Effects of LiBF4 Addition on the Lithium-Ion Conductivity of LiBH4
Abstract
:1. Introduction
2. Experimental Section
2.1. Solid-State Synthesis
2.2. Structural Characterization
2.3. Electrochemical Impedance Spectroscopy
2.4. Linear Sweep Voltammetry
3. Results and Discussion
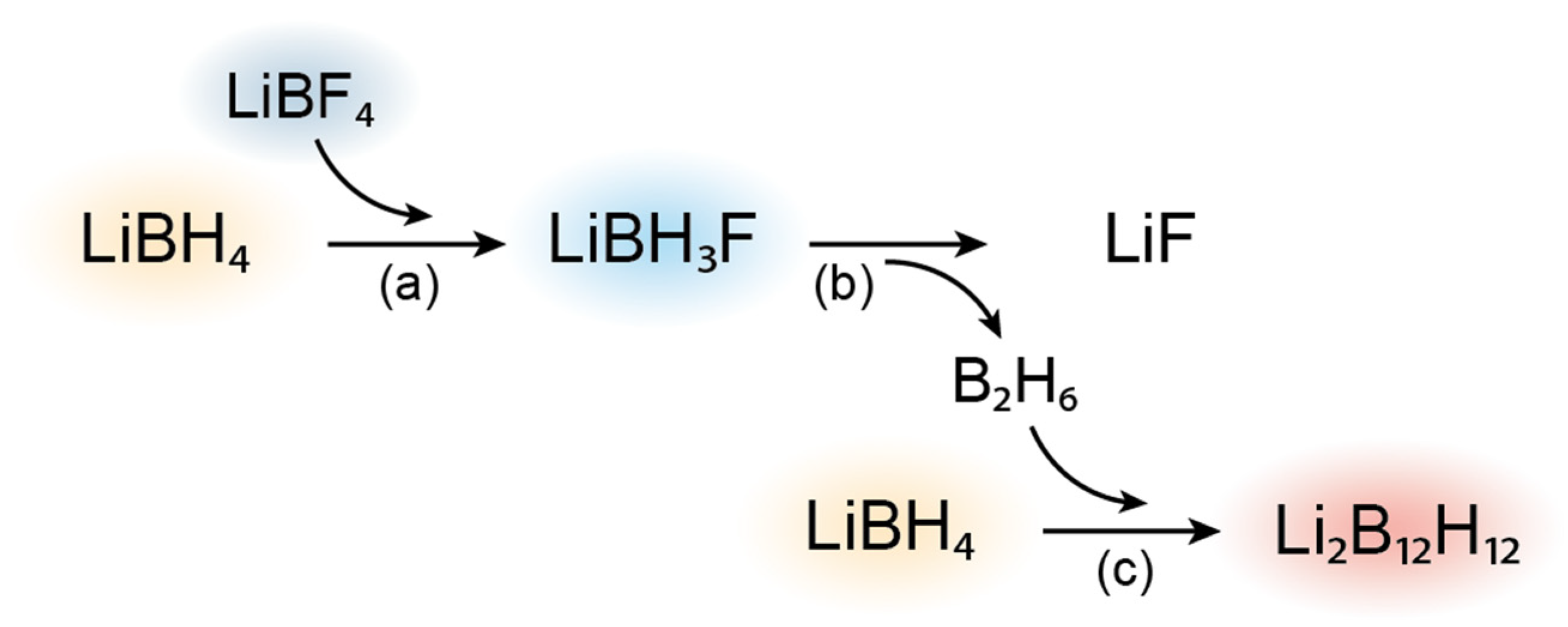
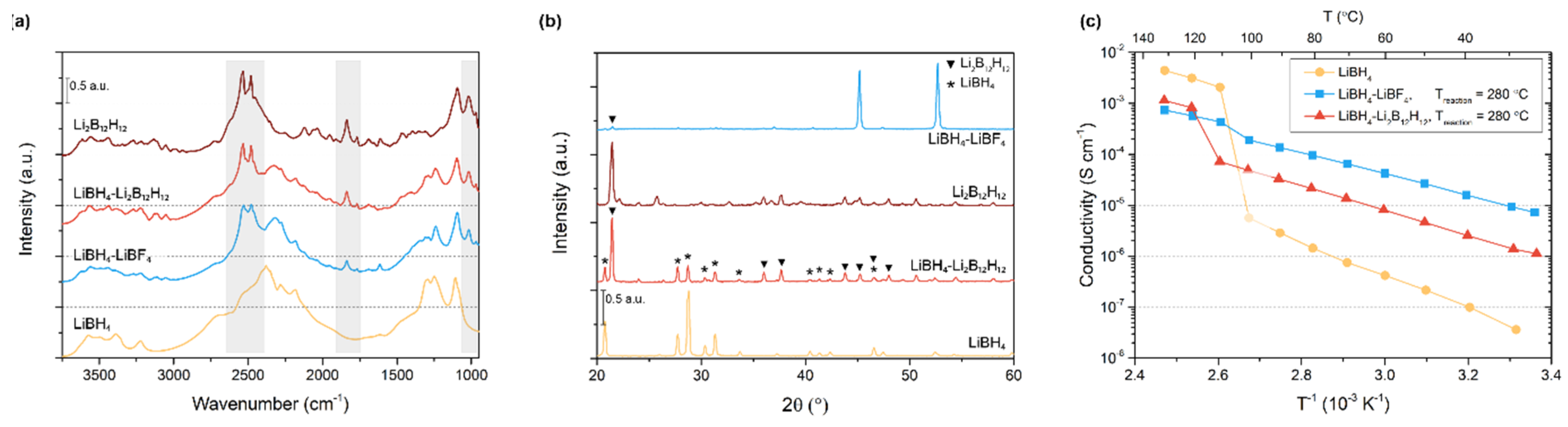
3.1. Structural Characterization and Conductivity of LiBH4-LiBF4 Mixtures
3.2. LiBH4-Li2B12H12 Composites
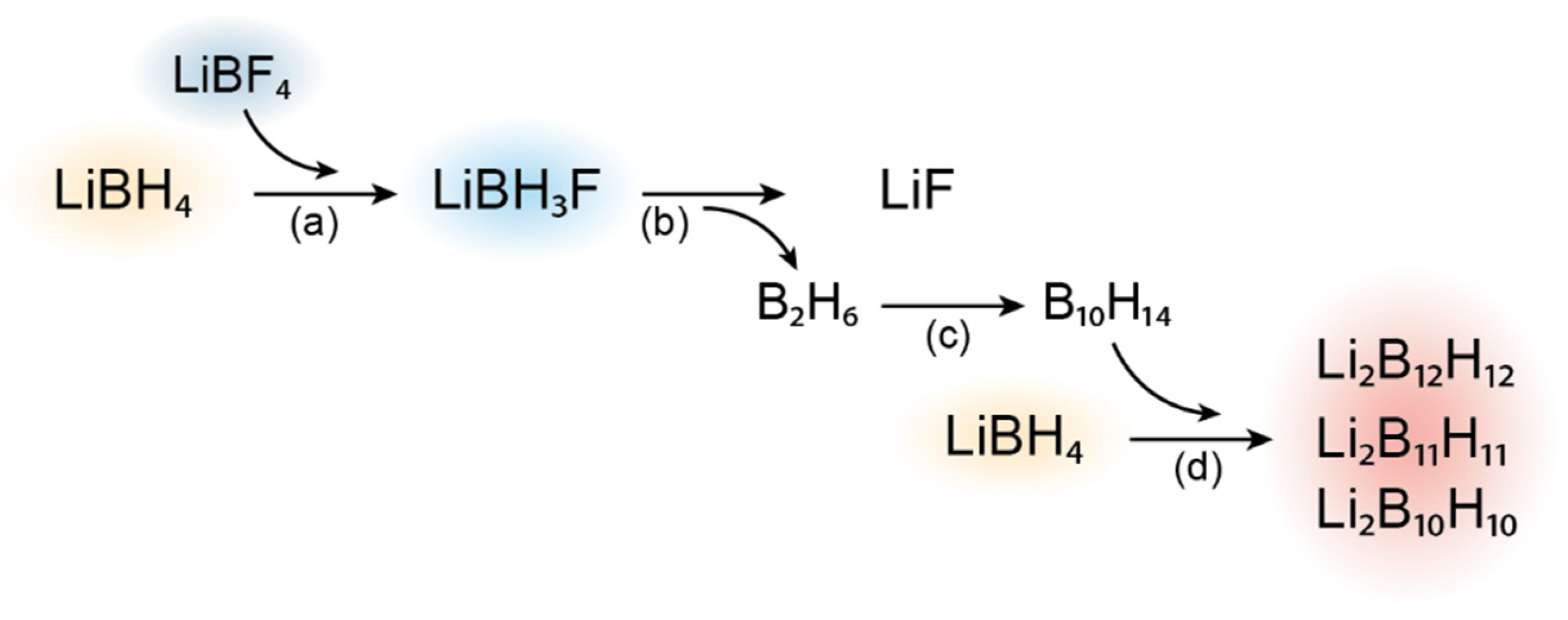
3.3. Alternative Decomposition Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lach, J.; Wróbel, K.; Wróbel, J.; Czerwiński, A. Applications of carbon in rechargeable electrochemical power sources: A review. Energies 2021, 14, 2649. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.Y.; He, Z.J.; Li, Y.J.; Mao, J.; Dai, K.H.; Yan, C.; Zheng, J.C. An advance review of solid-state battery: Challenges, progress and prospects. Sustain. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Subramanian, K.; Alexander, G.V.; Karthik, K.; Patra, S.; Indu, M.S.; Sreejith, O.V.; Viswanathan, R.; Narayanasamy, J.; Murugan, R. A brief review of recent advances in garnet structured solid electrolyte based lithium metal batteries. J. Energy Storage 2021, 33, 102157. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Recent progress in solid oxide and lithium ion conducting electrolytes research. Ionics 2006, 12, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.; Inaguma, Y.; Jung, W.H.; Chen, L.; Nakamura, T. High lithium ion conductivity in the perovskite-type compounds Ln12Li12TiO3 (Ln=La,Pr,Nd,Sm). Solid State Ion. 1994, 70–71, 203–207. [Google Scholar] [CrossRef]
- Seino, Y.; Ota, T.; Takada, K.; Hayashi, A.; Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 2014, 7, 627–631. [Google Scholar] [CrossRef]
- Rangasamy, E.; Liu, Z.; Gobet, M.; Pilar, K.; Sahu, G.; Zhou, W.; Wu, H.; Greenbaum, S.; Liang, C. An iodide-based Li7P2S8I superionic conductor. J. Am. Chem. Soc. 2015, 137, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Nakamori, Y.; Orimo, S.I.; Maekawa, H.; Takamura, H. Lithium superionic conduction in lithium borohydride accompanied by structural transition. Appl. Phys. Lett. 2007, 91, 2–5. [Google Scholar] [CrossRef]
- Lu, Z.; Ciucci, F. Metal Borohydrides as Electrolytes for Solid-State Li, Na, Mg, and Ca Batteries: A First-Principles Study. Chem. Mater. 2017, 29, 9308–9319. [Google Scholar] [CrossRef]
- Asakura, R.; Duchêne, L.; Kühnel, R.S.; Remhof, A.; Hagemann, H.; Battaglia, C. Electrochemical Oxidative Stability of Hydroborate-Based Solid-State Electrolytes. ACS Appl. Energy Mater. 2019, 2, 6924–6930. [Google Scholar] [CrossRef]
- Kisu, K.; Kim, S.; Oguchi, H.; Toyama, N.; Orimo, S.I. Interfacial stability between LiBH4-based complex hydride solid electrolytes and Li metal anode for all-solid-state Li batteries. J. Power Sour. 2019, 436, 226821. [Google Scholar] [CrossRef]
- Duchêne, L.; Remhof, A.; Hagemann, H.; Battaglia, C. Status and prospects of hydroborate electrolytes for all-solid-state batteries. Energy Storage Mater. 2020, 25, 782–794. [Google Scholar] [CrossRef]
- Verdal, N.; Udovic, T.J.; Stavila, V.; Tang, W.S.; Rush, J.J.; Skripov, A.V. Anion reorientations in the superionic conducting phase of Na2B12H12. J. Phys. Chem. C 2014, 118, 17483–17489. [Google Scholar] [CrossRef]
- Tang, W.S.; Matsuo, M.; Wu, H.; Stavila, V.; Zhou, W.; Talin, A.A.; Soloninin, A.V.; Skoryunov, R.V.; Babanova, O.A.; Skripov, A.V.; et al. Liquid-Like Ionic Conduction in Solid Lithium and Sodium Monocarba-closo-Decaborates Near or at Room Temperature. Adv. Energy Mater. 2016, 6, 1502237. [Google Scholar] [CrossRef]
- Gulino, V.; Brighi, M.; Murgia, F.; Ngene, P.; De Jongh, P.; Černý, R.; Baricco, M. Room temperature Solid-State Lithium-ion Battery using LiBH4-MgO composite Electrolyte. ACS Appl. Energy Mater. 2021, 4, 1228–1236. [Google Scholar] [CrossRef]
- Zettl, R.; De Kort, L.; Gombotz, M.; Wilkening, H.M.; De Jongh, P.E.; Ngene, P. Combined Effects of Anion Substitution and Nanoconfinement on the Ionic Conductivity of Li-Based Complex Hydrides. J. Phys. Chem. C 2020, 124, 2806–2816. [Google Scholar] [CrossRef] [Green Version]
- De Kort, L.M.; Harmel, J.; De Jongh, P.E.; Ngene, P. The effect of nanoscaffold porosity and surface chemistry on the Li-ion conductivity of LiBH4-LiNH2/metal oxide nanocomposites. J. Mater. Chem. A 2020, 8, 20687–20697. [Google Scholar] [CrossRef]
- De Kort, L.M.; Gulino, V.; de Jongh, P.E.; Ngene, P. Ionic conductivity in complex metal hydride-based nanocomposite materials: The impact of nanostructuring and nanocomposite formation. J. Alloys Compd. 2021, 901, 163474. [Google Scholar] [CrossRef]
- Oguchi, H.; Matsuo, M.; Hummelshøj, J.S.; Vegge, T.; Nørskov, J.K.; Sato, T.; Miura, Y.; Takamura, H.; Maekawa, H.; Orimo, S. Experimental and computational studies on structural transitions in the LiBH4-LiI pseudobinary system. Appl. Phys. Lett. 2009, 94, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, M.; Takamura, H.; Maekawa, H.; Li, H.-W.; Orimo, S. Stabilization of lithium superionic conduction phase and enhancement of conductivity of LiBH4 by LiCl addition. Appl. Phys. Lett. 2009, 94, 084103. [Google Scholar] [CrossRef]
- Maekawa, H.; Matsuo, M.; Takamura, H.; Ando, M.; Noda, Y. Halide-Stabilized LiBH4, a Room-Temperature Lithium Fast-Ion Conductor. J. Am. Chem. Soc. 2009, 131, 894–895. [Google Scholar]
- Rude, L.H.; Groppo, E.; Arnbjerg, L.M.; Ravnsbaek, D.B.; Malmkjaer, R.A.; Filinchuk, Y.; Baricco, M.; Besenbacher, F.; Jensen, T.R. Iodide substitution in lithium borohydride, LiBH4-LiI. J. Alloys Compd. 2011, 509, 8299–8305. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, M.; Remhof, A.; Martelli, P.; Caputo, R.; Ernst, M.; Miura, Y.; Sato, T.; Oguchi, H.; Maekawa, H.; Takamura, H. Complex hydrides with (BH4)- and (NH2)- anions as new lithium fast-ion conductors. J. Am. Chem. Soc. 2009, 131, 16389–16391. [Google Scholar] [CrossRef]
- Yan, Y.; Kühnel, R.S.; Remhof, A.; Duchêne, L.; Reyes, E.C.; Rentsch, D.; Łodziana, Z.; Battaglia, C. A Lithium Amide-Borohydride Solid-State Electrolyte with Lithium-Ion Conductivities Comparable to Liquid Electrolytes. Adv. Energy Mater. 2017, 7, 1700294. [Google Scholar] [CrossRef]
- Gulino, V.; Dematteis, E.M.; Corno, M.; Palumbo, M.; Baricco, M. Theoretical and Experimental Studies of LiBH4–LiBr Phase Diagram. ACS Appl. Energy Mater. 2021, 4, 7327–7337. [Google Scholar]
- Gulino, V.; Brighi, M.; Dematteis, E.M.; Murgia, F.; Nervi, C.; Cerny, R.; Baricco, M. Phase Stability and Fast Ion Conductivity in the Hexagonal LiBH4–LiBr–LiCl Solid Solution. Chem. Mater. 2019, 31, 5133–5144. [Google Scholar] [CrossRef] [Green Version]
- Rude, L.H.; Filsø, U.; D’Anna, V.; Spyratou, A.; Richter, B.; Hino, S.; Zavorotynska, O.; Baricco, M.; Sørby, M.H.; Hauback, B.C. Hydrogen-fluorine exchange in NaBH4-NaBF4. Phys. Chem. Chem. Phys. 2013, 15, 18185–18194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Wang, P.; Fang, Z.; Cheng, H. Thermodynamically tuning LiBH4 by fluorine anion doping for hydrogen storage: A density functional study. Chem. Phys. Lett. 2008, 450, 318–321. [Google Scholar] [CrossRef]
- Corno, M.; Pinatel, E.; Ugliengo, P.; Baricco, M. A computational study on the effect of fluorine substitution in LiBH4. J. Alloy. Compd. 2011, 509, S679–S683. [Google Scholar] [CrossRef]
- Heyn, R.H.; Saldan, I.; Sørby, M.H.; Frommen, C.; Arstad, B.; Bougza, A.M.; Fjellvåg, H.; Hauback, B.C. Structural and spectroscopic characterization of potassium fluoroborohydrides. Phys. Chem. Chem. Phys. 2013, 15, 11226–11230. [Google Scholar] [CrossRef] [Green Version]
- Saldin, V.I.; Sukhovey, V.V.; Savchenko, N.N.; Slobodyuk, A.B.; Ignatieva, L.N. Thermal studies of sodium tetrahydroborate–potassium tetrafluoroborate mixtures. Russ. J. Inorg. Chem. 2016, 61, 630–637. [Google Scholar] [CrossRef]
- Richter, B.; Ravnsbæk, D.B.; Sharma, M.; Spyratou, A.; Hagemann, H.; Jensen, T.R. Fluoride substitution in LiBH4; Destabilization and decomposition. Phys. Chem. Chem. Phys. 2017, 19, 30157–30165. [Google Scholar] [CrossRef]
- Brighi, M.; Murgia, F.; Černý, R. Closo-Hydroborate Sodium Salts as an Emerging Class of Room-Temperature Solid Electrolytes. Cell Rep. Phys. Sci. 2020, 1, 100217. [Google Scholar] [CrossRef]
- Zhu, M.; Pang, Y.; Lu, F.; Shi, X.; Yang, J.; Zheng, S. In Situ Formed Li-B-H Complex with High Li-Ion Conductivity as a Potential Solid Electrolyte for Li Batteries. ACS Appl. Mater. Interfaces 2019, 11, 14136–14141. [Google Scholar] [CrossRef]
- Ohba, N.; Miwa, K.; Aoki, M.; Noritake, T.; Towata, S.I.; Nakamori, Y.; Orimo, S.I.; Züttel, A. First-principles study on the stability of intermediate compounds of LiBH4. Phys. Rev. B 2006, 74, 075110. [Google Scholar] [CrossRef] [Green Version]
- Zavorotynska, O.; Corno, M.; Damin, A.; Spoto, G.; Ugliengo, P.; Baricco, M. Vibrational properties of MBH4 and MBF4 crystals (M = Li, Na, K): A combined DFT, infrared, and Raman study. J. Phys. Chem. C 2011, 115, 18890–18900. [Google Scholar] [CrossRef]
- D’Anna, V.; Spyratou, A.; Sharma, M.; Hagemann, H. FT-IR spectra of inorganic borohydrides. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Filinchuk, Y.; Hagemann, H. Structure and properties of NaBH4·2H2O and NaBH4. Eur. J. Inorg. Chem. 2008, 112, 3127–3133. [Google Scholar] [CrossRef] [Green Version]
- Raman, C.V. The vibration spectrum of lithium fluoride and the evaluation of its specific heat. Proc. Indian Acad. Sci. Sect. A 1962, 55, 131–152. [Google Scholar] [CrossRef]
- Breuer, S.; Pregartner, V.; Lunghammer, S.; Wilkening, H.M.R. Dispersed Solid Conductors: Fast Interfacial Li-Ion Dynamics in Nanostructured LiF and LiF γ-Al2O3 Composites. J. Phys. Chem. C 2019, 123, 5222–5230. [Google Scholar] [CrossRef]
- Li, C.; Gu, L.; Maier, J. Enhancement of the Li conductivity in LiF by introducing glass/crystal interfaces. Adv. Funct. Mater. 2012, 22, 1145–1149. [Google Scholar] [CrossRef]
- Miyazaki, R.; Karahashi, T.; Kumatani, N.; Noda, Y.; Ando, M.; Takamura, H.; Matsuo, M.; Orimo, S.I.; Maekawa, H. Room temperature lithium fast-ion conduction and phase relationship of LiI stabilized LiBH4. Solid State Ion. 2011, 192, 143–147. [Google Scholar] [CrossRef]
- Sveinbjörnsson, D.; Myrdal, J.S.; Blanchard, D.; Bentzen, J.J.; Hirata, T.; Mogensen, M.B.; Norby, P.; Orimo, S.I.; Vegge, T. Effect of Heat Treatment on the Lithium Ion Conduction of the LiBH4–LiI Solid Solution. J. Phys. Chem. C 2013, 117, 3249–3257. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Merrifield, R.E.; Miller, H.C.; Knoth, W.H.; Downing, J.R. Chemistry of Boranes. III. 1 The Infrared and Raman Spectra of B 12 H 12-and Related Anions. J. Am. Chem. Soc. 1962, 84, 2506–2508. [Google Scholar] [CrossRef]
- Sharma, M.; Sethio, D.; D’Anna, V.; Hagemann, H. Theoretical study of B12Hn F (12-N)2-. Int. J. Hydrogen Energy 2015, 40, 12721–12726. [Google Scholar] [CrossRef]
- Jensen, S.R.; Paskevicius, M.; Hansen, B.R.; Jakobsen, A.S.; Møller, K.T.; White, J.L.; Allendorf, M.D.; Stavila, V.; Skibsted, J.; Jensen, T.R. Hydrogenation properties of lithium and sodium hydride- closo -borate, [B10H10]2- and [B12H12]2-, composites. Phys. Chem. Chem. Phys. 2018, 20, 16266–16275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadikin, Y.; Brighi, M.; Schouwink, P.; Černý, R. Superionic Conduction of Sodium and Lithium in Anion-Mixed Hydroborates Na3BH4B12H12 and (Li0.7Na0.3)3BH4B12H12. Adv. Energy Mater. 2015, 5, 1501016. [Google Scholar] [CrossRef]
- Sadikin, Y.; Skoryunov, R.V.; Babanova, O.A.; Soloninin, A.V.; Lodziana, Z.; Brighi, M.; Skripov, A.V.; Cerny, R. Anion Disorder in K3BH4B12H12 and Its Effect on Cation Mobility. J. Phys. Chem. C 2017, 121, 5503–5514. [Google Scholar] [CrossRef]
- Toyama, N.; Kim, S.; Oguchi, H.; Sato, T.; Takagi, S.; Tazawa, M.; Nogami, G.; Orimo, S.I. Lithium ion conductivity of complex hydrides incorporating multiple closo-type complex anions. J. Energy Chem. 2019, 38, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, O.; Remhof, A.; Hwang, S.-J.; Züttel, A. Role of Li2B12H12 for the Formation and Decomposition of LiBH4. Chem. Mater. 2010, 22, 3265–3268. [Google Scholar] [CrossRef]
- Gulino, V.; Wolczyk, A.; Golov, A.A.; Eremin, R.A.; Palumbo, M.; Nervi, C.; Blatov, V.A.; Proserpio, D.M.; Baricco, M. Combined DFT and geometrical–topological analysis of Li-ion conductivity in complex hydrides. Inorg. Chem. Front. 2020, 7, 3115–3125. [Google Scholar] [CrossRef]
- Asakura, R.; Reber, D.; Duchêne, L.; Payandeh, S.; Remhof, A.; Hagemann, H.; Battaglia, C. 4 V Room-Temperature All-Solid-State Sodium Battery Enabled By a Passivating Cathode/Hydroborate Solid Electrolyte Interface. Energy Environ. Sci. 2020, 13, 5048–5058. [Google Scholar] [CrossRef]
- Yoshida, K.; Sato, T.; Unemoto, A.; Matsuo, M.; Ikeshoji, T.; Udovic, T.J.; Orimo, S.I. Fast sodium ionic conduction in Na2B10H10-Na2B12H12 pseudo-binary complex hydride and application to a bulk-type all-solid-state battery. Appl. Phys. Lett. 2017, 110, 103901. [Google Scholar] [CrossRef] [Green Version]
- Duchêne, L.; Kühnel, R.S.; Rentsch, D.; Remhof, A.; Hagemann, H.; Battaglia, C. A highly stable sodium solid-state electrolyte based on a dodeca/deca-borate equimolar mixture. Chem. Commun. 2017, 53, 4195–4198. [Google Scholar] [CrossRef] [Green Version]
- Polak, R.J.; Obenland, C. Pyrolysis of diborane. Ind. Eng. Chem. Prod. Res. Dev. 1964, 3, 234–238. [Google Scholar]
- Dobson, J.; Maruca, R.; Schaeffer, R. Studies of Boranes. XXX. Reaction of Pentaborane (9) with Diborane (6). Isolation of Several New Boron Hydrides. Inorg. Chem. 1970, 9, 2161–2166. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, R.; Li, H.; Zhang, Y.; Wang, Y.; Wu, C.; Yan, Y.; Chen, Y. Li-Ion Conductivity Enhancement of LiBH4·xNH3 with in Situ Formed Li2O Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 31635–31641. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Grinderslev, J.B.; Lee, Y.S.; Jørgensen, M.; Cho, Y.W.; Černý, R.; Jensen, T.R. Ammonia-assisted fast Li-ion conductivity in a new hemiammine lithium borohydride, LiBH4·1/2NH3. Chem. Commun. 2020, 56, 3971–3974. [Google Scholar] [CrossRef] [PubMed]

| Sample | Ea (eV) | Pre-Exponential Factor |
|---|---|---|
| LiBH4 (orthorhombic) | 0.68 (±0.05) | 15 (±2) |
| LiBH4-LiBF4 (280 °C) | 0.44 (±0.01) | 11.1 (±0.3) |
| LiBH4-Li2B12H12 (280 °C) | 0.41 (±0.01) | 11.8 (±0.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Kort, L.M.; Gulino, V.; Blanchard, D.; Ngene, P. Effects of LiBF4 Addition on the Lithium-Ion Conductivity of LiBH4. Molecules 2022, 27, 2187. https://doi.org/10.3390/molecules27072187
de Kort LM, Gulino V, Blanchard D, Ngene P. Effects of LiBF4 Addition on the Lithium-Ion Conductivity of LiBH4. Molecules. 2022; 27(7):2187. https://doi.org/10.3390/molecules27072187
Chicago/Turabian Stylede Kort, Laura M., Valerio Gulino, Didier Blanchard, and Peter Ngene. 2022. "Effects of LiBF4 Addition on the Lithium-Ion Conductivity of LiBH4" Molecules 27, no. 7: 2187. https://doi.org/10.3390/molecules27072187
APA Stylede Kort, L. M., Gulino, V., Blanchard, D., & Ngene, P. (2022). Effects of LiBF4 Addition on the Lithium-Ion Conductivity of LiBH4. Molecules, 27(7), 2187. https://doi.org/10.3390/molecules27072187






