Synergistic Effect of Lithocholic Acid with Gentamicin against Gram-Positive Bacteria but Not against Gram-Negative Bacteria
Abstract
:1. Introduction
2. Results
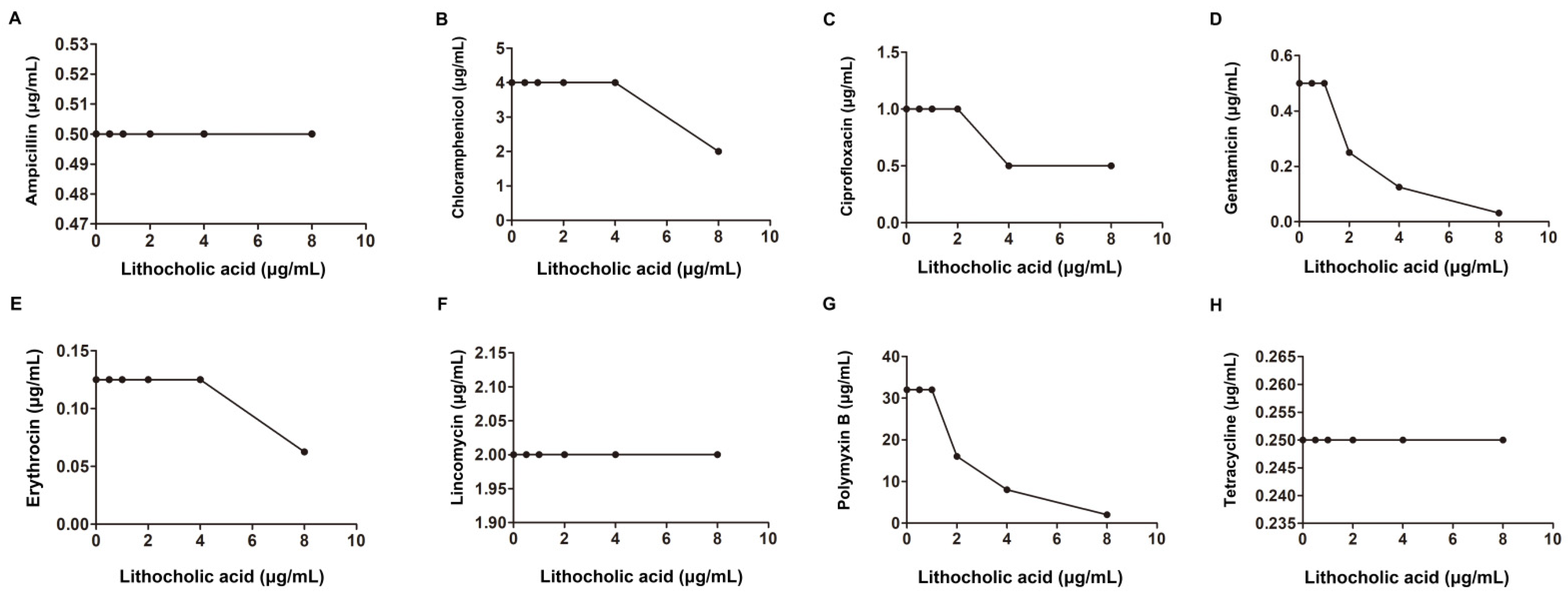
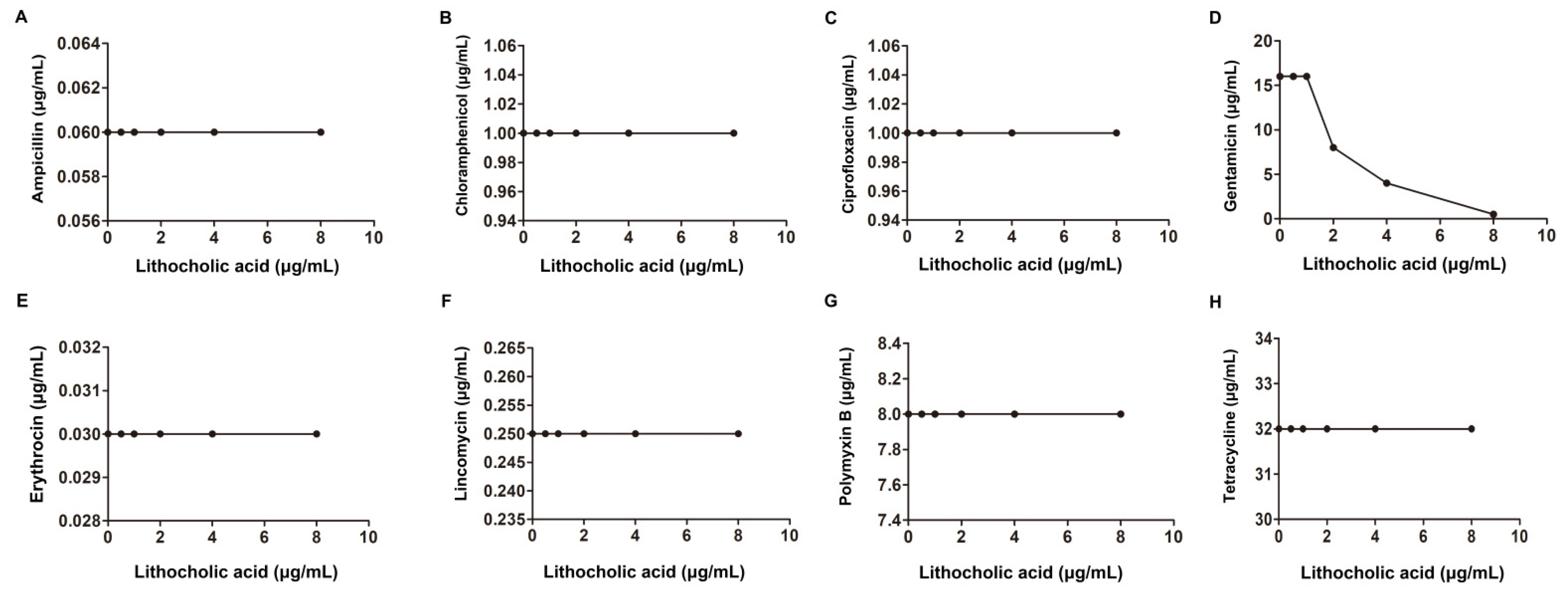
2.1. LCA Improves L. monocytogenes Sensitivity to Genta In Vitro
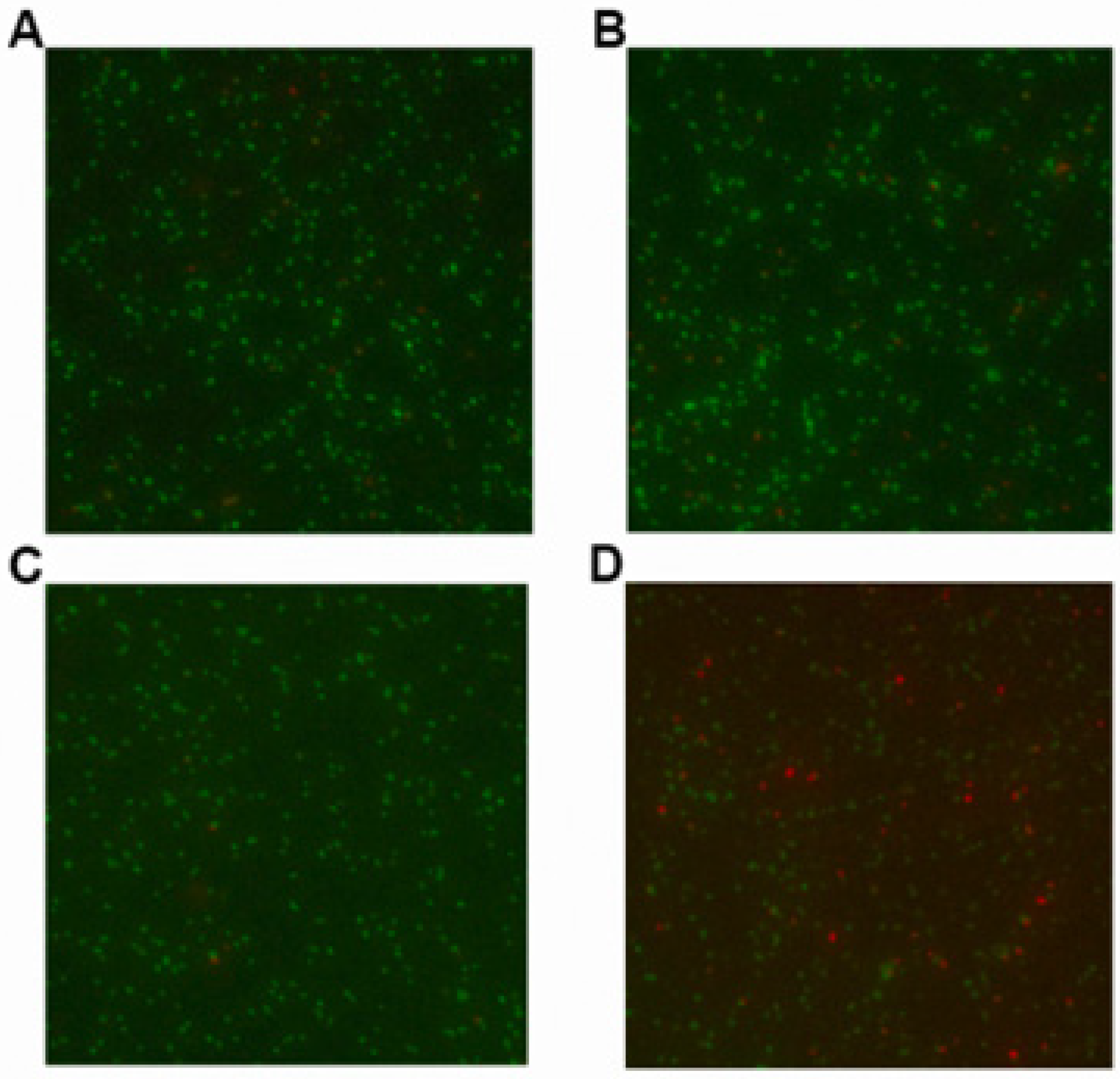
2.2. LCA in Combination with Genta Cause Cell Membrane Injure of L. monocytogenes
2.3. LCA Combined with Genta Induces Morphological Changes in L. monocytogenes
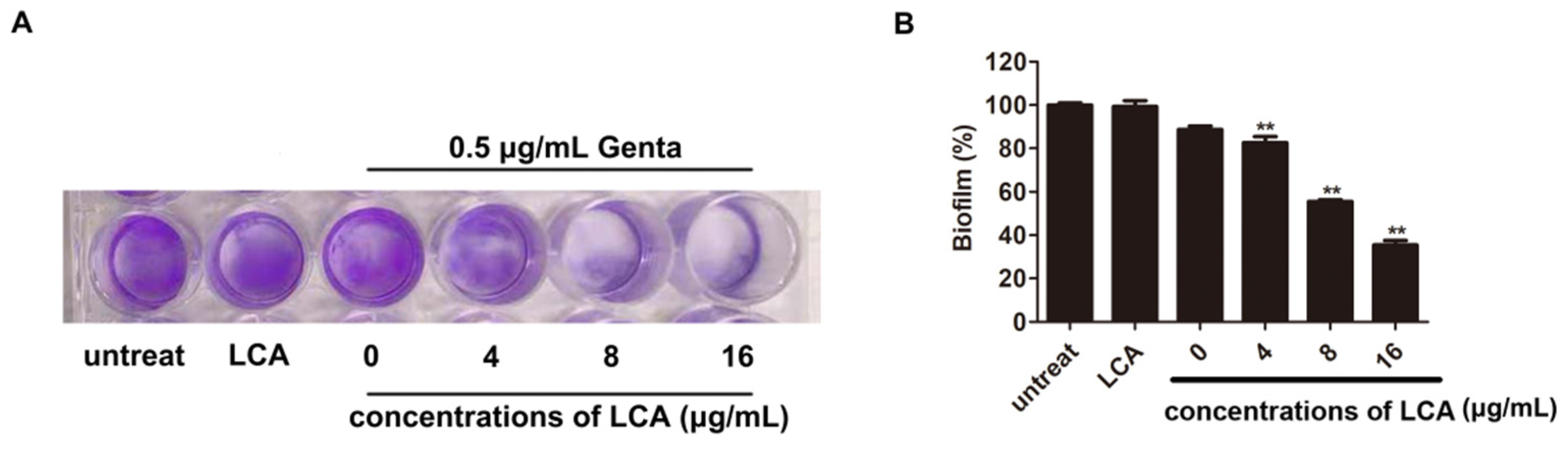
2.4. LCA Improves the Inhibition of L. monocytogenes Biofilm Formation by Genta
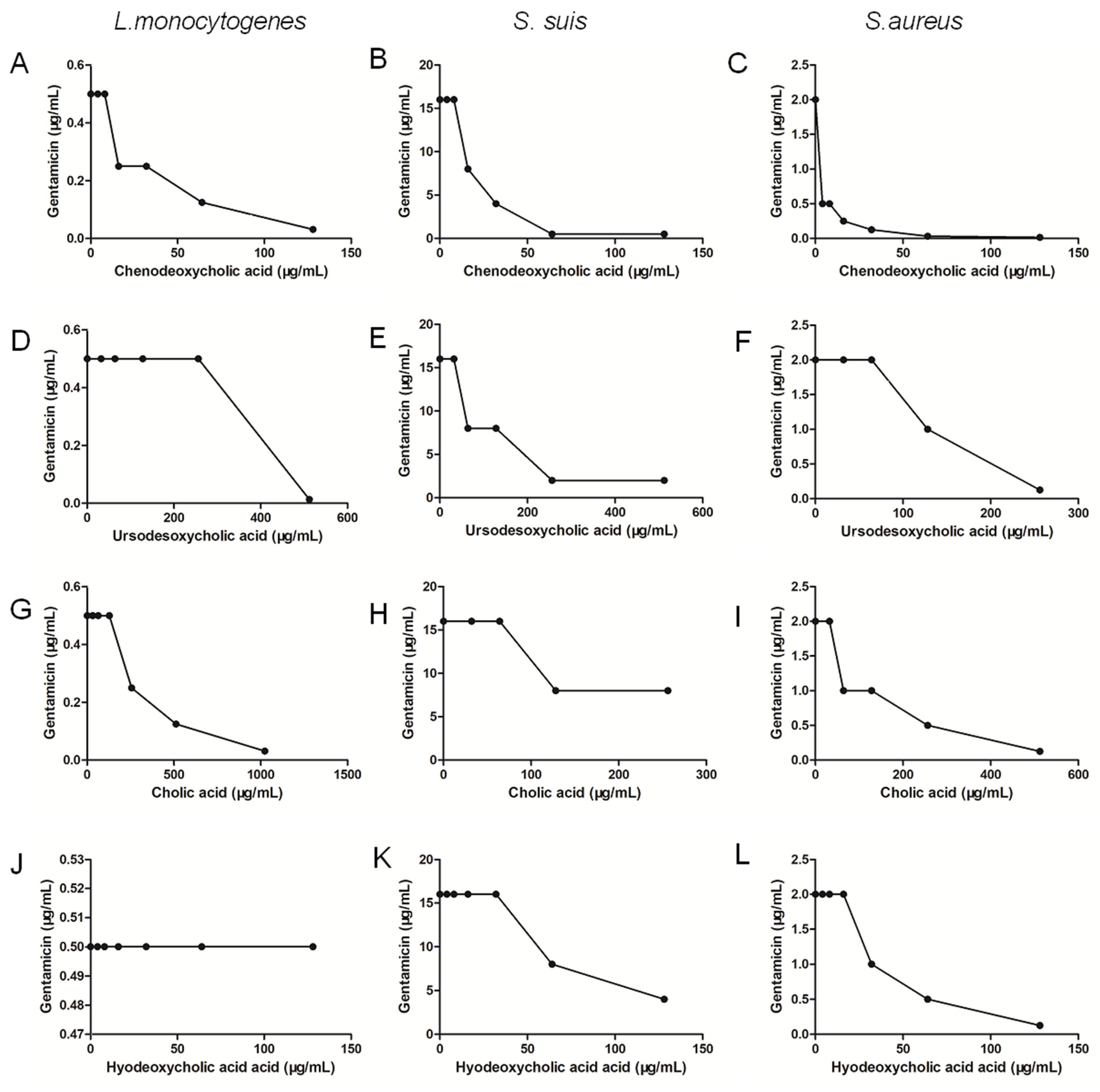
2.5. Chenodeoxycholic Acid Also Improves the Bactericidal Effect of Genta against Gram-Positive Bacteria
3. Discussion
4. Materials and Methods
4.1. Microbial Strains, Reagents and Growth Conditions
4.2. MIC and FIC Index Determination
4.3. Growth Curve Assay
4.4. BacLight LIVE/DEAD Staining Experiments
4.5. Scanning Electron Microscopy Analysis
4.6. Biofilm Inhibition Assays
4.7. FIC Index Determination between Cholic Acid Derivative and Genta
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Käferstein, F. Foodborne diseases in developing countries: Aetiology, epidemiology and strategies for prevention. Int. J. Environ. Health Res. 2003, 13 (Suppl. 1), S161–S168. [Google Scholar] [CrossRef] [PubMed]
- Bisholo, K.Z.; Ghuman, S.; Haffejee, F. Food-borne disease prevalence in rural villages in the Eastern Cape, South Africa. Afr. J. Prim. Health Care Fam. Med. 2018, 10, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Evivie, S.E.; Muhammad, Z.; Luo, G.W.; Liang, H.Z.; Wang, N.N.; Huo, G.C. In vitro assessment of the antimicrobial potentials of Lactobacillus helveticus strains isolated from traditional cheese in Sinkiang China against food-borne pathogens. Food Funct. 2016, 7, 789–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madjunkov, M.; Chaudhry, S.; Ito, S. Listeriosis during pregnancy. Obstet. Gynecol. Surv. 1998, 53, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Liu, D.; Xu, L.; Tenguria, S.; Drolia, R.; Gallina, N.L.F.; Cox, A.D.; Koo, O.-K.; Bhunia, A.K. Biofilm-isolated Listeria monocytogenes exhibits reduced systemic dissemination at the early (12-24 h) stage of infection in a mouse model. NPJ Biofilms Microbiomes 2021, 7, 18. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macia, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Kim, Y.-M. Antibiotics Application Strategies to Control Biofilm Formation in Pathogenic Bacteria. Curr. Pharm. Biotechnol. 2020, 21, 270–286. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef] [Green Version]
- Fiorucci, S.; Distrutti, E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol. Med. 2015, 21, 702–714. [Google Scholar] [CrossRef]
- Bariya, D.; Anand, V.; Mishra, S. Recent advances in the bile acid based conjugates/derivatives towards their gelation applications. Steroids 2021, 165, 108769. [Google Scholar] [CrossRef]
- Dobson, T.E.; Maxwell, A.R.; Ramsubhag, A. Antimicrobial cholic acid derivatives from the Pitch Lake bacterium Bacillus amyloliquefaciens UWI-W23. Steroids 2018, 135, 50–53. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, P.G.; Lemos, T.L.; Almeida, M.C.; de Souza, J.M.; Bizerra, A.M.; Santiago, G.M.; da Costa, J.G.; Coutinho, H.D. Lithocholic acid and derivatives: Antibacterial activity. Steroids 2015, 104, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lajczak-McGinley, N.K.; Porru, E.; Fallon, C.M.; Smyth, J.; Curley, C.; McCarron, P.A.; Tambuwala, M.M.; Roda, A.; Keely, S.J. The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis. Physiol. Rep. 2020, 8, e14456. [Google Scholar] [CrossRef] [PubMed]
- Masuno, H.; Kazui, Y.; Tanatani, A.; Fujii, S.; Kawachi, E.; Ikura, T.; Ito, N.; Yamamoto, K.; Kagechika, H. Development of novel lithocholic acid derivatives as vitamin D receptor agonists. Bioorganic Med. Chem. 2019, 27, 3674–3681. [Google Scholar] [CrossRef]
- Kong, F.; Niu, X.; Liu, M.; Wang, Q. Bile acids LCA and CDCA inhibited porcine deltacoronavirus replication in vitro. Vet. Microbiol. 2021, 257, 109097. [Google Scholar] [CrossRef]
- Travier, L.; Guadagnini, S.; Gouin, E.; Dufour, A.; Chenal-Francisque, V.; Cossart, P.; Olivo-Marin, J.-C.; Ghigo, J.-M.; Disson, O.; Lecuit, M. ActA promotes Listeria monocytogenes aggregation, intestinal colonization and carriage. PLoS Pathog. 2013, 9, e1003131. [Google Scholar] [CrossRef] [Green Version]
- Ishaq, A.R.; Manzoor, M.; Hussain, A.; Altaf, J.; Rehman, S.U.; Javed, Z.; Afzal, I.; Noor, A.; Noor, F. Prospect of microbial food borne diseases in Pakistan: A review. Braz. J. Biol. 2021, 81, 940–953. [Google Scholar] [CrossRef]
- Scharff, R.L. Economic burden from health losses due to foodborne illness in the United States. J. Food Prot. 2012, 75, 123–131. [Google Scholar] [CrossRef]
- Ivanek, R.; Gröhn, Y.T.; Tauer, L.W.; Wiedmann, M. The cost and benefit of Listeria monocytogenes food safety measures. Crit. Rev. Food Sci. Nutr. 2004, 44, 513–523. [Google Scholar] [CrossRef]
- Taylor, P.K.; Yeung, A.T.; Hancock, R.E. Antibiotic resistance in Pseudomonas aeruginosa biofilms: Towards the development of novel anti-biofilm therapies. J. Biotechnol. 2014, 191, 121–130. [Google Scholar] [CrossRef]
- Alkawash, M.A.; Soothill, J.S.; Schiller, N.L. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. Apmis 2006, 114, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.F.; Pollard, J.E.; Wright, J.O.; Savage, P.B.; Epand, R.M. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrob. Agents Chemother. 2010, 54, 3708–3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatmurge, N.S.; Hazra, B.G.; Pore, V.S.; Shirazi, F.; Chavan, P.S.; Deshpande, M.V. Synthesis and antimicrobial activity of beta-lactam-bile acid conjugates linked via triazole. Bioorganic Med. Chem. Lett. 2008, 18, 2043–2047. [Google Scholar] [CrossRef] [PubMed]
- de Grandi, A.Z.; Pinto, U.M.; Destro, M.T. Dual-species biofilm of Listeria monocytogenes and Escherichia coli on stainless steel surface. World J. Microbiol. Biotechnol. 2018, 34, 61. [Google Scholar] [CrossRef]
- Allison, K.R.; Brynildsen, M.P.; Collins, J.J. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 2011, 473, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Yi, T.; Shen, Z.; Teng, Z.; Wang, J. Aloe-emodin Attenuates Pathogenicity by Interfering With the Oligomerization of α-Toxin. Front. Cell. Infect. Microbiol. 2019, 9, 157. [Google Scholar] [CrossRef]
- Lu, G.; Xu, L.; Zhang, T.; Deng, X.; Wang, J. A potential bio-control agent from baical skullcap root against listeriosis via the inhibition of sortase A and listeriolysin O. J. Cell. Mol. Med. 2019, 23, 2042–2051. [Google Scholar] [CrossRef]
- Shen, X.; Niu, X.; Li, G.; Deng, X.; Wang, J. Amentoflavone Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo. Appl. Environ. Microbiol. 2018, 84, e01804-18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wang, J.; Guo, Y.; Liu, X.; Liu, S.; Niu, X.; Wang, Y.; Deng, X. Discovery of a potential MCR-1 inhibitor that reverses polymyxin activity against clinical mcr-1-positive Enterobacteriaceae. J. Infect. 2019, 78, 364–372. [Google Scholar] [CrossRef]
- Soudeiha, M.A.H.; Dahdouh, E.A.; Azar, E.; Sarkis, D.K.; Daoud, Z. In Vitro Evaluation of the Colistin-Carbapenem Combination in Clinical Isolates of A. baumannii Using the Checkerboard, Etest, and Time-Kill Curve Techniques. Front. Cell. Infect. Microbiol. 2017, 7, 209. [Google Scholar] [CrossRef]
- De Prijck, K.; Peeters, E.; Nelis, H.J. Comparison of solid-phase cytometry and the plate count method for the evaluation of the survival of bacteria in pharmaceutical oils. Lett. Appl. Microbiol. 2008, 47, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Wang, J.; Zhao, Y.; Zhong, Q.; Li, G.; Fu, Z.; Lu, S. Phage Endolysin LysP108 Showed Promising Antibacterial Potential against Methicillin-resistant. Front. Cell. Infect. Microbiol. 2021, 11, 668430. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, M.; Pan, J.; Shen, X.; Liu, W.; Zhang, X.; Li, H.; Deng, X. Quercetin impairs Streptococcus pneumoniae biofilm formation by inhibiting sortase A activity. J. Cell. Mol. Med. 2018, 22, 6228–6237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisuria, V.B.; Okshevsky, M.; Déziel, E.; Tufenkji, N. Proanthocyanidin Interferes with Intrinsic Antibiotic Resistance Mechanisms of Gram-Negative Bacteria. Adv. Sci. 2019, 6, 1802333. [Google Scholar] [CrossRef] [PubMed]




| Species | Sources | MIC (μg/mL) | Antibiotic | MIC (μg/mL) | FIC Index | Classification of the Interaction | |
|---|---|---|---|---|---|---|---|
| LCA Alone | Alone | Combination | |||||
| S. aureus USA 300 | American Type Culture Collection | Ampicillin | 128 | 8 | 0.18 | synergism | |
| Erythromycin | 32 | 4 | 0.25 | ||||
| Gentamicin | 2 | 0.06 | 0.15 | ||||
| Ciprofloxacin | 2 | 0.5 | 0.37 | ||||
| 256 | Lincomycin | 1 | 0.5 | 0.62 | |||
| Tetracycline | 0.25 | 0.125 | 0.62 | additivity | |||
| Chloromycetin | 4 | 2 | 0.62 | ||||
| Polymyxin B | 128 | 16 | 0.25 | synergism | |||
| L. monocytogenes EGD | Provided by Masao Mitsuyama | Ampicillin | 0.5 | 0.5 | 1.25 | additivity | |
| Erythromycin | 0.125 | 0.125 | 1.25 | ||||
| Gentamicin | 0.5 | 0.125 | 0.5 | synergism | |||
| Ciprofloxacin | 0. | 0.5 | 0.75 | additivity | |||
| 32 | Lincomycin | 2 | 2 | 1.25 | |||
| Tetracycline | 0.25 | 0.25 | 1.25 | ||||
| Chloromycetin | 4 | 4 | 1.25 | ||||
| Polymyxin B | 32 | 8 | 0.5 | synergism | |||
| S. suis. Type 2 | An isolated strain from pig | Ampicillin | 0.06 | 0.06 | 1.25 | additivity | |
| Erythromycin | 0.03 | 0.03 | 1.25 | ||||
| Gentamicin | 16 | 4 | 0.5 | synergism | |||
| Ciprofloxacin | 1 | 1 | 1.25 | additivity | |||
| 16 | Lincomycin | 0.25 | 0.25 | 1.25 | |||
| Tetracycline | 32 | 32 | 1.25 | ||||
| Chloromycetin | 1 | 1 | 1.25 | ||||
| Polymyxin B | 8 | 8 | 1.25 | ||||
| Species | Sources | MIC (μg/mL) | Antibiotic | MIC (μg/mL) | FIC Index | Classification of the Interaction | |
|---|---|---|---|---|---|---|---|
| LCA Alone | Alone | Combination | |||||
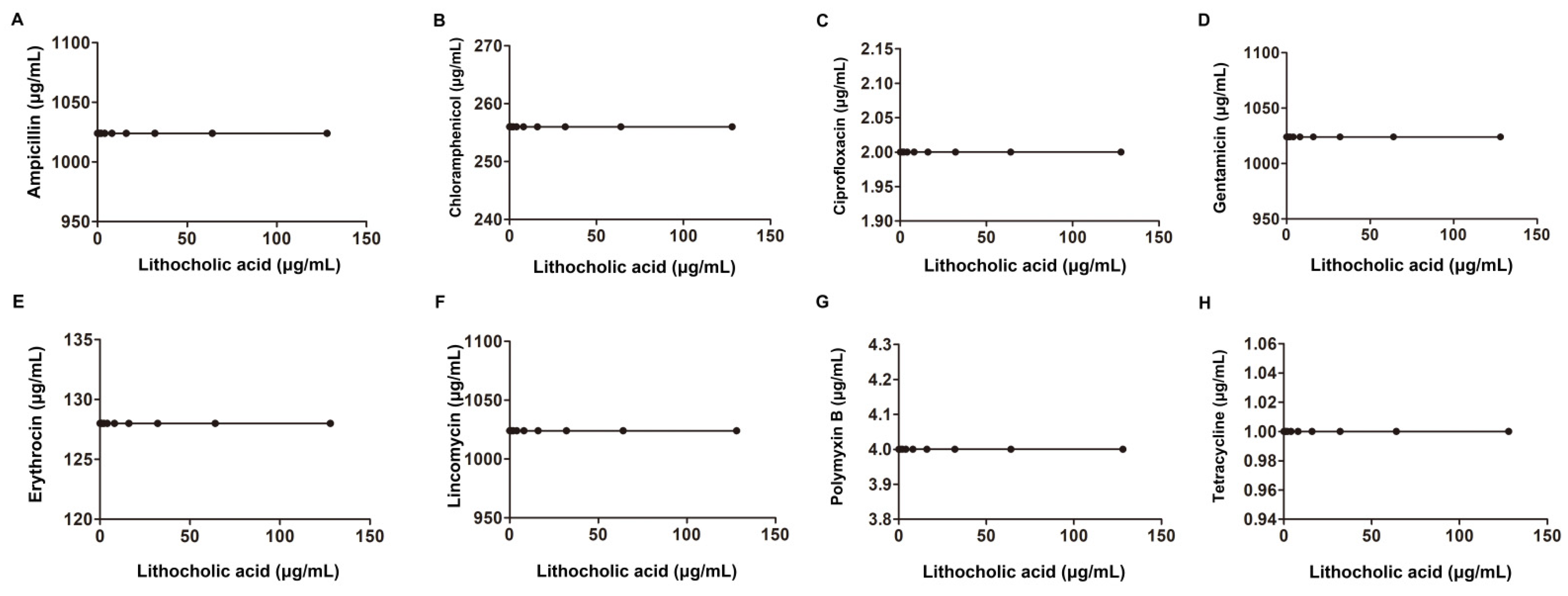
| Salmonella SL1344 | Derived from the virulent strain SL1344 | Ampicillin | 2 | 2 | 1.01 | additivity | |
| Erythromycin | 256 | 256 | 1.01 | ||||
| Gentamicin | 8 | 8 | 1.01 | ||||
| Ciprofloxacin | 0.015 | 0.015 | 1.01 | ||||
| 256 | Lincomycin | 1024 | 1024 | 1.01 | |||
| Tetracycline | 1 | 1 | 1.01 | ||||
| Chloromycetin | 4 | 4 | 1.01 | ||||
| Polymyxin B | 2 | 2 | 1.01 | ||||
| E. coli ATCC25922 | American Type Culture Collection | Ampicillin | 1024 | 1024 | 1.01 | additivity | |
| Erythromycin | 128 | 128 | 1.01 | ||||
| Gentamicin | 1024 | 1024 | 1.01 | ||||
| Ciprofloxacin | 2 | 2 | 1.01 | ||||
| 256 | Lincomycin | 1024 | 1024 | 1.01 | |||
| Tetracycline | 1 | 1 | 1.01 | ||||
| Chloromycetin | 256 | 256 | 1.01 | ||||
| Polymyxin B | 4 | 4 | 1.01 | ||||
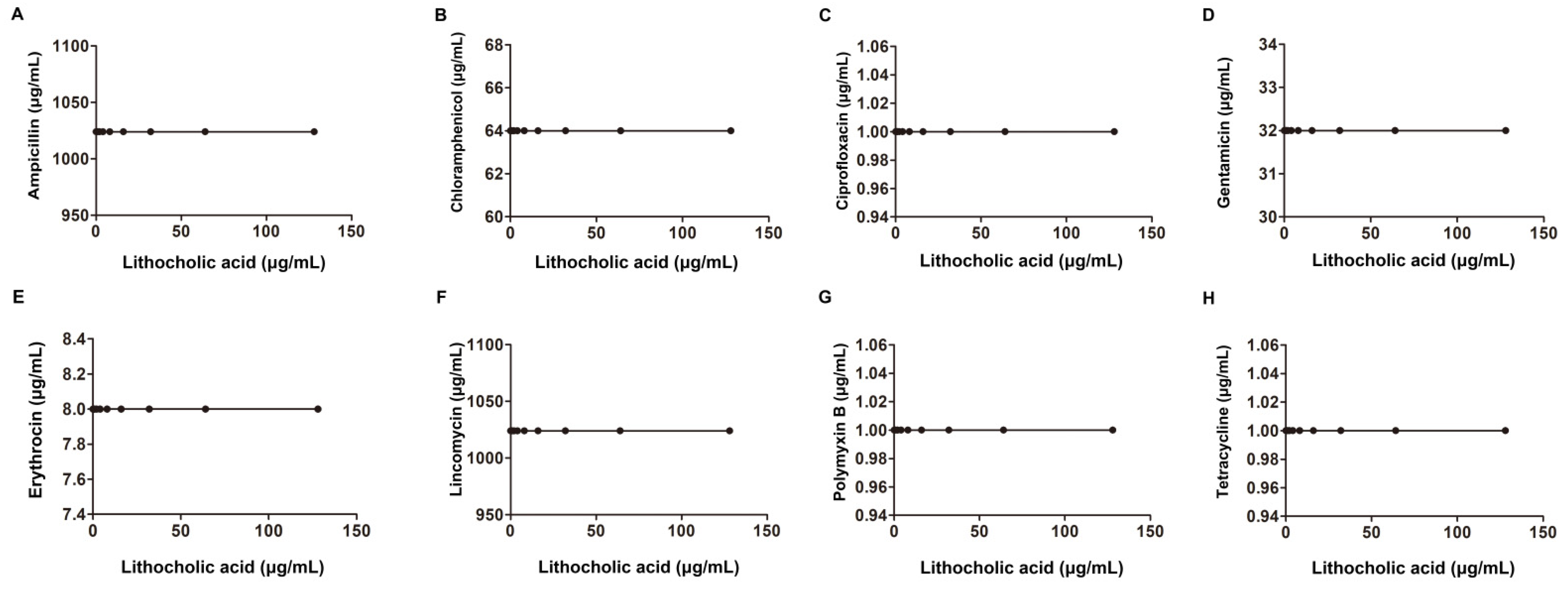
| A. baumannii ATCC19606 | American Type Culture Collection | Ampicillin | 1024 | 1024 | 1.01 | additivity | |
| Erythromycin | 8 | 8 | 1.01 | ||||
| Gentamicin | 32 | 32 | 1.01 | ||||
| Ciprofloxacin | 1 | 1 | 1.01 | ||||
| 256 | Lincomycin | 1024 | 1024 | 1.01 | |||
| Tetracycline | 1 | 1 | 1.01 | ||||
| Chloromycetin | 64 | 64 | 1.01 | ||||
| Polymyxin B | 1 | 1 | 1.01 | ||||
| Species | Cholic Acid Derivative | MIC (μg/mL) | Antibiotic | MIC (μg/mL) | FIC Index | Classification of the Interaction | |
|---|---|---|---|---|---|---|---|
| Cholic Acid Derivative Alone | Alone | Combination | |||||
| L. monocytogenes EGD | chenodeoxycholic acid | 256 | Gentamicin | 0.5 | 0.125 | 0.5 | synergism |
| S. suis. Type 2 | 256 | 16 | 0.5 | 0.28 | |||
| S. aureus USA 300 | 256 | 2 | 0.03 | 0.26 | |||
| L. monocytogenes EGD | ursodeoxycholic acid | 1024 | Gentamicin | 0.5 | 0.5 | 1.06 | additivity |
| S. suis. Type 2 | 1024 | 16 | 8 | 0.56 | |||
| S. aureus USA 300 | 512 | 2 | 2 | 1.125 | |||
| L. monocytogenes EGD | cholic acid | 2048 | Gentamicin | 0.5 | 0.5 | 1.03 | additivity |
| S. suis. Type 2 | 512 | 16 | 16 | 1.12 | |||
| S. aureus USA 300 | 1024 | 2 | 1 | 0.56 | |||
| L. monocytogenes EGD | hyodeoxycholic acid | 256 | Gentamicin | 0.5 | 0.5 | 1.25 | additivity |
| S. suis. Type 2 | 256 | 16 | 8 | 0.75 | |||
| S. aureus USA 300 | 256 | 2 | 0.5 | 0.5 | synergism | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, H.; Wang, L.; Liu, S.; Hu, W.; Wang, J.; Deng, X.; Gao, J. Synergistic Effect of Lithocholic Acid with Gentamicin against Gram-Positive Bacteria but Not against Gram-Negative Bacteria. Molecules 2022, 27, 2318. https://doi.org/10.3390/molecules27072318
Lv H, Wang L, Liu S, Hu W, Wang J, Deng X, Gao J. Synergistic Effect of Lithocholic Acid with Gentamicin against Gram-Positive Bacteria but Not against Gram-Negative Bacteria. Molecules. 2022; 27(7):2318. https://doi.org/10.3390/molecules27072318
Chicago/Turabian StyleLv, Hongfa, Lianping Wang, Shuang Liu, Wei Hu, Jianfeng Wang, Xuming Deng, and Jinying Gao. 2022. "Synergistic Effect of Lithocholic Acid with Gentamicin against Gram-Positive Bacteria but Not against Gram-Negative Bacteria" Molecules 27, no. 7: 2318. https://doi.org/10.3390/molecules27072318
APA StyleLv, H., Wang, L., Liu, S., Hu, W., Wang, J., Deng, X., & Gao, J. (2022). Synergistic Effect of Lithocholic Acid with Gentamicin against Gram-Positive Bacteria but Not against Gram-Negative Bacteria. Molecules, 27(7), 2318. https://doi.org/10.3390/molecules27072318






