Oxidative Stress and DNA Damage Effect of Dioscorea hispida Dennst. on Placental Tissues of Rats
Abstract
:1. Introduction
2. Results
2.1. UHPLC-ESI-MS Analysis
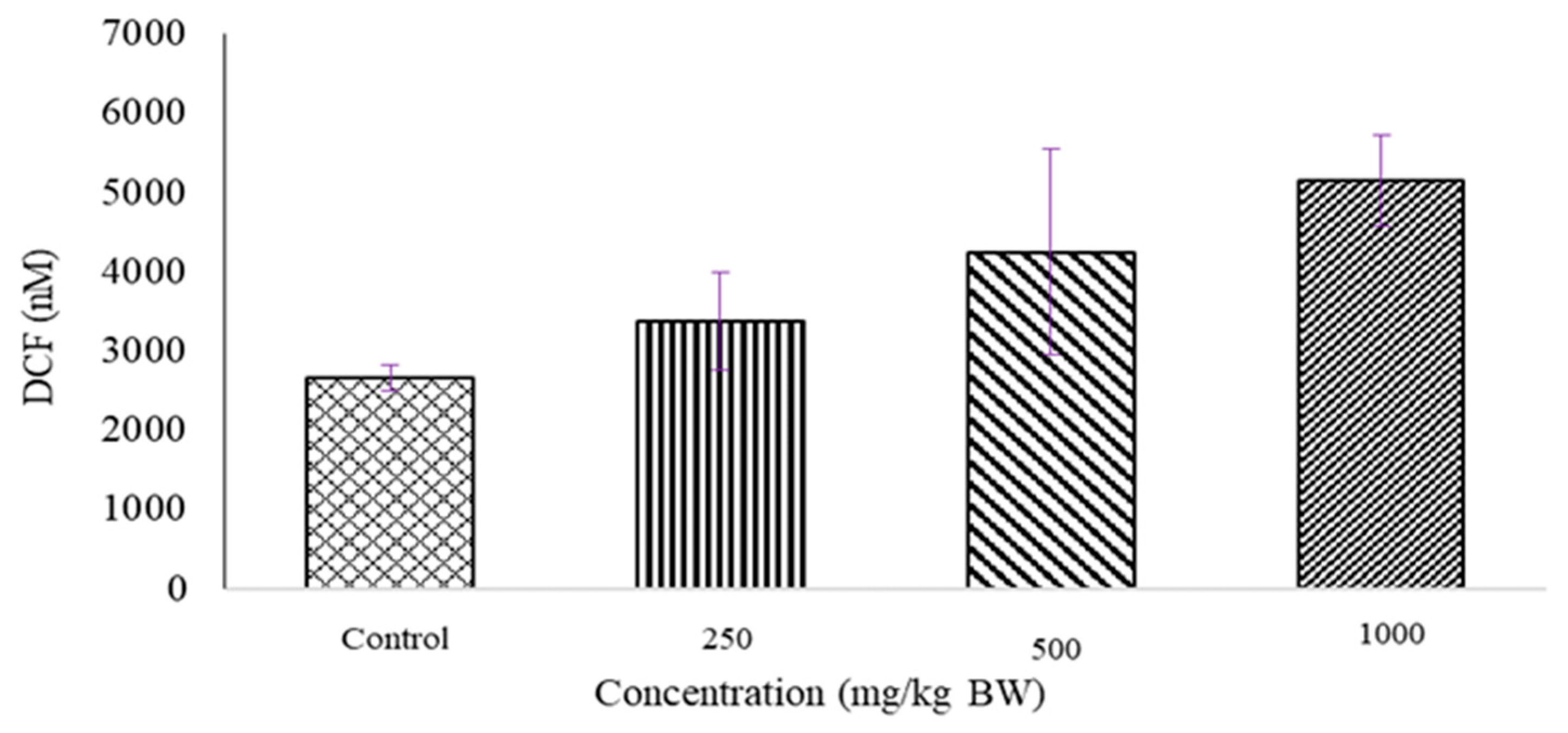
2.2. Determination of ROS Level
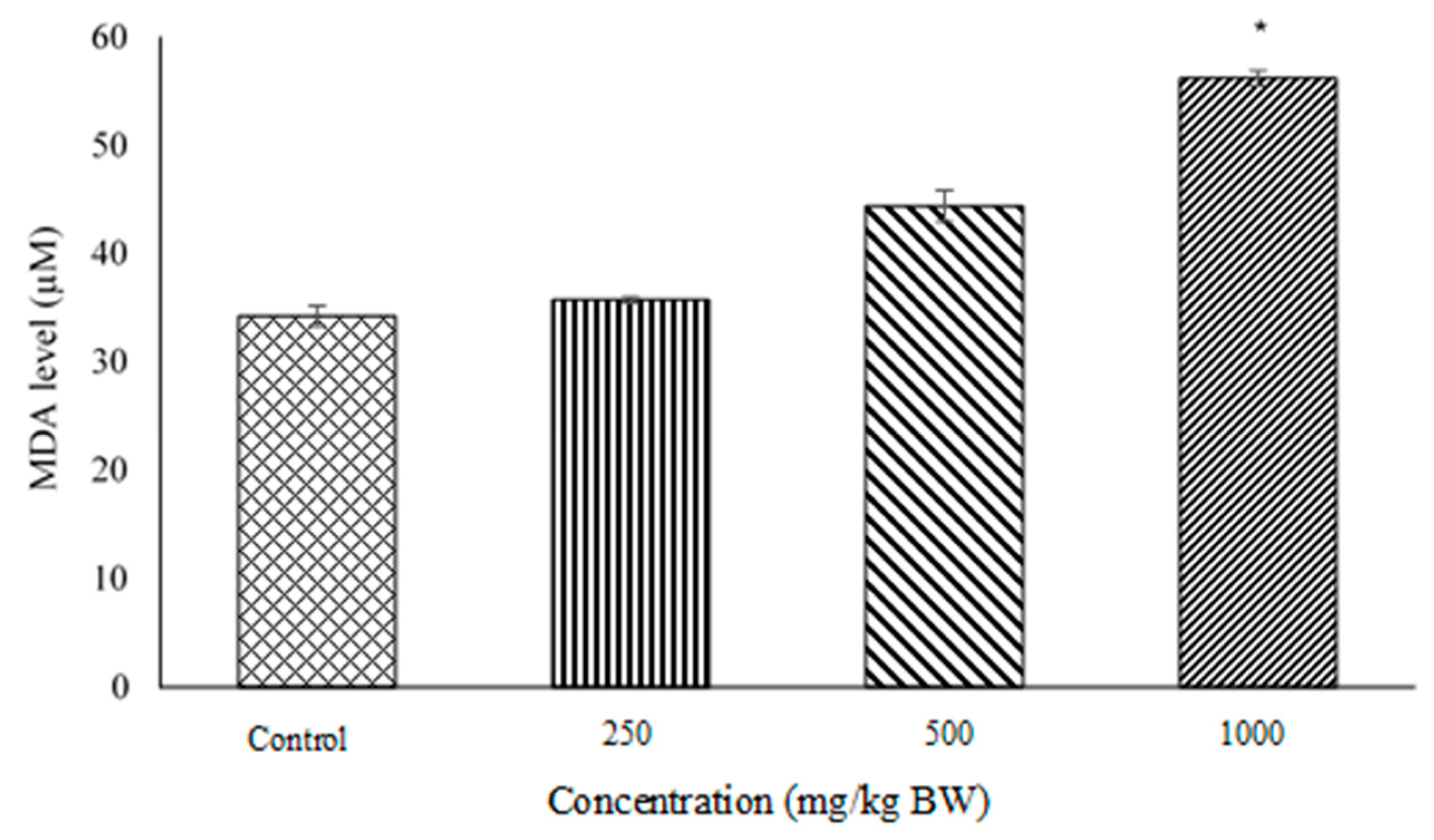
2.3. Lipid Peroxidation and Malondialdehyde (MDA) Quantification Assays
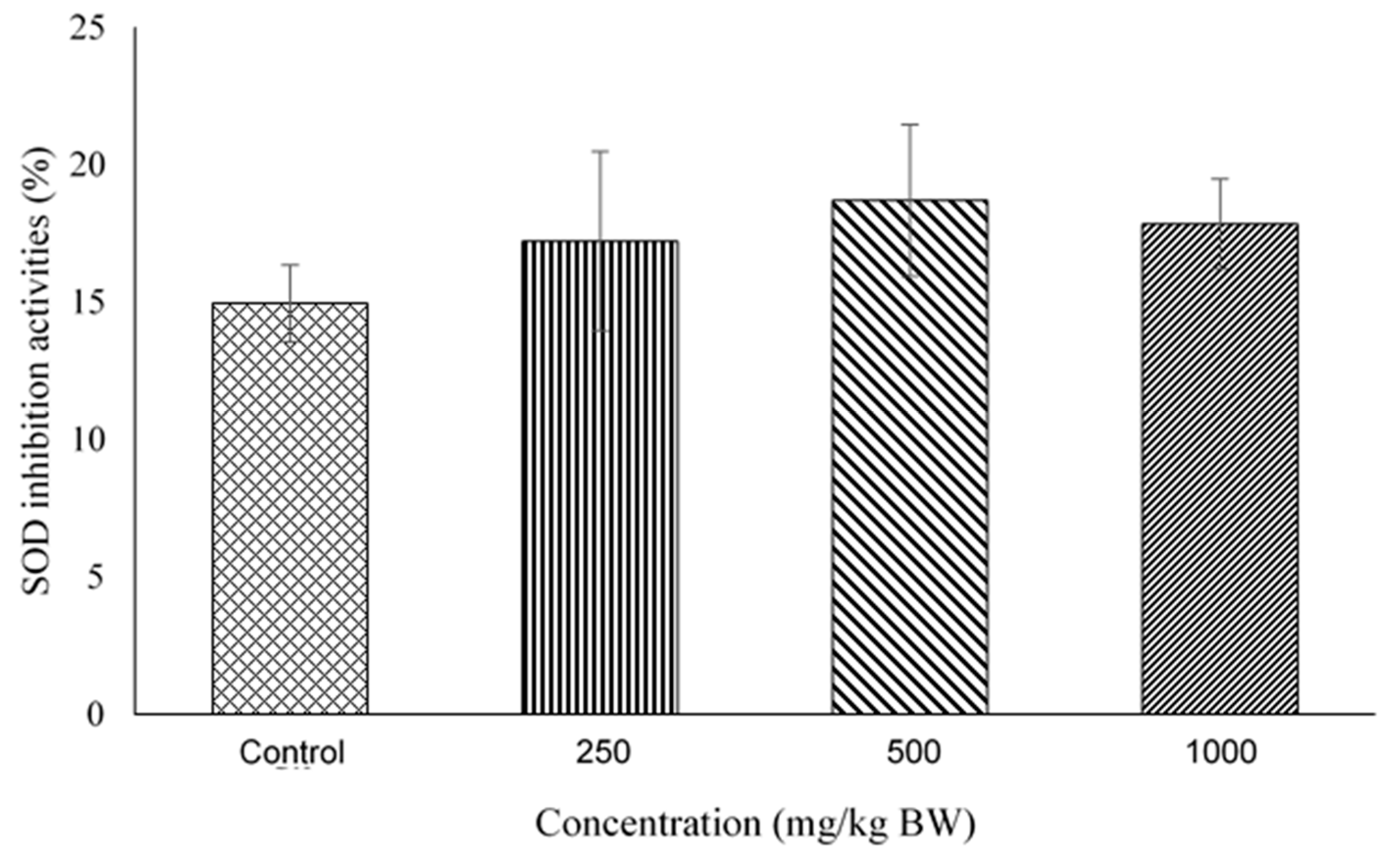
2.4. Superoxide Dismutase (SOD) Activity Assay
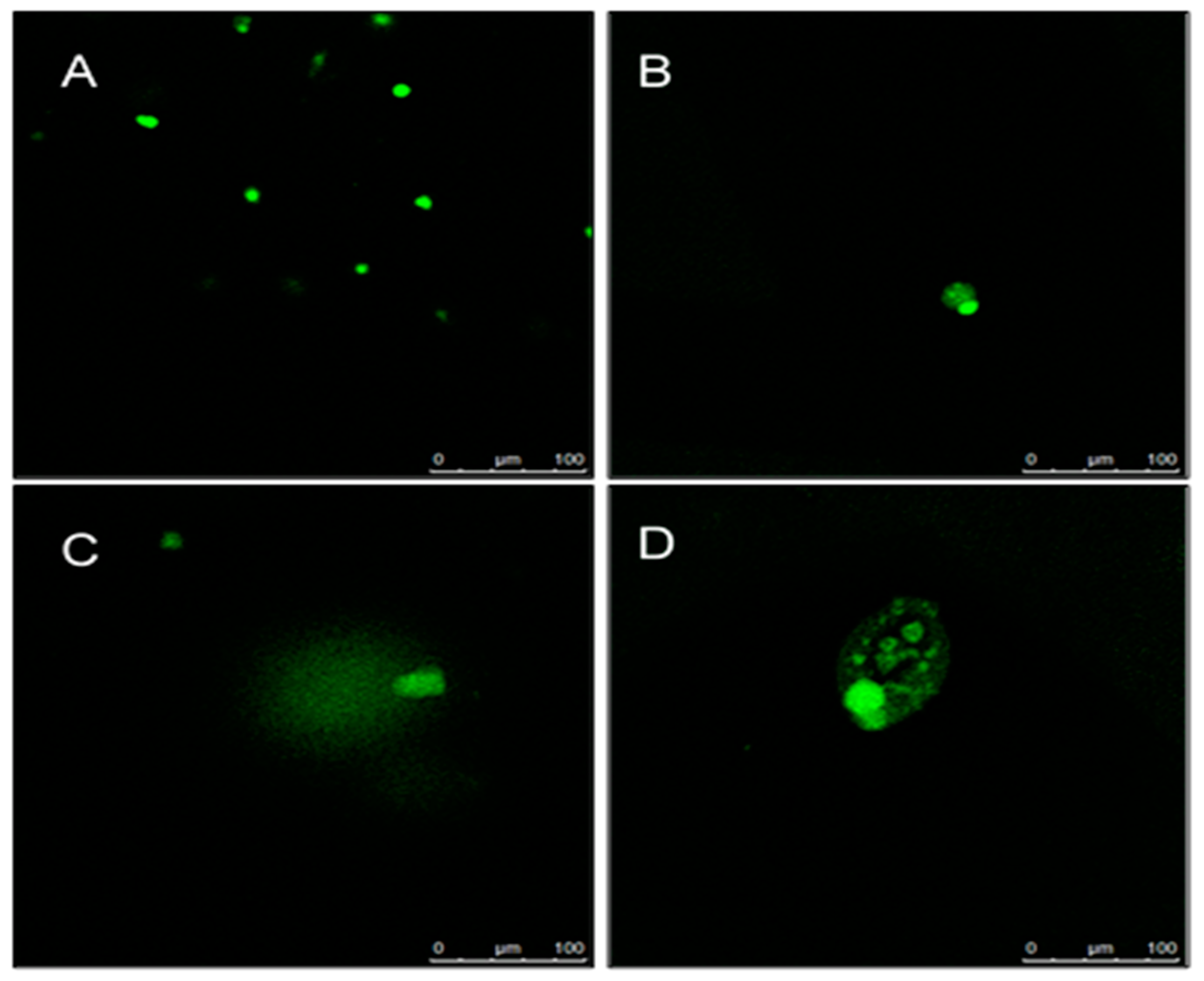
2.5. Determination of DNA Damage Using Comet Assay
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extract Preparation
4.3. Total Polyphenols
4.4. UHPLC-ESI-MS Analysis
4.5. Animals and Experimental Design
4.6. Determination of ROS Level
4.7. Lipid Peroxidation and Malondialdehyde (MDA) Quantification Assays
4.8. Superoxide Dismutase (SOD) Activity Assay
4.9. Determination of DNA Damage Using Comet Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Laforgia, N.; Di Mauro, A.; Guarnieri, G.F.; Varvara, D.; De Cosmo, L.; Panza, R.; Capozza, M.; Baldassarre, M.E.; Resta, N. The Role of Oxidative Stress in the Pathomechanism of Congenital Malformations. Oxidative Med. Cell. Longev. 2018, 2018, 7404082. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Stein, T.P.; Scholl, T.O.; Schluter, M.D.; Leskiw, M.J.; Chen, X.; Spur, B.W.; Rodriguez, A. Oxidative Stress Early in Pregnancy and Pregnancy Outcome. Free Radic. Res. 2008, 42, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Scholl, T.O.; Stein, T.P. Oxidant Damage to DNA and Pregnancy Outcome. J. Matern. Fetal Med. 2001, 10, 182–185. [Google Scholar] [CrossRef]
- Clapés, S.; Fernández, T.; Suárez, G. Oxidative Stress and Birth Defects in Infants of Women with Pregestational Diabetes. MEDICC Rev. 2013, 15, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Park, B.; Kim, Y.J.; Lee, H.; Ha, E.; Park, H. Effect of Oxidative Stress on Birth Sizes: Consideration of Window from Mid Pregnancy to Delivery. Placenta 2009, 30, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B. Food and Medicinal Values of Certain Species of Dioscorea with Special Reference to Assam. J. Pharmacogn. Phytochem. 2015, 3, 15–18. [Google Scholar]
- Tajuddin, S.; Mat, N.; Yunus, A.G.; Shamsul Bahri, A.R. Anatomical Study of Stem, Petiole, Leaf, Tuber, Root and Flower of Dioscorea hispida Dennst. (Dioscoreaceae) by Using Optical Microscope, SEM and TEM. J. Agrobiotechnol. 2013, 4, 33–42. [Google Scholar]
- Azman, I.; Mutalib, S.A.; Yusoff, S.F.M.; Fazry, S.; Noordin, A.; Kumaran, M.; Mat Lazim, A. Novel Dioscorea hispida Starch-Based Hydrogels and Their Beneficial Use as Disinfectants. J. Bioact. Compat. Polym. 2016, 31, 42–59. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.-H.; Wang, M.; Ali, Z.; Smillie, T.J.; Zweigenbaum, J.; Khan, I.A. Characterization of Steroidal Saponins from Dioscorea villosa and D. cayenensis Using Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. Planta Med. 2014, 80, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Frawley, J.; Adams, J.; Steel, A.; Broom, A.; Gallois, C.; Sibbritt, D. Women’s Use and Self-Prescription of Herbal Medicine during Pregnancy: An Examination of 1835 Pregnant Women. Women’s Health Issues 2015, 25, 396–402. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, S.; Zheng, L.; Yin, L.; Xu, L.; Peng, J. A 90-Day Subchronic Toxicological Assessment of Dioscin, a Natural Steroid Saponin, in Sprague-Dawley Rats. Food Chem. Toxicol. 2012, 50, 1279–1287. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, X.; Huang, W.; Gong, G.; Li, D.; He, Y.; Zhao, Y. Acute Toxicity and Sub-Chronic Toxicity of Steroidal Saponins from Dioscorea zingiberensis C.H.Wright in Rodents. J. Ethnopharmacol. 2009, 126, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, T.M. Potential Toxicities of Herbal Therapies in the Developing Fetus. Dev. Reprod. Toxicol. 2003, 68, 496–498. [Google Scholar] [CrossRef]
- Muhammad, H.; Fariza, S.; Md Saad, W.M.; Thani, N.S.I.A.; Ibnu Rasid, E.N.; Mahomoodally, M.F.; Awang, N. Histopathological Changes in Placenta and Liver of Pregnant Rats Administered with Aqueous Extract of Dioscorea hispida var. daemona (Roxb). Food Chem. Toxicol. 2019, 131, 110538. [Google Scholar] [CrossRef]
- Anhwange, B.A.; Asemave, K.; Ikyenge, B.A.; Oklo, D.A. Hydrogen Cyanide Content of Manihort utilissima, Colocasia esculenta, Dioscorea bulbifera and Dioscorea domentorum Tubers Found in Benue State. Int. J. Chem. 2011, 4, 69–71. [Google Scholar] [CrossRef]
- Takehara, Y.; Yoshioka, T.; Sasaki, J. Changes in the Levels of Lipoperoxide and Antioxidant Factors in Human Placenta during Gestation. Acta Med. Okayama 1990, 44, 103–111. [Google Scholar]
- Zong, W.X.; Ditsworth, D.; Bauer, D.E.; Wang, Z.Q.; Thompson, C.B. Alkylating DNA Damage Stimulates a Regulated Form of Necrotic Cell Death. Genes Dev. 2004, 18, 1272–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Esterbauer, H.; Eckl, P.; Ortner, A. Possible Mutagens Derived from Lipids and Lipid Precursors. Mutat. Res. Genet. Toxicol. 1990, 238, 223–233. [Google Scholar] [CrossRef]
- Tuma, D.J.; Kearley, M.L.; Thiele, G.M.; Worrall, S.; Haver, A.; Klassen, L.W.; Sorrell, M.F. Elucidation of Reaction Scheme Describing Malondialdehyde-Acetaldehyde-Protein Adduct Formation. Chem. Res. Toxicol. 2001, 14, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Guo, X.; Fu, S.; Zhang, X.; Liang, X. Characterization of Steroidal Saponins in Crude Extracts from Dioscorea zingiberensis C. H. Wright by Ultra-Performance Liquid Chromatography/Electrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2010, 53, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Kang, Y.; Xu, Z.; Zang, C.; Fang, B.; Liu, X. Dioscin Prevents the Mitochondrial Apoptosis and Attenuates Oxidative Stress in Cardiac H9c2 Cells. Drug Res. 2014, 64, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Sun, Y.; Song, S.; Qi, Y.; Tao, X.; Xu, L.; Yin, L.; Han, X.; Xu, Y.; Li, H.; et al. Protective Effects of Dioscin against Lipopolysaccharide-Induced Acute Lung Injury through Inhibition of Oxidative Stress and Inflammation. Front. Pharmacol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Tao, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Protective Effect of Dioscin against Doxorubicin-Induced Cardiotoxicity via Adjusting MicroRNA-140-5p-Mediated Myocardial Oxidative Stress. Redox Biol. 2018, 16, 189–198. [Google Scholar] [CrossRef]
- Zidenga, T.; Leyva-Guerrero, E.; Moon, H.; Siritunga, D.; Sayre, R. Extending Cassava Root Shelf Life via Reduction of Reactive Oxygen Species Production. Plant Physiol. 2012, 159, 1396–1407. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
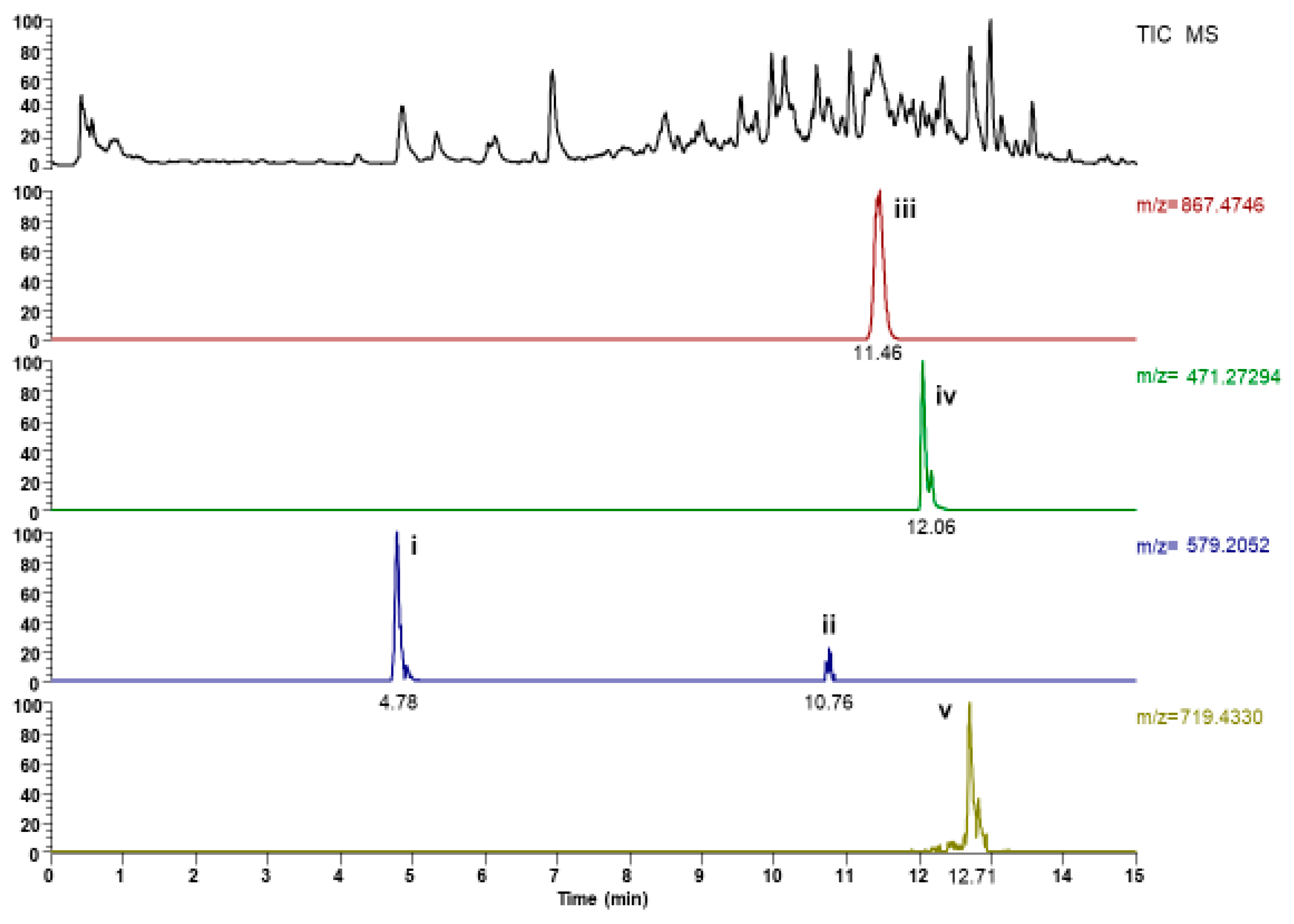

| Peak No | Retention Time Rt (min) | Compound | Formula | Selected Ion | m/z Observed | Error (ppm) |
|---|---|---|---|---|---|---|
| i. | 4.78 | (+)Syringavesinol-4-O-β-d-glucopyranoside-isomer | C28H35O13 | [M − H]− | 579.20844 | 2.11 |
| ii. | 10.76 | (+)Syringavesinol-4-O-β-d-glucopyranoside-isomer | C28H35O13 | [M − H]− | 579.20807 | 1.47 |
| iii. | 11.46 | Dioscin | C45H71O16 | [M − H]− | 867.47941 | 6.62 |
| iv. | 12.06 | Disogenin-3,6-dione | C28H39O6 | [M − H]− | 471.27484 | 1.54 |
| v. | 12.71 | Progenin III | C40H63O11 | [M − H]− | 719.43701 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, H.; Bakar, T.A.S.T.A.; Yusery, M.F.A.; Awang, N.; Saad, W.M.M.; Ibnu Rasid, E.N.; Mahomoodally, M.F.; Omar, M.H. Oxidative Stress and DNA Damage Effect of Dioscorea hispida Dennst. on Placental Tissues of Rats. Molecules 2022, 27, 2190. https://doi.org/10.3390/molecules27072190
Muhammad H, Bakar TASTA, Yusery MFA, Awang N, Saad WMM, Ibnu Rasid EN, Mahomoodally MF, Omar MH. Oxidative Stress and DNA Damage Effect of Dioscorea hispida Dennst. on Placental Tissues of Rats. Molecules. 2022; 27(7):2190. https://doi.org/10.3390/molecules27072190
Chicago/Turabian StyleMuhammad, Hussin, Tengku Aideed Syah Tg Abu Bakar, Muhamad Faizul Adhzim Yusery, Norizah Awang, Wan Mazlina Md. Saad, Elda Nurafnie Ibnu Rasid, Mohamad Fawzi Mahomoodally, and Maizatul Hasyima Omar. 2022. "Oxidative Stress and DNA Damage Effect of Dioscorea hispida Dennst. on Placental Tissues of Rats" Molecules 27, no. 7: 2190. https://doi.org/10.3390/molecules27072190
APA StyleMuhammad, H., Bakar, T. A. S. T. A., Yusery, M. F. A., Awang, N., Saad, W. M. M., Ibnu Rasid, E. N., Mahomoodally, M. F., & Omar, M. H. (2022). Oxidative Stress and DNA Damage Effect of Dioscorea hispida Dennst. on Placental Tissues of Rats. Molecules, 27(7), 2190. https://doi.org/10.3390/molecules27072190






