E.; Fokin, V.V. Copper-catalyzed azide–alkynecycloaddition (CuAAC) and beyond: New reactivity of copper(i) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Antipin, I.S.; Alfimov, M.V.; Arslanov, V.V.; Burilov, V.A.; Vatsadze, S.Z.; Voloshin, Y.Z.; Volcho, K.P.; Gorbatchuk, V.V.; Gorbunova, Y.G.; Gromov, S.P.; et al. Functional supramolecular systems: Design and applications. Russ. Chem. Rev. 2021, 90, 895–1107. [Google Scholar] [CrossRef]
- Solovieva, S.E.; Burilov, V.A.; Antipin, I.S. Thiacalix[4]arene’s Lower Rim Derivatives: Synthesis and Supramolecular Properties. Macroheterocycles 2017, 10, 134–146. [Google Scholar] [CrossRef][Green Version]
- Burilov, V.A.; Nugmanov, R.I.; Ibragimova, R.R.; Solovieva, S.E.; Antipin, I.S. ‘Click chemistry’ in the synthesis of new amphiphilic 1,3-alternate thiacalixarenes. Mend. Comm. 2015, 25, 177–179. [Google Scholar] [CrossRef]
- Burilov, V.; Mironova, D.; Sultanova, E.; Garipova, R.; Evtugyn, V.; Solovieva, S.; Antipin, I. NHC Polymeric Particles Obtained by Self-Assembly and Click Approach of Calix[4]Arene Amphiphiles as Support for Catalytically Active Pd Nanoclusters. Molecules 2021, 26, 6864. [Google Scholar] [CrossRef]
- Burilov, V.A.; Garipova, R.I.; Solovieva, S.E.; Antipin, I.S. Synthesis of Bifunctional Derivatives of Calix[4]arene Bearing Azidoalkyl Fragments in Cone Stereoisomeric Form. Dokl. Chem. 2020, 490, 1–5. [Google Scholar] [CrossRef]
- Huang, Z.-T.; Wang, G.-Q.; Yang, L.-M.; Lou, Y.-X. The Selective Chloromethylation of 25,27-Dihydroxy-26,28-Dimethoxycalix[4]arene and Nucleophilic Substitution Therefrom. Synth. Commun. 1995, 25, 1109–1118. [Google Scholar] [CrossRef]
- Chen, L.; Hu, T.-S.; Zhu, J.; Wu, H.; Yao, Z.-J. Application of a Regioselective Mannich Reaction on Naringenin and its Use in Fluorescent Labeling. Synlett 2006, 8, 1225–1229. [Google Scholar]
- Gutsche, C.D. Calixarenes; Royal Society of Chemistry: Cambridge, UK, 1989. [Google Scholar]
- McHedlov-Petrossyan, N.O.; Vodolazkaya, N.A.; Surov, Y.N.; Samoylov, D.V. 2,4,5,7-Tetranitrofluorescein in solutions: Novel type of tautomerism in hydroxyxanthene series as detected by various spectral methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 2747–2760. [Google Scholar] [CrossRef] [PubMed]
- Markuszewski, R.; Diehl, H. The infrared spectra and structures of the three solid forms of fluorescein and related compounds. Talanta 1980, 27, 937–946. [Google Scholar] [CrossRef]
- Schubert, M.A.; Muller-Goymann, C.C. Solvent injection as a new approach for manufacturing lipid nanoparticles-evaluation of the method and process parameters. Eur. J. Pharm. Biopharm. 2003, 55, 125–131. [Google Scholar] [CrossRef]
- Batistela, V.R.; da Costa Cedran, J.; de Oliveira, H.P.M.; Scarminio, I.S.; Ueno, L.T.; da Hora Machado, A.E.; Hioka, N. Protolytic fluorescein species evaluated using chemometry and DFT studies. Dye. Pigment. 2010, 86, 15–24. [Google Scholar] [CrossRef]
- Mchedlov-Petrossyan, N.O.; Cheipesh, T.A.; Shekhovtsov, S.V.; Redko, A.N.; Rybachenko, V.I.; Omelchenko, I.V.; Shishkin, O.V. Ionization and tautomerism of methyl fluorescein and related dyes. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2015, 150, 151–161. [Google Scholar] [CrossRef]
- Abboud, J.-L.M.; Foces-Foces, C.; Notario, R.; Trifonov, R.E.; Volovodenko, A.P.; Ostrovskii, V.A.; Alkorta, I.; Elguero, J. Basicity of N-H- and N-Methyl-1,2,3-triazoles in the Gas Phase, in Solution, and in the Solid State—An Experimental and Theoretical Study. Eur. J. Org. Chem. 2001, 2001, 3013–3024. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Springer: Boston, MA, USA, 1999; p. 698. [Google Scholar]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-Based Small-Molecule Fluorescent Probes: Rational Design and Bioimaging Applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Zhou, L.-Y.; Xu, L. Photocatalysis in Supramolecular Fluorescent Metallacycles and Metallacages. Chem. Asian J. 2021, 16, 3805–3816. [Google Scholar] [CrossRef]
- Saha, J.; Datta Roy, A.; Dey, D.; Chakraborty, S.; Bhattacharjee, D.; Paul, P.K.; Hussain, S.A. Investigation of Fluorescence Resonance Energy Transfer between Fluorescein and Rhodamine 6G. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2015, 149, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K.; Joshi, H.C.; Pant, T.C. Migration effects on excitation energy transfer by decay analysis using a nanosecond fluorimeter. Chem. Phys. Lett. 1988, 148, 472–478. [Google Scholar] [CrossRef]
- Bojarski, P.; Kułak, L.; Kubicki, J. Donor fluorescence decay in the presence of forward and reverse excitation energy transport in two-component disordered systems. Chem. Phys. Lett. 2000, 318, 379–384. [Google Scholar] [CrossRef]
- Zou, Y.-Q.; Chen, J.-R.; Liu, X.-P.; Lu, L.-Q.; Davis, R.L.; Jørgensen, K.A.; Xiao, W.-J. Highly Efficient Aerobic Oxidative Hydroxylation of Arylboronic Acids: Photoredox Catalysis Using Visible Light. Angew. Chem. Int. Ed. 2012, 51, 784–788. [Google Scholar] [CrossRef]
- Pitre, S.P.; McTiernan, C.D.; Ismaili, H.; Scaiano, J.C. Mechanistic Insights and Kinetic Analysis for the Oxidative Hydroxylation of Arylboronic Acids by Visible Light Photoredox Catalysis: A Metal-Free Alternative. J. Am. Chem. Soc. 2013, 135, 13286–13289. [Google Scholar] [CrossRef]
- Kumar, I.; Sharma, R.; Kumar, R.; Kumar, R.; Sharma, U. C70 Fullerene-Catalyzed Metal-Free Photocatalytic ipso-Hydroxylation of Aryl Boronic Acids: Synthesis of Phenols. Adv. Synth. Catal. 2018, 360, 2013. [Google Scholar] [CrossRef]
- Guo, W.; Lu, L.-Q.; Wang, Y.; Wang, Y.-N.; Chen, J.-R.; Xiao, W.-J. Metal-Free, Room-Temperature, Radical Alkoxycarbonylation of Aryldiazonium Salts through Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2015, 54, 2265–2269. [Google Scholar] [CrossRef]
- Zhang, T.-X.; Li, J.-J.; Li, H.-B.; Guo, D.-S. Deep Cavitand Calixarene–Solubilized Fullerene as a Potential Photodynamic Agent. Front. Chem. 2021, 9, 710808. [Google Scholar] [CrossRef]
- Chen, X.; Häkkinen, H. Protected but Accessible: Oxygen Activation by a Calixarene-Stabilized Undecagold Cluster. J. Am. Chem. Soc. 2013, 135, 12944–12947. [Google Scholar] [CrossRef]
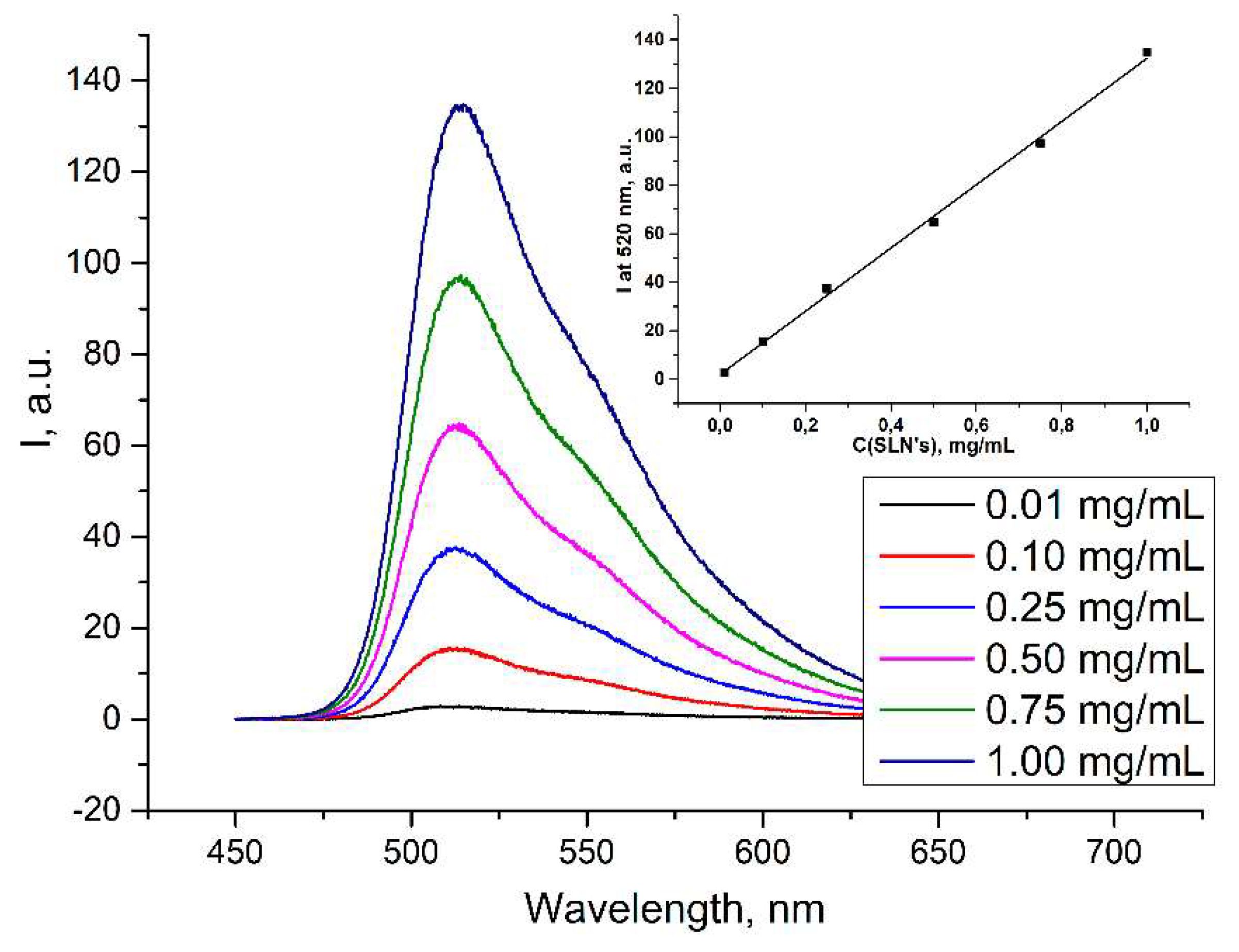


| C of SLNs, mg/mL | d, nm | PDI | ζ, mV |
|---|---|---|---|
| 0.01 | 152 ± 19 | 0.339 ± 0.073 | −21 ± 4 |
| 0.1 | 98 ± 1 | 0.194 ± 0.008 | |
| 0.25 | 97 ± 1 | 0.146 ± 0.017 | |
| 0.5 | 100 ± 1 | 0.145 ± 0.013 | −30 ± 1 |
| 0.7 | 109 ± 1 | 0.137 ± 0.009 | |
| 1.0 | 116 ± 1 | 0.134 ± 0.012 | −31 ± 1 |
| 3.0 | 541 ± 22 | 0.455 ± 0.043 | −25 ± 3 |
| pH | d, nm | PDI | ζ, mV |
|---|---|---|---|
| 2 | 92 ± 1 | 0.115 ± 0.009 | +30 ± 8 |
| 4 | 458 ± 147 | 0.499 ± 0.134 | +8 ± 1 |
| 5 | 3357 ± 3647 | 0.927 ± 0.110 | −18 ± 3 |
| 6 | 749 ± 440 | 0.673 ± 0.257 | −16 ± 1 |
| 6.5 | 144 ± 1 | 0.095 ± 0.019 | −14 ± 1 |
| 7.1 | 226 ± 2 | 0.253 ± 0.005 | −24 ± 1 |
| 7.5 | 100 ± 1 | 0.133 ± 0.010 | −27 ± 1 |
| 8 | 101 ± 1 | 0.162 ± 0.006 | −27 ± 1 |
| 8.6 | 78 ± 1 | 0.192 ± 0.003 | −27 ± 1 |
| 9 | 77 ± 1 | 0.241 ± 0.011 | −29 ± 1 |
| 9.5 | 57 ± 1 | 0.328 ± 0.011 | −29 ± 1 |
| 10 | 64 ± 1 | 0.387 ± 0.017 | −20 ± 2 |
| System | Phenol Yield, % 2 |
|---|---|
| Fluorescein | 6 |
| Rh6G | 3 |
| Fluorescein + Rh6G | 22 |
| SLNs | 64 |
| SLNs+Rh6G | 96 |
| SLNs+Rh6G no light | 1 |
| no photocatalyst | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burilov, V.A.; Artemenko, A.A.; Garipova, R.I.; Amirova, R.R.; Fatykhova, A.M.; Borisova, J.A.; Mironova, D.A.; Sultanova, E.D.; Evtugyn, V.G.; Solovieva, S.E.; et al. New Calix[4]arene—Fluoresceine Conjugate by Click Approach—Synthesis and Preparation of Photocatalytically Active Solid Lipid Nanoparticles. Molecules 2022, 27, 2436. https://doi.org/10.3390/molecules27082436
Burilov VA, Artemenko AA, Garipova RI, Amirova RR, Fatykhova AM, Borisova JA, Mironova DA, Sultanova ED, Evtugyn VG, Solovieva SE, et al. New Calix[4]arene—Fluoresceine Conjugate by Click Approach—Synthesis and Preparation of Photocatalytically Active Solid Lipid Nanoparticles. Molecules. 2022; 27(8):2436. https://doi.org/10.3390/molecules27082436
Chicago/Turabian StyleBurilov, Vladimir A., Alina A. Artemenko, Ramilya I. Garipova, Rezeda R. Amirova, Aigul M. Fatykhova, Julia A. Borisova, Diana A. Mironova, Elza D. Sultanova, Vladimir G. Evtugyn, Svetlana E. Solovieva, and et al. 2022. "New Calix[4]arene—Fluoresceine Conjugate by Click Approach—Synthesis and Preparation of Photocatalytically Active Solid Lipid Nanoparticles" Molecules 27, no. 8: 2436. https://doi.org/10.3390/molecules27082436
APA StyleBurilov, V. A., Artemenko, A. A., Garipova, R. I., Amirova, R. R., Fatykhova, A. M., Borisova, J. A., Mironova, D. A., Sultanova, E. D., Evtugyn, V. G., Solovieva, S. E., & Antipin, I. S. (2022). New Calix[4]arene—Fluoresceine Conjugate by Click Approach—Synthesis and Preparation of Photocatalytically Active Solid Lipid Nanoparticles. Molecules, 27(8), 2436. https://doi.org/10.3390/molecules27082436






