1. Introduction
Safflower (
Carthamus tinctorius) is an herbaceous and thistle-like annual plant in the sunflower family. It is widely cultivated in Xinjiang, northwest China, for vegetable oil extracted from the seeds. The safflower seed oil (SSO) contains abundant essential unsaturated fatty acids, especially linoleic acid (73–85%), which is ranked number one among the kingdom of oilseed crops [
1,
2]. SSO is also rich in phenolic compounds, tocopherols, and phytosterols, as the main lipid concomitants, which endows it with great nutritional value and health-promoting functions [
3,
4]. Polyphenols have outstanding antioxidant and antibacterial effects [
5]. In addition, SSO is rich in tocopherols, especially α-tocopherol, valued for their antioxidant properties to protect polyunsaturated fatty acids against oxidation [
6]. Safflower seed oil is also a rich source of phytosterols, in particular β-sitosterol and stigmasterol, providing important effects in anti-inflammatory and antitumor activities [
7,
8]. However, the abundant polyunsaturated fatty acids affect the shelf life of SSO because they are prone to oxidative degradation.
Deacidification, an important refining process in plant seed oil refining, can remove free fatty acids and improve the storage stability of plant seeds oils. Particularly, alkali refining deacidification has been widely used for decades in the edible oil industry [
9,
10], which is more prone to saponification of neutral oils and causes serious loss of bioactive components, such as sterols and tocopherols among others. Even worse, it generates a massive amount of wastewater. Hence the refining process with alkali deacidification has a drawbacks, and this limits its application in the production of superior-quality edible oils [
11]. Ultrasonic-assisted ethanol extraction (UAEE) is performed without alkali addition, at lower operating temperature, and with less processing time comparing to alkali refining, thus it facilitates the retention of lipid concomitants and eliminates the massive wastewater generation [
12,
13]. Therefore, ultrasonic-assisted ethanol extraction is a preferred method of refining and has high application potential in the production of high-quality plant seeds oils.
Three important indices for the evaluation of the oxidative stability of plant seed oils are acid, peroxide and anisidine values. Acid value is the degree of oil deterioration, reflecting the variety and content of free fatty acids [
14,
15]. Peroxide value indicates the degree of the initial oxidation products contained in the oil [
16]. The products such as lipid hydroperoxides are extremely unstable and easy to further decompose to small molecular compounds such as aldehydes, ketones and acids, etc. The degree of decomposition of those products is usually evaluated using the anisidine value, which represents the degree of deep oxidation of animal and vegetable lipids [
17]. Peroxide value and anisidine value are together added up to represent the total oxidation value, which comprehensively evaluates the step-by-step oil oxidation processes [
18]. SSO contains a large number of unsaturated fatty acids, which are readily oxidized and become rancid under the ambient environmental conditions of external oxygen content, temperature, light, and other factors. In recent years, many scholars have found that there is a certain relationship between the antioxidant capacity and lipid concomitants of vegetable oil [
19,
20]. For instance, polyphenols and tocopherols in walnut oil were positively correlated with the free radical scavenging rate, demonstrated that the bioactive components in walnut oil facilitated the antioxidant capacity of walnut oil [
21]. Similarly, phenolic compounds in flaxseed oil are closely related to the stability of the oil products due to their antioxidant property [
22].
In this study, we aimed to develop an efficient UAEE process for acquiring good-quality SSO. We used deacidification rate as the main index to optimize the UAEE process of SSO by the random centroid optimization (RCO) method. Furthermore, we tested the essential physicochemical properties, fatty acid composition, content of lipid minor components, and in vitro antioxidant capacity of SSO. We also performed a direct comparison for quality parameters of SSO produced by the UAEE and the alkali refining processing method.
3. Materials and Methods
3.1. Materials and Chemicals
Safflower seed crude oil was gifted from Yaquina Agricultural Development Co., Ltd. (Yining, China). α-Tocopherol and stigmasterol (standard, ≥95%) were purchased from Sigma-Aldrich (Shanghai, China); 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS, ≥98%), 1,1-diphenyl-2-picrylhydrazyl (DPPH, ≥98%), and Trolox (≥98%) were purchased from Yuanye Biotechnology Co., Ltd. (Shanghai, China). Folin–Ciocalteu reagent was purchased from Solarbio Technology Co., Ltd. (Beijing, China); petroleum ether, isopropanol, glacial acetic acid, isooctane, absolute ethanol, sodium hydroxide, methanol, n-hexane, and other chemical reagents used were of either analytical or chromatographic grade and were purchased from Zhiyuan Chemical Reagent Co., Ltd. (Tianjin, China).
3.2. Ultrasonic-Assisted Ethanol Extracton
Two methods were employed in this study to develop and optimize the efficiency and quality of the SSO extracted. UAEE and alkali refining deacidification were performed in parallel to compare the contents of extracted antioxidants and other bioactive compounds such as phenolic compounds, phytosterols, and tocopherols.
The extraction process of SSO with UAEE was performed in a SB-520DT ultrasonic cleaning device (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China). The operating procedure was as follows: 100 mL of crude safflower seed oil was degummed and dehydrated. The resultant residue was weighed and mixed with different concentrations of ethanol in water (v/v). The formed solution was then placed in an ultrasonic device for ultrasonic-assisted extraction with a varying number of extractions and time periods for each extraction. When finished, the mixed solution was centrifuged at 4000 r/min for 10 min. The above operation was repeated until the free fatty acid was almost removed. The extracted oil, at the bottom of the liquid solution, was collected, and transferred to a round bottom flask for the subsequent removal of solvent residue in vacuo in a rotary evaporator device.
3.3. Deacidification of SSO with Alkali Refining
Alkali refining deacidification was performed according to the method described by Ying et al. with minor modifications [
11]. The degummed oil was heated to 65 °C, then alkali aqueous solution was added while stirring. When the oil and soapstock was separated, the stirring was stopped, and the upper oil layer was separated after centrafuge. The alkali-treated SSO was transferred to a separating funnel and washed with deionized water until the discharged water was neutral. Finally, the collected SSO oil was dehydrated to yield alkali-refining-deacidified oil.
3.4. Random Centroid Optimization (RCO)
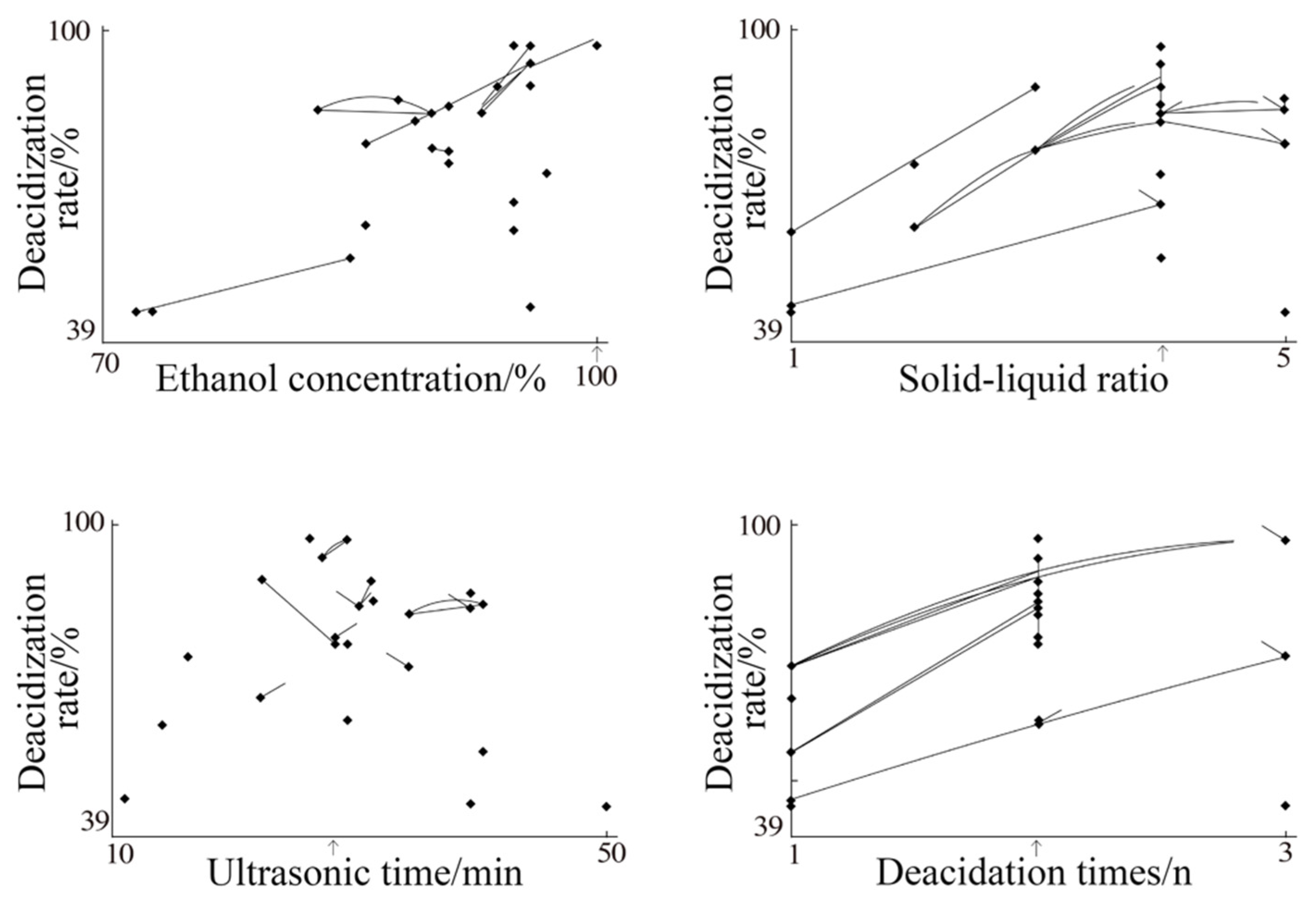
The effects of different factors on the deacidification of SSO were evaluated using RCO method. This universal optimization method is particularly useful for investigating multiple factors and targets and is currently used in the process optimization of food product development. In this study, the deacidification conditions and the range of parameter values were in accordance with those in the related literature [
13,
33]. The influencing factors were varied as follows: aqueous ethanol concentration, 70–100%; solid–liquid ratio, 1:1–1:5 (
w/v); ultrasonic time, 10–50 min; and 1–3 cycles of deacidification, as shown in
Table 9. The four variables and the ranges of their values were inputted to the RCO program (RCOPTNS), and 13 sets of proposed paramter values were yielded from the random search. These sets of parameters were then applied to the treatment of SSO samples in the UAEE process.
After the SSO treament, the parameter values from the UAEE process were determined and then inputted to the program of the centroid search for further optimized sets of parameter values. This round of centroid search proposed 11 sets of conditions for further experiments. These new sets of parameters’ values were further evaluated and the resultant values were re-inputted into the random search program. Finally, the obtained results were compiled and mapped for directing the optimum UAEE conditions. The extraction conditions of SSO using UAEE process were applied in triplicate following the results from mapping, and their output was then compared with that of traditional alkali refining.
3.5. Fatty Acid Analysis
The fatty acid compositions of the extracted SSO were determined by gas chromatography and mass spectrometry (GC/MS, GC 7890A, MS 5975C, Agilent, Santa Clara, CA, USA). The GC/MS conditions are detailed below. The carrier gas used for GC-FID analysis was hydrogen with a constant flow rate of 3.7 mL/min. The injector was maintained at 270 °C; capillary column, polydicyanopropylsiloxane strong polar stationary phase; column length, 100 m; inner diameter, 0.25 mm; and film thickness, 0.2 μm. The detector temperature was 280 °C. The split ratio was 1:100. The oven temperature was kept at 100 °C for 13 min, then programmed to rise to 180 °C at the speed of 10 °C/min and was kept for 6 min; then it rose to 200 °C at the speed of 1 °C/min and was kept for 20 min, and finally it rose to 230 °C at the speed of 4 °C/min and was kept for 10.5 min.
3.6. Determination of Acid Value, Peroxide Value, and Anisidine Value
The standards of the ISO (International Organization for Standardization) were used for the determination of acid value (ISO 660, 2009), peroxide value (ISO 3960, 2007), and anisidine value (ISO 6885, 2006). The total oxidation value was calculated according to the following Equation (1):
where
POV is the peroxide value, mmol/kg;
p − AV is the anisidine value.
The deacidification rate of safflower seed oil was obtained by the following Equation (2):
where
S1 is the acid value of the oil sample prior to deacidification, mg/g;
S2 indicates the acid value after deacidification, mg/g.
3.7. Determination of Lipid Concomitants
3.7.1. α-Tocopherol
The content of α-tocopherol was determined using the Agilent 1260 Infinity HPLC system (Agilent Technologies Co. Ltd., Palo Alto, CA, USA) according to the method described by Gama et al. [
34]. The HPLC system conditions were as follows: diamonsil-C18 column (4.6 mm × 250 mm, 5 μm); UV wavelength, 295 nm; mobile phase, methanol in isocratic elution; injection volume, 20 μL; column temperature, 30 °C; and flow rate, 1.0 mL/min. Peaks were quantified by area compared to the α-tocopherol standard (1.0 mg/L). The curve equation was y = 134,491x + 417,039, R
2 = 1.0000. α-Tocopherol was isolated from the oil sample as follows: methanol was added to 1.0 g of safflower seed oil with a solid–liquid ratio of 1:1.25; vortex oscillation time, 2 min; ultrasonic extraction, 20 min; and then centrifugation at 4000 rpm for 5 min. The supernatant was collected. This operation was repeated three times. The supernatant lyes were combined, concentrated to dryness in vacuo. Subsequently, 1 mL of methanol was added to the residue, mixed, and filtered through a 0.22 μm filter membrane, which was then analyzed on an HPLC system.
3.7.2. Determination of Total Phytosterol Content
The determination of the total sterol content in SSO was performed according to the following method after a minor modification from Yu et al. [
35]. An SSO sample (2.0 g) was added to 6 mL of 2 M potassium hydroxide in 95% ethanol aqueous solution. The resultant solution was mixed and heated to 60 °C for 60 min for saponification. A mixture of n-hexane with deionized water (3:2,
v/v) was then added, mixed well, and centrifuged at 4500 rpm for 5 min. The supernatant was isolated and concentrated to dryness in vacuo, and then mixed with 2 mL of absolute ethanol and refrigerated at 4 °C for use. An aliquot of the above solution (0.1 mL) was mixed with 2 mL of phosphorus ion reagent (PS-FE reagent) and 2 mL of ethanol, and then measured at 520 nm using a Shimadzu UV2000 Spectrophotometer (Shimadzu, Kyoto, Japan).
The standard curve of stigmasterol was prepared as follows: Stigmasterol (100 μg/mL) in ethanol was prepared as a stock solution. Aliquots of 0.0, 0.5, 1.0, 1.5, 2.0, and 2.5 mL of the stock stigmasterol ethanol solution were transferred to six volumetric flasks, respectively. Then, 2 mL of PS-FE reagent was added to each of the flasks, which were filled with absolute ethanol to a total solution volume of 6 mL. The absorbance value of each resulted solution was mearsured with UV at 520 nm wavelength. The corresponding standard curve equation was obtained as y = 0.001x − 0.006, (R2 = 0.9952), in which y is the concentration and x is the absorbance value of a stigmasteral solution.
3.7.3. Determination of the Total Content of Phenolic Compounds
The total phenolic content of SSO was measured according to the method described by Durmaz [
36] with slight modifications. Briefly, 1.5 g of each extract was dissolved in 1.5 mL of hexane and extracted with 1.5 mL of methanol aqueous solution (80%,
v/v) three times. The aqueous layers were combined and centrifuged at 3000 rpm for 10 min. Supernatant of the centrifuged sample was collected. A 500 μL solution of the extract was transferred to a 10 mL calibration flask. Folin–Ciocalteu reagent (0.5 mL) and 2 mL of saturated sodium carbonate solution (4%) were also added to the calibration flask, shaken for 3 min, and then stored in the dark for 1 h. The measurement was carried out at 760 nm using a Shimadzu UV2000 Spectrophotometer (Shimadzu, Kyoto, Japan). Gallic acid was used as the reference standard. Each sample was analyzed in triplicate. Calibration curves of gallic acid were obtained using the least-squares method, resulting in the equation y = 0.0211x + 0.0076 (R
2 = 0.9998), with a concentration range of 10–60 μg/mL. The results were expressed as mg gallic acid equivalents/kg (mg GAE/kg).
3.8. Determination of Antioxidant Activity In Vitro
The antioxidant activity of the extracted SSO was evaluated using two assays: 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, and 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging assay according to the method described by Ncab et al. [
37] with slight modifications. Each sample was prepared by dissolving 5.0 g of SSO in 5.0 mL of methanol, which was then centrifuged at 4500 rpm for 5 min, and the supernatant (methanol solution) was collected. The extaction was repeated two more times. The supernatant was combined and refrigerated at 4 °C for the antioxidant assays. This extraction process was repeated in triplicate to obtain three methanol extracts (supernatant) from each SSO sample.
3.8.1. Determination of DPPH Radical Scavenging Capacity
A 2.0 mL sample of supernatant (prepared as described above) from each sample was mixed with 2.0 mL of a DPPH solution (0.1 mmol/L) in methanol and kept in a dark at room temperature for 30 min. The decrease in absorbance was measured at 517 nm on a Shimadzu UV2000 Spectrophotometer (Shimadzu, Kyoto, Japan). Trolox was used as standard and the results were expressed in μmol Trolox equivalent/100 g of SSO (μmol TE/100 g). Each sample was measured in triplicate.
3.8.2. Determination of ABTS Radical Scavenging Ccapacity
The ABTS assay was performed in this study, and the ABTS free radical solution was prepared as following. A solution of ABTS (7.0 mM) was mixed with a solution of potassium persulfate (K2S2O8, 2.4 mM), and the mixed solution was kept in the dark for 12–16 h. The mixed solution was then diluted with methanol (1:1, v/v) at pH 7.4 until an absorbance of 0.7 at 734 nm was reached steadily.
Then, 100 μL of a methanol extract (supernatant) from each SSO sample was mixed with 1.0 mL of ABTS free radical solution and 1.0 mL of methanol in the dark. After 6 min, the absorbance was measured by a Shimadzu UV2000 Spectrophotometer (Shimadzu, Kyoto, Japan). Trolox was the standard reference, and results were presented in μmol Trolox equivalent/100 g of SSO (μmol TE/100 g). Each sample of a methanol extract was measured in triplicate.
3.9. Statistical Analysis
Each group of experiments was repeated three times. The results are shown as mean ± SD (standard deviation). ANOVA significance analysis and Pearson correlation analysis were carried out using SPSS 16.0 (IBM, Armonk, NY, USA).
4. Conclusions
In this study, ultrasonic-assisted ethanol extraction (UAEE) technology was for the first time applied to the refining deacidification of safflower seed oil. The optimization of the UAEE process was performed with a random centroid optimization program (RCO). The optimum UAEE conditions obtained were as follows: ethanol concentration, 100%; solid–liquid ratio, 1:4; ultrasonic time, 29 min; and 2 cycles of deacidification. The safflower seed oil was rich in unsaturated fatty acids, mainly linoleic acid, and bioactive compounds such as polyphenols and phytosterols. Compared with alkali refining deacidification, the UAEE method effectively retained bioactive compounds, improved the physicochemical properties of the safflower seed oil, and enhanced antioxidant capacity. Overall, crude SSO after the UAEE process showed desirable physicochemical characteristics, satisfactory amounts of bioactive compounds, and good in vitro antioxidant capacity. Therefore, the UAEE-processed SSO may be a good source of edible oils, and the UAEE method appeared to be preferably suitable for deacidification of safflower seed oil.
Furthermore, alkali refining is a traditional deacidification process widely used currently in practical industrial production. However, due to its high operating temperature, high refining energy consumption, massive generation of waste water, and great loss of bioactive components, a new method is urgently needed to overcome the drawbacks in SSO processing using alkali refining. The UAEE process developed in this study has the advantages of low operating temperature, short treatment time, and high retention rate of bioactive ingredients. Thus, it has the advantages of being fast, economical, and environmental friendly. Therefore, UAEE has a broad application prospect.