Abstract
Momordica charantia L., a member of the Curcubitaceae family, has traditionally been used as herbal medicine and as a vegetable. Functional ingredients of M. charantia play important roles in body health and human nutrition, which can be used directly or indirectly in treating or preventing hyperglycemia-related chronic diseases in humans. The hypoglycemic effects of M. charantia have been known for years. In this paper, the research progress of M. charantia phytobioactives and their hypoglycemic effects and related mechanisms, especially relating to diabetes mellitus, has been reviewed. Moreover, the clinical application of M. charantia in treating diabetes mellitus is also discussed, hoping to broaden the application of M. charantia as functional food.
1. Introduction
Due to globalization, industrialization and changes of human environment, hyperglycemia is widely prevalent [1]. Diabetes mellitus has been estimated to be the fifth leading cause of death globally, characterized by hyperglycemia due to defects in insulin action, insulin secretion, or both [2,3]. Characterized by altered carbohydrate, lipid and protein metabolism, diabetes mellitus is the leading cause of renal, neurological, and gastrointestinal manifestations in developed and developing countries [4,5,6,7]. Type 1 diabetes mellitus (T1DM), chiefly genetic, is characterized by disruption of pancreas function and absolute insulin insufficiency [8]. Insulin resistance plays a critical role in the development of type 2 diabetes mellitus (T2DM) and related complications [9]. T2DM is the major type of diabetes mellitus, affecting 90% of overall diabetes patients. Therefore, optimizing diabetes mellitus therapy is a modern critical medical and social challenge [8]. In particular, effective control of postprandial blood glucose levels may play key roles in diabetes care [10].
Many pharmacological approaches have been used to improve hyperglycemia, mainly through stimulating insulin release, increasing glucose transport activity, inhibiting gluconeogenesis, and reducing absorption of glucose from the intestine [10]. Currently, available therapies may be used as monotherapy or in combination to provide better glycemic regulation [11]. In addition to dietary management, hypoglycemic drugs are often used to treat T2DM [8]. However, the available hypoglycemic reagents have inadequate efficacy and some serious mechanism-based side effects, including hypoglycemia, gastrointestinal stimulation, and edema [12,13]. Owing to thesevere side-effects of synthetic hypoglycemic drugs, more effective and safer hypoglycemic agents from natural sources are greatly needed [14,15,16,17,18].
Natural phytoconstituents with hypoglycemic effect mainly contain peptides, lipids, glycopeptides, flavonoids, alkaloids, terpenoids, phenolics, glycosides, steroids, chalcones, carotenoids, tannins, saponins, iridoids, ursolic acid and imidazolines [19]. Momordica charantia L., also known as bitter melon or bitter gourd, belonging to the Cucurbitaceae family, is widely distributed in tropical and subtropical regions, such as Asia, South America, Africa, parts of the Amazon basin, and the Caribbean [20]. Notably, M. charantia seeds and fruits are rich in proteins, the quality of which meet the amino acids requirements/standards laid down by WHO for preschool children [21]. Due to various health-promoting properties, such as hypoglycemic, anti-cancerous, antimicrobial, antioxidant, antifertility, antimutagenic, antihelminthic, and immunomodulatory activities, M. charantia has been used as traditional medicinal plant in treating toothache, gout, jaundice, leprosy, furuncle, dysmenorrhea, piles, pneumonia, psoriasis, diarrhea, eczema, and rheumatism [22,23,24].
Due to remarkable hypoglycemic properties, M. charantia has great potential as an dietary ingredient and in medical foods for diabetic and prediabetic patients, as well as for the regulation of body weight and lipid metabolism [25]. This review discusses the potential applications of M. charantia bioactive compounds in the management of hyperglycemia and related chronic diseases, and clarifies the possible action of their mechanisms, hoping to supply valuable references for the development of M. charantia bioactives as hypoglycemic agents.
2. Bioactive Compounds of M. charantia with Hypoglycemic Potentials
As a highly nutritive vegetable, M. charantia contains numerous active phytochemicals, including proteins, carbohydrates, fatty acids, essential oils, amino acids, vitamins, phenolic acids, minerals, alkaloids, flavonoids, triterpenoids, quinines, saponins, triterpene glycosides, and other bioactive components [25]. Many types of bioactive compounds with hypoglycemic potentials have been isolated from M. charantia, among which cucurbitane-type triterpene glycosides, charantin, and momordicin were the well-studied compounds (Figure 1 and Figure 2). Polypeptide-p, purified from M. charantia fruit and seeds, showed effective hypoglycemic activities when administered subcutaneously to langurs, gerbils, and humans [26]. The 68-residue of insulin receptor (IR)-binding protein (mcIRBP) and adMc1proteins from M. charantia exhibited hypoglycemic effects in mice [27]. The 9cis,11trans,13trans-conjugated linolenic acid (9c,11t,13t-CLN) regulated lipid and glucose homeostasis via inducing acyl CoA oxidase (ACO) activity and serving as peroxisome proliferator activated receptor α (PPARα) activators [28]. Charantin, momordenol, and momordicilin are important active compounds possessing insulin-like chemical structure and properties [7]. Momordicine II and kuguaglycoside G could also stimulate insulin secretion [29,30].
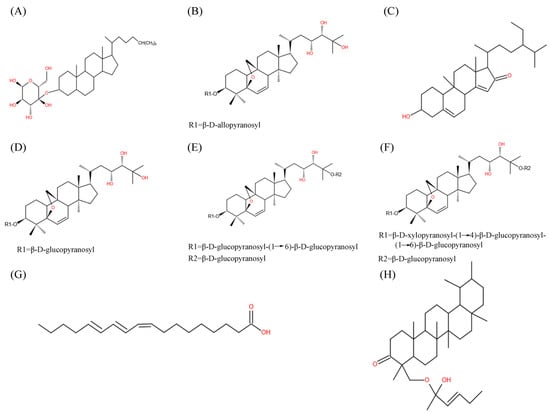
Figure 1.
Chemical structure of main M. charantia active substance. (A–H) referred to charantin, karaviloside XI, momordenol, momordicoside Q, momordicoside S, momordicoside T, 9c,11t,13t-CLN, and momordicilin, respectively.
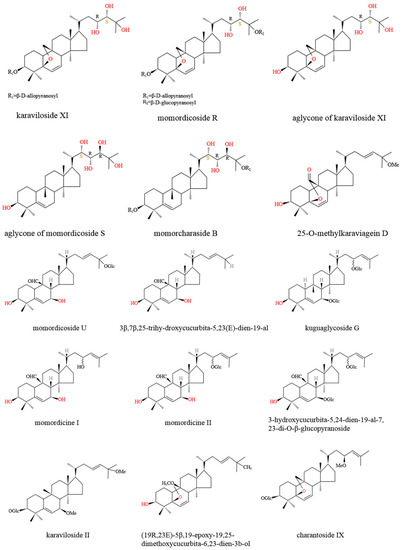
Figure 2.
Chemical structure of main M. charantia cucurbitane-type triterpenoids.
M. charantia triterpenoids (Figure 1) were found to control the balance of blood glucose via increasing adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) activity, which further enhanced glucose uptake and fatty acid oxidation, as well as inhibited lipid synthesis and hepatic glucose output [31]. Momordicosides (Q, R, S, U, and T) and karaviloside XI all exhibited many biologic effects beneficial to diabetes, such as enhancing the entry of inducible glucose into cells and stimulating fatty acid oxidation and glucose disposal [29,32,33]. The 5β,19-epoxy3β,25-dihydroxycucurbita- 6,23(E)-diene and 3β,7β,25-trihydroxycucurbita-5,23(E)-dien-19-al, purified from ether fraction of M. charantia methanol extract, showed blood hypoglycemic effects at the dosage of 400 mg/kg in diabetes-induced male ddY mice [33]. Protein tyrosine phosphatase 1B (PTP1B) served as a negative regulator of insulin through the dephosphorylation of the activated insulin receptor, which could be an effective target for the therapy of type 2 diabetes [34]. Cucurbitane-type triterpenoids, such as 25-O-methylkaraviagein D, karaviloside II, and (19R,23E)-5β,19-epoxy-19,25-dimethoxycucurbita-6,23-dien-3β–ol, all showed remarkable inhibitory activity against PTP1B and α-amylase (Figure 2) [15].
3. Antidiabetic Activity of M. charantia
M. charantia could effectively combat diabetes mellitus (Table 1). In obese diabetic db/db mice, M. charantia fruit water extract alone or together with platycodin-D significantly decreased obesity-related changes, and the water extract:platycodin-D (1:4) showed the most dramatic and synergic obesity-inhibiting effects [35]. In alloxan diabetic albino rats, acetone extract of whole M. charantia fruit lowered blood glucose from 13 to 50% after 8–30 days treatment [36]. Administration of M. charantia fruit methanol extract for 28 days lowered blood glucose levels in a dose-dependent manner in both normal and diabetic animals [37]. In the normal glucose primed rat model, alcoholic extract of M. charantia fruit significantly depressed plasma glucose levels by 10–15% at 1 h [38]. In both normal and streptozotocin-induced diabetic rats, subcutaneous administration of protein extract of M. charantia fruit pulp significantly decreased plasma glucose concentrations in a dose-dependent manner [39].
In normal rats, M. charantia pulp juice (250 mg/2 mL water) lowered fasting blood glucose levels significantly (p < 0.05 at 120 min), and the effect was even more pronounced with saponin-free methanol extract (150 mg/2 mL water) [40]. In diet-induced obesity C57BL/6 mice (male, 8 wk old), freeze-dried M. charantia fruit powder was seen to significantly reduce body weight, as the final body weights of mice receiving 10% M. charantia powder were almost the same as the control mice [41]. In normoglycemic Sprague-Dawley rats, aqueous extracts of M. charantia fruit (100 mg/kg) greatly reduced blood glucose levels [42].
In patients with T2DM, M. charantia extracts of unripe fruit could effectively lower the average fasting glucose level in an age- and sex-independent manner, showing no serious adverse events [43]. In a study of 52 individuals with prediabetes, M. charantia fruit extracts lowered elevated fasting plasma glucose [44]. Moreover, M. charantia fruit pulps at 2000 mg/day showed a modest hypoglycemic effect in patients with T2DM, and fructosamine levels were significantly reduced [45]. Among 112 patients with T2DM, administration of M. charantia fruit powder (2 or 4 g/day) significantly improved blood lipids, atherogenic index, body weight, and systolic blood pressure [46].
3.1. Improving Insulin Secretory and Resistance
M. charantia fruit extract could significantly increase islet size, number of β-cells, and total β-cell area, and also induce the regeneration of β-cells in the pancreatic islets of diabetic rats [47]. Furthermore, M. charantia fruit juice significantly increased the number of pancreatic β cells through reviving β cells and recovering partially destroyed β cells in streptozocin (STZ)-induced diabetic rats, but showed no effect on pancreatic α and δ cells [48]. In alloxan diabetic albino rats, acetone extract of M. charantia fruit showed antihyperglycemic activities through stimulating the recovery of pancreatic islet β cells [36]. Saponin-free methanol extract of M. charantia pulp juice (150 mg/2 mL water) showed significant hypoglycemic effects both in fasting (p < 0.05 at 120 min) and in postprandial states in non-insulin-dependent diabetes mellitus (NIDDM) model rats, through improving the insulin secretory capacity of B cells and enhancing insulin action, indicating the presence of non-sapogenin hypoglycemic compound(s) in M. charantia fruit pulp [40].
In MIN6 β-cells, M. charantia extracts rich in saponin significantly stimulated insulin secretion [29]. In particular, momordicine II and kuguaglycoside G also stimulated insulin secretion at concentrations of 10 μg/mL and 25 μg/mL, respectively [29]. The dried powder of M. charantia fruit pulp could also increase insulin secretion [49]. In INS-1 cells and rat pancreatic islets, M. charantia green fruit methanol extract and its ethyl acetate fraction could increase ATP content, augment insulin secretion in a dose-dependent manner, increase serum insulin levels after glucose loading, and decrease blood glucose levels significantly [50]. In MIN6 β-cells, purified momordicoside U (15.8–197.2 μM) moderately enhanced insulin secretion [30]. In both normal and streptozotocin-induced diabetic rats, protein extract of M. charantia fruit pulp raised plasma insulin concentrations 2-fold at 4 h following subcutaneous administration [39]. The protein extract of M. charantia fruit pulp (10 μg/mL) increased insulin secretion in perfused rat pancreases, and exerted insulin secretagogue and insulinomimetic activities to lower blood glucose concentrations [39]. In male high-fat-fed (HFD) Wistar rats, M. charantia fruit extract notably improved insulin sensitivity, and reduced fasting insulin [51].
The mcIRBP exhibited hypoglycemic effects in mice through interaction with insulin receptors (IR) [27]. In particular, the mcIRBP-19 (spanning residues 50–68 of mcIRBP) could enhance the binding of insulin to IR, stimulate phosphorylation of PDK1 and Akt, as well as stimulate the uptake of glucose in cells and clearance of glucose in diabetic mice (Figure 3) [52]. M. charantia fruit extract supplementation together with a high-fat diet (HFD) improved the insulin-stimulated tyrosine phosphorylation of insulin receptor subtrate-1 (IRS-1) [51]. In rats fed high fat diets, M. charantia freeze-dried unripe fruit juice (0.75%) could improve insulin resistance, as well as lower serum insulin and leptin [53].
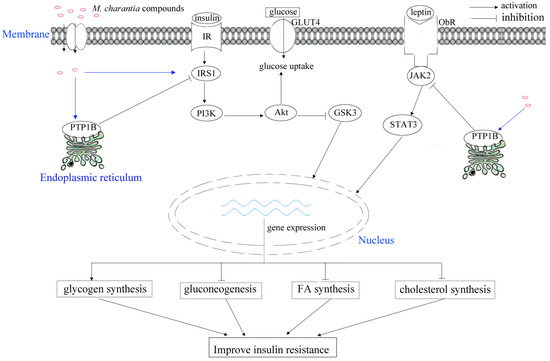
Figure 3.
Schematic illustrating of M. charantia active substance towards diabetes mellitus.

Table 1.
Effects of M. charantia active components on diabetes mellitus.
Table 1.
Effects of M. charantia active components on diabetes mellitus.
| Active Components | Dose | Model | Effect | References |
|---|---|---|---|---|
| water extract: platycodin-D (1:4) | _ | obese diabetic db/db mice | decrease obesity-related changes | [35] |
| acetone extract of whole fruit | 25–75 mg/100 g body weight | alloxan diabetic albino rats, | lower blood glucose, stimulate the recovery of pancreatic islet β cells | [36] |
| methanol extract of fruit | 200–600 mg/kg | normal and diabetic animals | lower blood glucose level, | [37] |
| alcoholic extract of fruit | 500 mg/kg | normal glucose primed rat | depress plasma glucose levels, enhance glycogen synthesis in liver | [38] |
| protein extract of fruit pulp | 5–10 mg/kg | normal and STZ-induced diabetic rats | exert insulin secretagogue and insulinomimetic activities, decrease plasma glucose concentrations, raise plasma insulin concentrations | [39] |
| fruit pulps | 2000 mg/day | patients with T2DM | hypoglycemic effect | [45] |
| powder | 2–4 g/day | patients with T2DM | improve blood lipids, atherogenic index, body weight, and systolic blood pressure, | [46] |
| fruit | _ | STZ-induced diabetic rats, male high-fat-fed Wistar rats, rat L6 myotubes, male Sprague-Dawley rats with diabetes | increase number of pancreatic β cells, improve insulin sensitivity, reduce fasting insulin, increase glucose uptakes, improve wound healing, increase diversity and shift overall structure of gut microbiota, stimulate amino acid uptake, normalise structural abnormalities of peripheral nerves, reduce glucose absorptions, improve body mass gain and LDL cholesterol values | [48,51,54,55,56,57,58] |
| Saponin-free methanol extract of juice | 150 mg/2 mL water | NIDDM model rats | improve insulin secretory capacity of B cells, enhance insulin action | [40] |
| saponin-rich fraction of fruit | 125 μg/mL | MIN6 β-cells | stimulate insulin secretion | [29] |
| dried powder of fruit pulp | 2000 mg/day | patients with T2DM | ameliorate diabetes associated CV risk, decrease level of glycosylated hemoglobin, increase insulin secretion | [46,49,59] |
| green fruit methanol extract and ethyl acetate fraction | INS-1 cells and rat pancreatic islets | increase ATP content, augment insulin secretion, increase serum insulin levels, decrease blood glucose levels | [50] | |
| momordicoside U | 15.8–197.2 μM | MIN6 β-cells | enhance insulin secretion | [30] |
| Momordicilin | block the active site of GSK-3, | [7] | ||
| mcIRBP | induce expression of GLUT4, stimulate phosphorylation of PDK1 and Akt, stimulate the uptake of glucose and clearance of glucose, | [27] | ||
| freeze-dried unripe fruit juice | 0.75% | rats fed high fat diets | improve insulin resistance, lower serum insulin and leptin, improve oral glucose tolerance, lower body weight and visceral fat mass, raise serum-free fatty acid concentration, reduce adiposity, | [53] |
| momordicosides (Q, R, S, and T) and karaviloside XI | _ | L6 myotubes, 3T3-L1 adipocytes, mice | enhance AMPK activity, stimulate GLUT4 translocation to the cell membrane, enhance fatty acid oxidation and glucose disposal | [32] |
| nanoparticles synthesized with filtrate of methanolic extract and silver nitrate | 50 mg/kg | STZ-induced diabetic rats | regulate signaling pathways, up-regulate expression level of glucokinase | [60] |
3.2. Regulating Glucose Uptake
Glucose transporters (GLUT) are widely distributed in body cells, facilitating the maintenance of the blood glucose level in the human body [61,62]. Sodium-coupled glucose transporters (SGLUTs) are scattered across the human body, and the selective inhibition of SGLUT1 could significantly slow postprandial gut uptake of glucose, as well as increase plasma levels of GLP-1 and GIP in healthy volunteers [63,64]. GLUT2 plays bidirectional roles in specific transportation of glucose in hepatocytes, as well as the absorption and reabsorption of glucose from enterocytes and renal tubules particularly [65]. Therefore, GLUT2 is considered as a competent target in treating diabetes mellitus [66]. The potential target proteins in diabetes contain dipeptidyl peptidase-IV (DPP-IV), GLUT, SGLTs, peroxisome proliferator-activated receptors, and α-glucosidase inhibitors [67]. The mcIRBP-19 could induce expression of GLUT4 (Figure 3) [52].
Six peptides (i.e., SMCG, DECC, TTIT, RTTI, ARNL and TVEV) (Figure 4), derived from the hypoglycemic protein adMc1 of M. charantia, were shown to be potential inhibitors of DPP-IV, SGLT1, and GLUT2 receptor proteins [66]. In L6 myotubes and 3T3-L1 adipocytes, momordicosides (Q, R, S, and T) and karaviloside XI could enhance AMPK activity and stimulate GLUT4 translocation to the cell membrane, which is an essential step for inducible glucose entry into cells [32]. In STZ-induced diabetic rats, daily oral administration of M. charantia fruit juice significantly reduced the Na+- and K+-dependent absorptions of glucose by jejunum [54]. Also in STZ-induced diabetic rats, M. charantia fruit juice could, like insulin, regulate glucose uptake into the jejunum membrane brush border vesicles and skeletal muscle cells [54]. In rat L6 myotubes, lyophilized extract of M. charantia fruit juice (5 µg/mL) stimulated the uptake of 14C-D-glucose, but high concentrations (10–200 µg/mL) inhibited the uptake [54]. M. charantia fruit protein extract enhanced glucose uptake into C2C12 myocytes and 3T3-L1 adipocytes, and significantly increased glucose uptake after 4–6 h of incubation in rat adipocytes [39]. Incubation of L6 rat myotubes with M. charantia fruit juice (1, 5 and 10 µg/mL) resulted in time-dependent increases in 3H-deoxy-D-glucose uptakes [55].
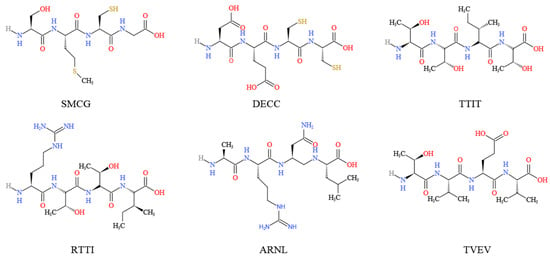
Figure 4.
Chemical structure of the main M. charantia peptides derived from the hypoglycemic protein adMc1.
3.3. Improving Glucose Metabolism
Diabetes mellitus is associated with irregular glucose homeostasis, so the effective control of blood glucose level is critical in preventing or reversing diabetic complications and improving life quality in diabetic patients [68]. In male HFD Wistar rats, M. charantia fruit extract notably improved glucose tolerance [51]. In STZ-induced diabetic rats, alcoholic extract of M. charantia fruit improved the oral glucose tolerance, and led to significant reduction in plasma glucose of 26% at 3.5 h [38]. In rats fed high-fat diets, M. charantia freeze-dried unripe fruit juice (0.75%) could improve oral glucose tolerance [53]. The hypoglycemic activities of M. charantia fruit extracts might partly be due to increased glucose utilization in the liver [38]. M. charantia leaf nanoparticles, synthesized with filtrate from methanolic extract with silver nitrate (1 mM), could significantly up-regulate the expression level of glucokinase in diabetic rats [60]. Momordicilin exhibited antidiabetic activities through blocking the active site of glycogen synthase kinase-3 (GSK-3), which can phosphorylate and inactivate glycogen synthase [7]. In normally fed rats, alcoholic extract of M. charantia fruit (500 mg/kg) enhanced glycogen synthesis (4–5 fold) from U-14C-glucose in the liver [38].
3.4. Modulating Lipid and Amino Acid Metabolism
In male HFD Wistar rats, M. charantia fruit extract notably reduced triacylglycerol, cholesterol and epidydimal fat [51]. Momordicoside(s) could enhance fatty acid oxidation and glucose disposal in both insulin-sensitive and insulin-resistant mice [32]. In STZ-induced diabetic rats, M. charantia fruit powder (10 or 50 g/kg diet for 6 weeks) could improve body mass gain and low-density lipoprotein (LDL) cholesterol values, which could be dampened by co-administered trivalent chromium (Cr) [56]. In rats fed high-fat diets, M. charantia freeze-dried unripe fruit juice (0.75%) could lower body weight and visceral fat mass, raise serum free fatty acid concentration, and reduce adiposity without affecting fat absorption [53].
Microbes inhabiting the gut may play important roles in hosts’ metabolism homeostasis and health maintenance [69,70,71]. Oral administration of M. charantia fruit significantly prevented hyperlipidemia, but the effects substantially diminished when co-treated with antibiotics [57]. In particular, M. charantia fruit moderately increased diversity and shifted the overall structure of gut microbiota via enhancing the relative abundance of short-chain fatty acid (SCFAs)-producing genera and increasing fecal SCFAs content [57]. The transplantation of gut flora from M. charantia fruit-treated donor mice significantly decreased serum lipids in male recipient mice [57]. In L6 rat myotubes, M. charantia fruit juice (1, 5 and 10 µg/mL) enhanced the N-methyl-amino-α-isobutyric acid uptakes in a time-dependent manner, as M. charantia fruit juice exerted a hypoglycemic effect partly through stimulating amino acid uptake into skeletal muscle cells like insulin [55].
3.5. Protective Effects of M. charantia
In STZ-induced diabetic rats, M. charantia fruit juice normalized the structural abnormalities of peripheral nerves, including the mean cross-sectional myelinated nerve fibers, axonal area, myelin area, and maximal fiber area [54]. In Cr-co-supplemented type 2 diabetic rats, M. charantia fruit powder could decrease Cr content in liver and kidneys through binding of Cr by polyphenol-type compounds [55,56]. In STZ-induced diabetic rats, M. charantia leaf nanoparticles (50 mg/kg) could alleviate diabetes nephropathy through regulating SOCS/JAK/STAT and PI3K/Akt/PTEN signaling pathways: levels of Akt, PI3k, TGF-β, JAK2, STAT3 were down-regulated; the expressions of PTEN, SOCS3 and SOCS4 were up-regulated [60]. In type 2 diabetic db/db mice, the gastro-resistant peptide mcIRBP-9 showed anti-inflammatory and reno-protective abilities, as well as controlling blood glucose and HbA1c levels [72]. The mcIRBP-9 could ameliorate diabetic nephropathy through reducing renal vascular leakage and histopathological changes, altering pathways involved in inflammatory and immune responses, as well as improving inflammatory characteristics of mice [72]. In particular, nuclear factor-κB (NF-κB) played an important role in regulating mcIRBP-9-affected immune pathways [72]. In obese and diabetic OLETF rats, treatment with M. charantia edible portion (3%) down-regulated the levels of proinflammatory cytokines in liver, muscle and epididymal fats [73]. Administration of dried powder of M. charantia fruit pulp (2000 mg/day) significantly reduced the levels of glycated hemoglobin A1c, 2-h glucose, areas under the curve (AUC) of glucose, weight, fat percentage, body mass index, and waist circumference [49]. In 112 patients with T2DM, M. charantia fruit powder ameliorated diabetes-associated cardiovascular risk factors more effectively than glibenclamide [46]. In 40 diabetic patients (over 18 years old), M. charantia administration (two capsules, three times a day after meals, for 3 months) slightly decreased levels of glycosylated hemoglobin (hemoglobin A1c or HbA1c) by 0.22% [59]. M. charantia active components could reduce oxidative stress, decrease insulin resistance, increase insulin release, reduce adiposity, modulate glycolysis and gluconeogenesis, as well as lower oxidative status [74].
Diabetic patients often suffer from chronic nonhealing wounds, such as foot ulcers, which often result in amputations [75,76,77]. In diabetic patients, hyperglycemia can cause arteries to narrow, result in poor oxygenation of wound tissue, and delay wound repair and regeneration [58,78]. Moreover, the wound-healing response can be further compromised by chronic hyperglycemia-induced damage to both the peripheral nerves and the immune system [58,79]. Diabetes also has deleterious effects on granulation tissue cells, especially fibroblasts and endothelial cells [80]. In male Sprague-Dawley rats with diabetes, M. charantia fruit appeared to benefit the formation of wound granulation tissue, as distinct cellular layers were well-formed [75]. Moreover, M. charantia fruit treatment increased angiogenesis in diabetic granulation tissue, which was marked by abundant microvessels and large blood vessels [58]. In particular, locally applied M. charantia fruit extract could prevent regression of granulation tissue and blood vessels, and improve wound healing in diabetic wounds, showing no effect on systemic blood glucose levels or insulin receptor substrate 1 [58].
3.6. Inhibitory Effects of Related Enzymes
The α-glucosidase, located in the brush-border membranes of human intestinal cells, is involved in carbohydrate metabolism and the post-translational processing of glycoproptein [81]. The α-amylase, an important secretory product generated by the pancreas and salivary glands, can catalyze the initial step of starch hydrolysis to a mixture of oligosaccharides through cleavaging the α-D (1–4) glycosidic bonds [82,83]. In particular, α-glucosidase and α-amylase have long been proposed as candidate drug targets for the modulation of postprandial hyperglycemia [84].
As natural inhibitors of α-glucosidase and α-amylase, M. charantia may be used as auxiliary hypoglycemic functional foods or drugs (Figure 5). Three cucurbitane-type triterpenoids, including 25-O-methylkaraviagein D, karaviloside II, and (19R,23E)-5β, 19-epoxy-19,25-dimethoxycucurbita-6,23-dien-3β–ol, could all inhibit α-glucosidase activity [15]. 25-O-Methylkaraviagein D showed remarkable inhibitory activity against PTP1B and α-amylase [15]. Capsules containing M. charantia extract also exerted anti-obesity activities through selectively and dose-dependently inhibiting the activity of 11β-Hydroxysteroid dehydrogenase type 1 (11β-HSD1), which is a microsomal enzyme converting glucocorticoid receptor-inert cortisone to active cortisol in metabolic tissues [85].
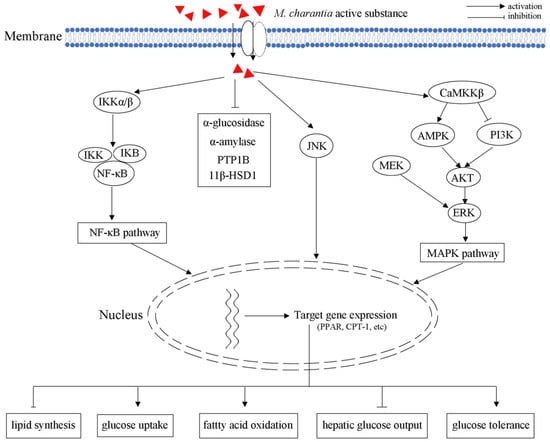
Figure 5.
Schematic illustrating of M. charantia active substance towards obesity.
3.7. Regulation of Signal Pathways
AMPK plays multiple critical roles in the body’s overall metabolic balance, response to exercise, hormonal stimulation, nutritional stress, as well as glucose-lowering drugs metformin and rosiglitazone [10,86]. AMPK consists of a catalytic α subunit and two non-catalytic subunits (β and γ), forming active 1:1:1 heterotrimers. Moreover, the activation of AMPK can induce the expression of PPARα and carnitine palmitoyltransferase I (CPT-1), which further increase fatty acid oxidation and improve insulin sensitivity [87]. In L6 myotubes and LKB1-deficient HeLa cells, M. charantia triterpenoids increased AMPK activity by 20–35% through regulating the upstream kinase CaMKKβ in a Ca2+-independent manner [31]. As an AMPK activator, M. charantia triterpenoids could increase the expression of AMPK, and further control the balance of blood glucose (Figure 5).
PPARs could be activated by a ligand, heterodimerize with retinoid X receptor, binding to a peroxisome proliferator responsive element (PPRE), and promote transcription of target genes participating in lipid catabolism. Therefore, PPARs play important roles in regulating lipid and glucose homeostasis through genomic action [88]. The 9c,11t,13t-CLN, isolated from wild M. charantia fruit, could significantly induce ACO activity in a peroxisome proliferator-responsive murine hepatoma cell line (H4IIEC3) [28]. As a PPARα activator, 9c,11t,13t-CLN regulated lipid and glucose homeostasis through PPARα signaling pathways in vivo [28]. In obese and diabetic OLETF rats, treatment with M. charantia edible portion (3%) significantly improved glucose tolerance and insulin sensitivity via inhibiting NF-κB and JNK pathways: the levels of phospho-insulin receptor substrate-1 (Tyr612) and phospho-Akt (Ser473) were increased; the activation of NF-κB in liver and muscle was decreased [73].
4. Challenges and Perspectives
M. charantia has received considerable attention in biological and biomedical research due to its remarkable biological activities, especially its antidiabetic/hypoglycemic effects. M. charantia is usually served in one of four dosage forms (fruit juice, entire fruit, freeze-dried powder, or capsule), the preparations are mainly crude extracts (extracted with water, ethanol, or methanol) and the effective monomer components are extracted from fruit, seeds, and leaves [89]. In particular, the typical hypoglycemic activities are mainly attributed to proteins/peptides, polysaccharides, phenolic compounds, triterpenoids, alkaloids, and charantins [90,91]. As one of the most important global health problems, diabetes mellitus could be treated with several M. charantia-derived bioactive compounds, mainly through inhibiting α-glucosidase and α-amylase, activating AMPK, JNK, and Akt signal pathways, activating PTP1B activities, and inhibiting the formation of advanced glycation end-products (AGE). The modulation of gut microbiota is essential for the hypoglycemic and anti-hyperlipidemic activities of M. charantia. M. charantia fruit juice has multiple influences on glucose and lipid metabolism, strongly counteracting the untoward effects of high-fat diets. Furthermore, antioxidant and anti-inflammatory activities also greatly contribute to its anti-hyperglycemic properties. Long-term oral administration of M. charantia fruit extracts at appropriate dosages may be benefit in improving diabetes. Identification of potential mechanism(s) by which M. charantia improves insulin sensitivity and insulin signaling may supply new therapeutic targets for the treatment of obesity/dyslipidemia-induced insulin resistance.
Traditional M. charantia remedies have supplied sources of useful hypoglycemic agents, but should continue to be investigated for possible drug alternatives. In vitro and animal studies have suggested the remarkable hypoglycemic activity of M. charantia, but limited human research is available to support its usage. M. charantia has traditionally been used for treating diabetes, but some clinical trials show conflicting results. In addition, very limited-quality evidence has shown that M. charantia adjunct preparations could improve glycemic control in T2DM patients [92]. Moreover, no large clinical trial has been performed on the efficacy and safety of M. charantia preparation. Therefore, rigorous research focusing on standardizing M. charantia formulation is greatly needed, as well as clinical trials with adequate sample size to determine its efficacy and safety. Diabetes mellitus is also associated with an increase in sialic acid concentration, but ingestion of M. charantia fruit juice (55 mL/24 h) showed no effect on levels of serum sialic acid in NIDDM patients [93,94,95]. Moreover, diabetes mellitus is also associated with disruption of biorhythms, but no related research was available for active components of M. charantia [8]. Dysfunction of bone marrow-derived endothelial progenitor cells contributes to poor vasculogenesis in diabetes mellitus [96]. However, the effect of M. charnatia active components on bone marrow-derived endothelial progenitor cells is not well understood and needs to be studied in more depth. Moreover, the effect of M. charantia active components on protein kinase C is not well understood, and is vital when considering diabetic vascular complications [97].
Due to the interaction of drugs with in vivo systems, rational drug use should consider medical, biological, and pharmaceutical factors to ensure high bioavailability and efficacy. In particular, the most active candidates against diabetes mellitus will be determined through measuring many biochemical parameters, including fasting blood glucose, lipid profile, insulin, glycosylated hemoglobin, serum urea and creatinine, plasma alanine and aspartate transaminases, as well as microscopical examinations of pancreatic sections. Moreover, the gastrointestinal resistance of M. charantia bioactive compounds and their thermal tolerance in vivo also need to be better understood. Therefore, further study is greatly needed to investigate the detailed hypoglycemic mechanism and possible linkage to unexpected side effects, aiming to establish a safety guideline for the consumption of M. charantia-derived products.
Author Contributions
Conceptualization, B.X., Z.L. and S.L.; writing—original draft preparation, B.X. and Z.L.; writing—review and editing, T.Z., J.Z. and S.W.; visualization, B.X. and Z.L.; supervision, C.-T.H. and S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research reported in this paper was funded by a grant from Hubei Province, China (2019ABA100), Open Fund of the Hubei Key Laboratory of Economic Forest Germplasm Improvement and Resources Comprehensive Utilization (201932103), and funding from Assessment and Comprehensive Utilization of Characteristic Biological Resources in Dabie Mountains (4022019006).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AGE | advanced glycation end products |
| AMPK | Adenosine 5′-monophosphate (AMP)-activated protein kinase |
| adMc1 | anti-hyperglycemic protein of M. charantia |
| AUC | areas under the curve |
| 9c,11t,13t-CLN | 9cis,11trans,13trans-conjugated linolenic acid |
| CPT-1 | carnitine palmitoyltransferase I |
| Cr | chromium |
| DPP-IV | dipeptidyl peptidase-IV |
| GLUT | Glucose transporters |
| GLP-1 | glucagon-like peptide 1 |
| GIP | glucose-dependent insulinotropic peptide |
| HFD | high-fat-fed |
| IDDM | insulin-dependent diabetes mellitus |
| IR | insulin receptor |
| IRS-1 | insulin receptor subtrate-1 |
| NF-κB | nuclear factor-κB |
| NIDDM | non-insulin-dependent diabetes mellitus |
| PDK1 | protein kinase-1 |
| STZ | streptozocin |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| mcIRBP | M. charantia insulin receptor (IR)-binding protein |
| PPARα | Peroxisome proliferator activated receptor α |
| PTP1B | protein tyrosine phosphatase 1B |
| PPRE | peroxisome proliferator responsive element |
| SCFAs | short-chain fatty acid |
| SGLUTs | Sodium-coupled glucose transporters |
| SGLT1 | sodium dependent glucose transporter 1 |
| TAG | triacylglycerol |
References
- Xiao, J.B.; Högger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Roglic, G.; Unwin, N.; Bennett, H.; Mathers, C.; Tuomilehto, J.; Nag, S.; Connolly, V.; King, H. The burden of mortality attributable to diabetes: Realistic estimates for the year 2000. Diabetes Care 2005, 28, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- ADA. Classification and diagnosis of diabetes. Diabetes Care 2015, 40, S11–S24. [Google Scholar]
- Zhao, Y.; Liu, Y.J.; Yi, F.Z.; Zhang, J.; Xu, Z.H.; Liu, Y.H.; Tao, Y. Type 2 diabetes mellitus impaired nasal immunity and increased the risk of hyposmia in COVID-19 mild pneumonia patients. Int. Immunopharmacol. 2021, 93, 107406. [Google Scholar] [CrossRef]
- Salamone, D.; Rivellese, A.A.; Vetrani, C. The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: The possible role of dietary fibre. Acta Diabetol. 2021, 58, 1131–1138. [Google Scholar] [CrossRef]
- Karagz, I.K.; Karagz, A.; Zkalayc, F.; Dogan, C.; Kocabay, G.; Elbay, A. Relation between platelet reactivity levels and diabetic retinopathy stage in patient with type 2 diabetes mellitus by using multiplate whole blood aggregometry. Semin. Ophthalmol. 2021, 36, 392–399. [Google Scholar] [CrossRef]
- Hazarika, R.; Parida, P.; Neog, B.; Yadav, R.N. Binding energy calculation of GSK-3 protein of human against some anti-diabetic compounds of Momordica charantia Linn (bitter melon). Bioinformation 2012, 8, 251–254. [Google Scholar] [CrossRef]
- Bunyatyan, N.D.; Bukhtiyarova, I.P.; Drogovoz, S.Z.; Kononenko, A.V.; Olefir, Y.V.; Prokof’ev, A.B.; Proskurina, I.A.; Goryachev, D.V. Influence of human biorhythms on the blood glucose level and the efficacy of hypoglycemic drugs (review). Pharm. Chem. J. 2017, 51, 399–401. [Google Scholar] [CrossRef]
- Lee, S.H.; Min, K.H.; Han, J.S.; Lee, D.H.; Park, D.B.; Jung, W.K.; Park, P.; Jeon, B.T.; Kim, S.K.; Jeon, Y.J. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012, 50, 575–582. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, C.F.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J.B. Bioactive compounds from marine macroalgae and their hypoglycemic benefits. Trends Food Sci. Technol. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.H.; Kang, E.S.; Kim, S.K. Antidiabetic agents from medicinal plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Khan, V.; Najmi, A.K.; Akhtar, M.; Aqil, M.; Mujeed, M.; Pillai, K.K. A pharmacological appraisal of medicinal plants with antidiabetic potential. J. Pharm. Bioallied Sci. 2012, 4, 27–42. [Google Scholar] [PubMed]
- Lee, S.H.; Kang, N.; Kim, E.A.; Heo, S.J.; Moon, S.H.; Jeon, B.T.; Jeon, Y.J. Antidiabetogenic and antioxidative effects of octaphlorethol a isolated from the brown algae Ishige foliacea in streptozotocin-induced diabetic mice. Food. Sci. Biotechnol. 2014, 23, 1261–1266. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xiao, J.B.; Xu, B.J. An insight into anti-diabetic properties of dietary phytochemicals. Phytochem. Rev. 2017, 16, 535–553. [Google Scholar] [CrossRef]
- Yue, J.; Xu, J.; Cao, J.; Zhang, X.S.; Zhao, Y.Q. Cucurbitane triterpenoids from Momordica charantia L. and their inhibitory activity against α-glucosidase, α-amylase and protein tyrosine phosphatase 1B (PTP1B). J. Funct. Foods 2017, 37, 624–631. [Google Scholar] [CrossRef]
- Mar, K.; Fassnacht, M.; Führer-Sakel, D.; Honegger, J.B.; Weber, M.M.; Kroiss, M. The diagnosis and management of endocrine side effects of immune checkpoint inhibitors. Dtsch. Arztebl. Int. 2021, 118, 33724917. [Google Scholar]
- Preiato, V.L.; Salvagni, S.; Ricci, C.; Ardizzoni, A.; Pelusi, C. Diabetes mellitus induced by immune checkpoint inhibitors: Type 1 diabetes variant or new clinical entity? review of the literature. Rev. Endocr. Metab. Dis. 2021, 22, 337–349. [Google Scholar] [CrossRef]
- Fauchier, L.; Fauchier, G.; Bisson, A.; Bodin, A.; Herbert, J.; Angoulvant, D.; Ducluzeau, P.H.; Lip, G.Y.H. Antidiabetic drugs use and new-onset atrial fibrillation in patients with diabetes mellitus. Eur. Heart J. 2021, 42, ehab724.0457. [Google Scholar] [CrossRef]
- Wu, C.H.; Hsieh, H.T.; Lin, J.A.; Yen, G.C. Alternanthera paronychioides protects pancreatic β-cells from glucotoxicity by its antioxidant, antiapoptotic and insulin secretagogue actions. Food Chem. 2013, 139, 362–370. [Google Scholar] [CrossRef]
- Leung, L.; Birtwhistle, R.; Kotecha, J.; Hannah, S.; Cuthbertson, S. Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): A mini review. Br. J. Nutr. 2009, 102, 1703–1708. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Niaz, B.; Arshad, M.U.; Tufail, T.; Hussain, M.B.; Javed, A. Bitter melon (Momordica charantia): A natural healthy vegetable. Int. J. Food Prop. 2018, 21, 1270–1290. [Google Scholar] [CrossRef]
- Tan, H.F.; Gan, C.Y. Polysaccharide with antioxidant, α-amylase inhibitory and ace inhibitory activities from Momordica charantia. Int. J. Biol. Macromol. 2016, 85, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J.H. Recent advances in Momordica charantia: Functional components and biological activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Assefa, A.D.; Keum, Y.S. Fatty acid and carotenoid composition of bitter melon (Momordica charantia L.) seed arils: A potentially valuable source of lycopene. J. Food Meas. Charact. 2017, 11, 1266–1273. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Yang, G.; Ho, C.; Li, S. Momordica charantia: A popular health-promoting vegetable with multifunctionality. Food Funct. 2017, 8, 1749–1762. [Google Scholar] [CrossRef]
- Khanna, P.; Jain, S.C.; Panagariya, A.; Dixit, V.P. Hypoglycemic activity of polypeptide-p from a plant source. J. Nat. Prod. 1981, 44, 648–655. [Google Scholar] [CrossRef]
- Lo, H.Y.; Ho, T.Y.; Li, C.C.; Chen, J.C.; Liu, J.J.; Hsiang, C.Y. A novel insulin receptor-binding protein from Momordica charantia enhances glucose uptake and glucose clearance in vitro and in vivo through triggering insulin receptor signaling pathway. J. Agric. Food Chem. 2014, 62, 8952–8961. [Google Scholar] [CrossRef]
- Chuang, C.Y.; Hsu, C.; Chao, C.Y.; Wein, Y.S.; Kuo, Y.H.; Huang, C.J. Fractionation and identification of 9c, 11t, 13t-conjugated linolenic acid as an activator of PPARα in bitter gourd (Momordica charantia L.). J. Biomed. Sci. 2006, 13, 763–772. [Google Scholar] [CrossRef]
- Keller, A.C.; Ma, J.; Kavalier, A.; He, K.; Brillantes, A.M.B.; Kennelly, E.J. Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine 2011, 19, 32–37. [Google Scholar] [CrossRef]
- Ma, J.; Whittaker, P.; Keller, A.; Mazzola, E.P.; Pawar, R.S.; White, K.D.; Callahan, J.H.; Kennelly, E.J.; Krynitsky, A.J.; Rader, J.I. Cucurbitane-type triterpenoids from Momordica charantia. Plant. Med. 2010, 76, 1758–1761. [Google Scholar] [CrossRef]
- Iseli, T.J.; Nigel, T.; Zeng, X.Y.; Cooney, G.J.; Kraegen, E.W.; Yao, S.; Ye, Y.; James, D.E.; Ye, J.M. Activation of AMPK by bitter melon triterpenoids involves CaMKKβ. PLoS ONE 2013, 8, e62309. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Ye, J.M.; Turner, N.; Hohnen-Behrens, C.; Ke, C.Q.; Tang, C.P.; Chen, T.; Weiss, H.C.; Gesing, E.R.; Rowland, A.; et al. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem. Biol. 2008, 15, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Harinantenaina, L.; Tanaka, M.; Takaoka, S.; Oda, M.; Mogami, O.; Uchid, M.; Askawa, Y. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem. Pharm. Bull. 2006, 54, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Jirousek, M.R.; Johnson, T.O.; Jacques, E. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov. 2002, 1, 696–709. [Google Scholar]
- Sohn, J.H.; Kim, J.W.; Jung, G.W.; Park, D.C.; Moon, S.B.; Cho, H.R.; Ku, S.K.; Choi, J.S. Synergic antiobesity effects of bitter melon water extract and platycodin-D in genetically obese mice. J. Environ. Biol. 2018, 39, 603–611. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, M. Regeneration of beta cells in islets of langerhans of pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (bitter gourd) fruits. Indian J. Exp. Biol. 2007, 45, 1055–1062. [Google Scholar]
- Kolawole, O.T.; Abiona, F.E.; Kolawole, S.O.; Ayankunle, A.A.; OIaniran, O.I. Effect of Momordica charantia fruit extract on normal and alloxan-diabetic rats. Int. J. Pharmacol. 2011, 7, 532–535. [Google Scholar] [CrossRef]
- Sarkar, S.; Pranava, M.; Marita, R.A. Demonstration of the hypoglycemic action of Momordica charantia in a validated animal model of diabetes. Pharmacol. Res. 1996, 33, 1–4. [Google Scholar] [CrossRef]
- Yibchok-Anun, S.; Adisakwattana, S.; Yao, C.Y. Slow acting protein extract from fruit pulp of Momordica charantia with insulin secretagogue and insulinomimetic activities. Biol. Pharm. Bull. 2006, 29, 1126–1131. [Google Scholar] [CrossRef]
- Ali, L.; AzadKan, K.; Rouf Mamun, M.I.; Mosihuzzaman, M.; Nahar, N.; Nur-e-Alam, M.; Rokeya, B. Studies on hypoglycemic effects of fruit pulp, seed, and whole plant of Momordica charantia on normal and diabetic model rats. Plant. Med. 1993, 59, 408–412. [Google Scholar] [CrossRef]
- Pina, F.; Brown, A.; Luebcke, E.; Perkins-Veazie, P.; Clarke, S.; Kuvibidila, S.; Hill, M.; Linghtfoot, S.; Smith, B.; Lucas, E. Momordica charantia improves body weight and glucose tolerance in mice fed high fat diet. FASEB J. 2009, 23, 563.37. [Google Scholar] [CrossRef]
- Burnett, A.A.J.; Singh, P.D.; Simon, O.; Mckoy, M.L.G. Effect of acute administration of Momordica charantia fruit extracts on blood glucose levels of normoglycemic rats. FASEB J. 2011, 25, 889–890. [Google Scholar]
- Kim, S.K.; Jung, J.; Jung, J.H.; Yoonb, N.A.; Kangb, S.S.; Rohb, G.S.; Hahma, J.R. Hypoglycemic efficacy and safety of Momordica charantia (bitter melon) in patients with type 2 diabetes mellitus. Complement. Ther. Med. 2020, 52, 102524. [Google Scholar] [CrossRef] [PubMed]
- Krawinkel, M.B.; Ludwig, C.; Swai, M.E.; Yang, R.Y.; Chun, K.P.; Habicht, S.D. Bitter gourd reduces elevated fasting plasma glucose levels in an intervention study among prediabetics in Tanzania. J. Ethnopharmacol. 2018, 216, 1–7. [Google Scholar] [CrossRef]
- Fuangchan, A.; Sonthisombat, P.; Seubnukarn, T.; Chanouan, P.; Chotchaisuwat, P.; Sirigulsatien, V.; Lngkaninan, K.; Plianbangchang, P.; Haines, S.T. Hypoglycemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patients. J. Ethnopharmacol. 2011, 134, 422–428. [Google Scholar] [CrossRef]
- Rahman, I.U.; Khan, R.; Rahman, K.; Bashir, M. Lower hypoglycemic but higher antiatherogenic effects of bitter melon than glibenclamide in type 2 diabetic patients. Nutr. J. 2015, 14, 13. [Google Scholar] [CrossRef]
- Cortez-Navarrete, M.; Méndez-Del Villar, M.; Ramos-González, E.J.; Pérez-Rubio, K.G. Momordica Charantia: A Review of Its effects on metabolic diseases and mechanisms of action. J. Med. Food 2021, 24, 1017–1027. [Google Scholar] [CrossRef]
- Ahmed, I.; Adeghate, E.; Sharma, A.K.; Pallot, D.J.; Singh, B. Effects of Momordica charantia fruit juice on islet morphology in the pancreas of the streptozotocin- diabetic rat. Diabetes. Res. Clin. Pr. 1998, 40, 145–151. [Google Scholar] [CrossRef]
- Cortez-Navarrete, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; González-Ortiz, M.; Villar, M.M.D. Momordica charantia Administration Improves Insulin Secretion in Type 2 Diabetes Mellitus. J. Med. Food. 2018, 21, 672–677. [Google Scholar] [CrossRef]
- Shimada, T.; Kato, F.; Dwijayanti, D.R.; Nagata, T.; Kinoshita, A.; Okuyama, T.; Nishizawa, M.; Mukai, E. Bitter melon fruit extract enhances intracellular ATP production and insulin secretion from rat pancreatic β-cells. Br. J. Nutr. 2022, 127, 377–383. [Google Scholar] [CrossRef]
- Sridhar, M.G.; Vinayagamoorthi, R.; Arul Suyambunathan, V.; Bobby, Z.; Selvaraj, N. Bitter gourd (Momordica charantia) improves insulin sensitivity by increasing skeletal muscle insulin-stimulated IRS-1 tyrosine phosphorylation in high-fat-fed rats. Br. J. Nutr. 2008, 99, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.Y.; Li, C.C.; Ho, T.Y.; Hsiang, C.Y. Identification of the bioactive and consensus peptide motif from Momordica charantia insulin receptor-binding protein. Food Chem. 2016, 204, 298–305. [Google Scholar] [CrossRef]
- Chen, Q.; Chan, L.L.; Li, E.T.S. Bitter melon (Momordica charantia) reduces adiposity, lowers serum insulin and normalizes glucose tolerance in rats fed a high fat diet. J. Nutr. 2003, 133, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Adeghate, E.; Cummings, E.; Singh, J. Beneficial effects and mechanism of action of Momordica charantia juice in the treatment of streptozotocin-induced diabetes mellitus in rat. Mol. Cell. Biochem. 2004, 261, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cummings, E.; Hundal, H.S.; Wackerhage, H.; Belle, M.; Adeghate, E.; Singh, J. Momordica charantia fruit juice stimulates glucose and amino acid uptakes in l6 myotubes. Mol. Cell. Biochem. 2004, 261, 99–104. [Google Scholar] [CrossRef]
- White, P.E.; Król, E.; Szwengiel, A.; Tubacka, M.; Szczepankiewicz, D.; Staniek, H.; Vincent, J.B.; Krejpcio, Z. Effects of bitter melon and a chromium propionate complex on symptoms of insulin resistance and type 2 diabetes in rat models. Biol. Trace Elem. Res. 2021, 199, 1013–1026. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Yu, J.; Tan, Y.; Guo, P.; Wu, C.M. The gut microbiota confers the lipid-lowering effect of bitter melon (Momordica charantia L.) in high-fat diet (HFD)-induced hyperlipidemic mice. Biomed. Pharmacother. 2020, 131, 110667. [Google Scholar] [CrossRef]
- Singh, R.; Garcia-Gomez, I.; Gudehithlu, K.P.; Singh, A.K. Bitter melon extract promotes granulation tissue growth and angiogenesis in the diabetic wound. Adv. Skin Wound Care 2017, 30, 16–26. [Google Scholar] [CrossRef]
- Dans, A.M.L.; Villarruz, M.V.C.; Jimeno, C.A.; Javelosa, M.A.U.; Chua, J.; Bautista, R.; Velez, G.G.B. The effect of Momordica charantia capsule preparation on glycemic control in Type 2 Diabetes Mellitus needs further studies. J. Clin. Epidemiol. 2007, 60, 554–559. [Google Scholar] [CrossRef]
- Elekofehinti, O.O.; Oyedokun, V.O.; Iwaloye, O.; Lawal, A.O.; Ejelonu, O.C. Momordica charantia silver nanoparticles modulate SOCS/JAK/STAT and P13K/AKT/PTEN signalling pathways in the kidney of streptozotocin-induced diabetic rats. J. Diabetes Metab. Dis. 2021, 20, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Duddela, S.; Nataraj Sekhar, P.; Padmavati, G.V.; Banerjee, A.K.; Murty, U.S.N. Probing the structure of human glucose transporter 2 and analysis of protein ligand interactions. Med. Chem. Res. 2010, 19, 836–853. [Google Scholar] [CrossRef]
- Guillam, M.T.; Hümmler, E.; Schaerer, E.; Wu, J.Y.; Birnbaum, M.J.; Beermann, F.; Schmidt, A.; Deriaz, N.; Thorens, B. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking glut-2. Nat. Genet. 1997, 17, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Barranco, C. SGLT2 blockers in T2DM. Nat. Rev. Cardiol. 2018, 15, 255. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, R.L.; Greenway, F.L.; Chen, L.; Liu, Y.; Breed, S.L.; Andrews, S.M.; Wald, J.A.; Walker, A.; Smith, C.D. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am. J. Physiol. -Gastrointest. Liver Physiol. 2015, 308, G946–G954. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.S.; Snyder, A.; Rajendran, V.M.; Coon, S. GLUT2 proteins are regulated by the AKT pathway under diabetic conditions in intestinal epithelial cells. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Mahrosh, H.S.; Mehmood, R.; Bukhari, S.A.; Afzal, G.; Arif, R. Investigation of hypoglycemic peptides derived from conserved regions of adMc1 to reveal their antidiabetic activities. BioMed Res. Int. 2021, 2021, 5550180. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, K.; Ahmed, B.; Al-Abbasi, F.A.; Anwar, F.; Verma, A. Deconvoluting the dual hypoglycemic effect of wedelolactone isolated from Wedelia calendulacea: Investigation via experimental validation and molecular docking. RSC Adv. 2018, 8, 18180–18196. [Google Scholar] [CrossRef]
- Musa, E.; Matjila, M.; Levitt., N.S. Kisspeptins and glucose homeostasis in pregnancy: Implications for gestational diabetes mellitus—A review article. Reprod. Sci. 2021, 29, 321–327. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A. Talking microbes: When gut bacteria interact with diet and host organs. Mol. Nutr. Food Res. 2016, 60, 58–66. [Google Scholar] [CrossRef]
- Kenny, D.J.; Plichta, D.R.; Shungin, D.; Koppel, N.; Hall, B.; Fu, B.; Vasan, R.S.; Shaw, S.Y.; Vlamakis, H.; Balskus, E.P.; et al. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe 2020, 28, 245–257. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu. Rev. Med. 2012, 62, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.Y.; Lo, H.Y.; Liu, I.C.; Lo, L.C.; Hsiang, C.Y.; Ho, T.Y. A gastro-resistant peptide from Momordica charantia improves diabetic nephropathy in db/db mice via its novel reno-protective and anti-inflammatory activities. Food Funct. 2022, 13, 1822–1833. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Choi, J.M.; Park, S.E.; Rheec, E.J.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Park, C.Y. Preventive effects of bitter melon (Momordica charantia) against insulin resistance and diabetes are associated with the inhibition of NF-κB and JNK pathways in high-fat-fed OLETF rats. J. Nutr. Biochem. 2015, 26, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Evidence from a Systematic Review and Meta-Analysis Pointing to the Antidiabetic Effect of Polyphenol-Rich Plant Extracts from Gymnema montanum, Momordica charantia and Moringa oleifera. Curr. Issues Mol. Biol. 2022, 44, 699–717. [Google Scholar] [CrossRef]
- Moulik, P.K.; Mtonga, R.; Gill, G. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003, 26, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Ghanassia, E.; Villon, L.; Tdd, J.F.; Boegner, C.; Avignon, A.; Sultan, A. Long-term outcome and disability of diabetic patients hospitalized for diabetic foot ulcers: A 6.5-year follow-up study. Diabetes Care 2008, 31, 1288–1292. [Google Scholar] [CrossRef]
- Larsson, J.; Eneroth, M.; Apelqvist, J.; Stenstrom, A. Sustained reduction in major amputations in diabetic patients: 628 amputations in 461 patients in a defined population over a 20-year period. Acta. Orthop. 2008, 79, 665–673. [Google Scholar] [CrossRef]
- Goyal, R.; Singhai, M. Tuberculosis and non-diabetic hyperglycemia: A challenge to public health management. Med. Hypotheses 2013, 81, 1170–1171. [Google Scholar] [CrossRef]
- Wang, W.T.; Lee, P.; Yeh, H.W.; Smirnova, I.V.; Choi, I.Y. Effects of acute and chronic hyperglycemia on the neurochemical profiles in the rat brain with streptozotocin-induced diabetes detected using in vivo 1 h MR spectroscopy at 9.4 t. J. Neurochem. 2012, 121, 407–417. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Schultz, G.S.; Lopez-Mola, E.; Nieto, G.G.; Siverio, M.G.; Martinez, L.H. Glucose toxic effects on granulation tissue productive cells: The diabetics’ impaired healing. BioMed Res. Int. 2013, 2013, 256043. [Google Scholar] [CrossRef]
- Jia, Y.N.; Xue, Z.H.; Wang, Y.J.; Lu, Y.P.; Li, R.L.; Li, N.N.; Wang, Q.R. Chemical structure and inhibition on α-glucosidase of polysaccharides from corn silk by fractional precipitation. Carbohyd. Polym. 2021, 252, 117185. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Zhu, J.; Ai, Y.F. Utilization of maltogenic α-amylase treatment to enhance the functional properties and reduce the digestibility of pulse starches. Food Hydrocoll. 2021, 120, 106932. [Google Scholar] [CrossRef]
- Belhadj, S.; Hentati, O.; Elfeki, A.; Hamden, K. Inhibitory activities of Ulva lactuca polysaccharides on digestive enzymes related to diabetes and obesity. Arch. Physiol. Biochem. 2013, 119, 81–87. [Google Scholar]
- Li, Y.X.; Kim, S.K. Utilization of seaweed derived ingredients as potential antioxidants and functional ingredients in the food industry: An overview. Food Sci. Biotechnol. 2011, 20, 1461–1466. [Google Scholar] [CrossRef]
- Blum, A.; Loerz, C.; Martin, H.J.; Staab-Weijnitz, C.A.; Maser, E. Momordica charantia extract, a herbal remedy for type 2 diabetes, contains a specific 11β-hydroxysteroid dehydrogenase type 1 inhibitor. J. Steroid Biochem. Mol. Biol. 2012, 128, 51–55. [Google Scholar] [CrossRef]
- Iseli, T.J.; Oakhill, J.S.; Bailey, M.F.; Wee, S.; Walter, M.; Denderen, B.J.V.; Castelli, L.A.; Katsis, F.; Witters, L.A.; Stapleton, D.; et al. AMP-activated protein kinase subunit interactions. J. Biol. Chem. 2008, 283, 4799–4807. [Google Scholar] [CrossRef]
- Bijland, S.; Mancini, S.J.; Salt, I.P. Role of amp-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013, 124, 491–507. [Google Scholar] [CrossRef]
- Zeng, W.F.; Yin, X.Z.; Jiang, Y.H.; Jin, L.T.; Liang, W. PPARα at the crossroad of metabolic–immune regulation in cancer. FESB J. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Liu, Z.; Gong, J.; Huang, W.; Lu, F.; Dong, H. The effect of Momordica charantia in the treatment of diabetes mellitus: A review. Evid-Based Compl. Alt. 2021, 2021, 3796265. [Google Scholar] [CrossRef]
- Pahlavani, N.; Roudi, F.; Zakerian, M.; Ferns, G.A.; Navashenaq, J.G.; Mashkouri, A.; Ghayour-Mobarhan, M.; Rahimi, H. Possible molecular mechanisms of glucose- lowering activities of Momordica charantia (karela) in diabetes. J. Cell. Biochem. 2019, 120, 10921–10929. [Google Scholar] [CrossRef]
- Sur, S.; Steele, R.; Aurora, R.; Varvares, M.; Schwetye, K.E.; Ray, R.B. Bitter Melon prevents the development of 4-NQO-induced oral squamous cell carcinoma in an immunocompetent mouse model by modulating immune signaling. Cancer Prev. Res. 2018, 11, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.L.; Kasali, F.M.; Deyno, S.; Mtewa, A.; Nagendrappa, P.B.; Tolo, C.U.; Ogwang, P.E.; Sesaazi, D. Momordica charantia L. lowers elevated glycaemia in type 2 diabetes mellitus patients: Systematic review and meta-analysis. J. Ethnopharmacol. 2019, 231, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Jensen, J.S.; Jensen, T.; Deckert, T. Serum sialic acid concentration is elevated in IDDM especially in early diabetic nephropathy. J. Intern. Med. 2010, 237, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Crook, M.A.; Pickup, J.C.; Lumb, P.J.; Georgino, F.; Webb, D.J.; Fuller, J.H. Relationship between plasma sialic acid concentration and microvascular and macrovascular complications in type 1 diabetes: The eurodiab complications study. Diabetes Care 2001, 24, 316–322. [Google Scholar] [CrossRef]
- Rahman, I.; Malik, S.A.; Bashir, M.; Khan, R.; Iqbal, M. Serum sialic acid changes in non-insulin-dependant diabetes mellitus (NIDDM) patients following bitter melon (Momordica charantia) and rosiglitazone (avandia) treatment. Phytomedicine 2009, 16, 401–405. [Google Scholar] [CrossRef]
- Sukmawati, D.; Fujimura, S.; Jitsukawa, S.; Hirano, R.I.; Ishii, T.; Sato, T.; Hayashi, A.; Itoh, S.; Mizuno, H.; Daida, H.; et al. Oxidative stress tolerance of early stage diabetic endothelial progenitor cell. Regen. Ther. 2015, 1, 38–44. [Google Scholar] [CrossRef][Green Version]
- Kizub, I.V.; Klymenko, K.I.; Soloviev, A.I. Protein kinase C in enhanced vascular tone in diabetes mellitus. Int. J. Cardiol. 2014, 174, 230–242. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).