Interaction of the Fungal Metabolite Harzianic Acid with Rare-Earth Cations (La3+, Nd3+, Sm3+, Gd3+)
Abstract
:1. Introduction
2. Results and Discussion
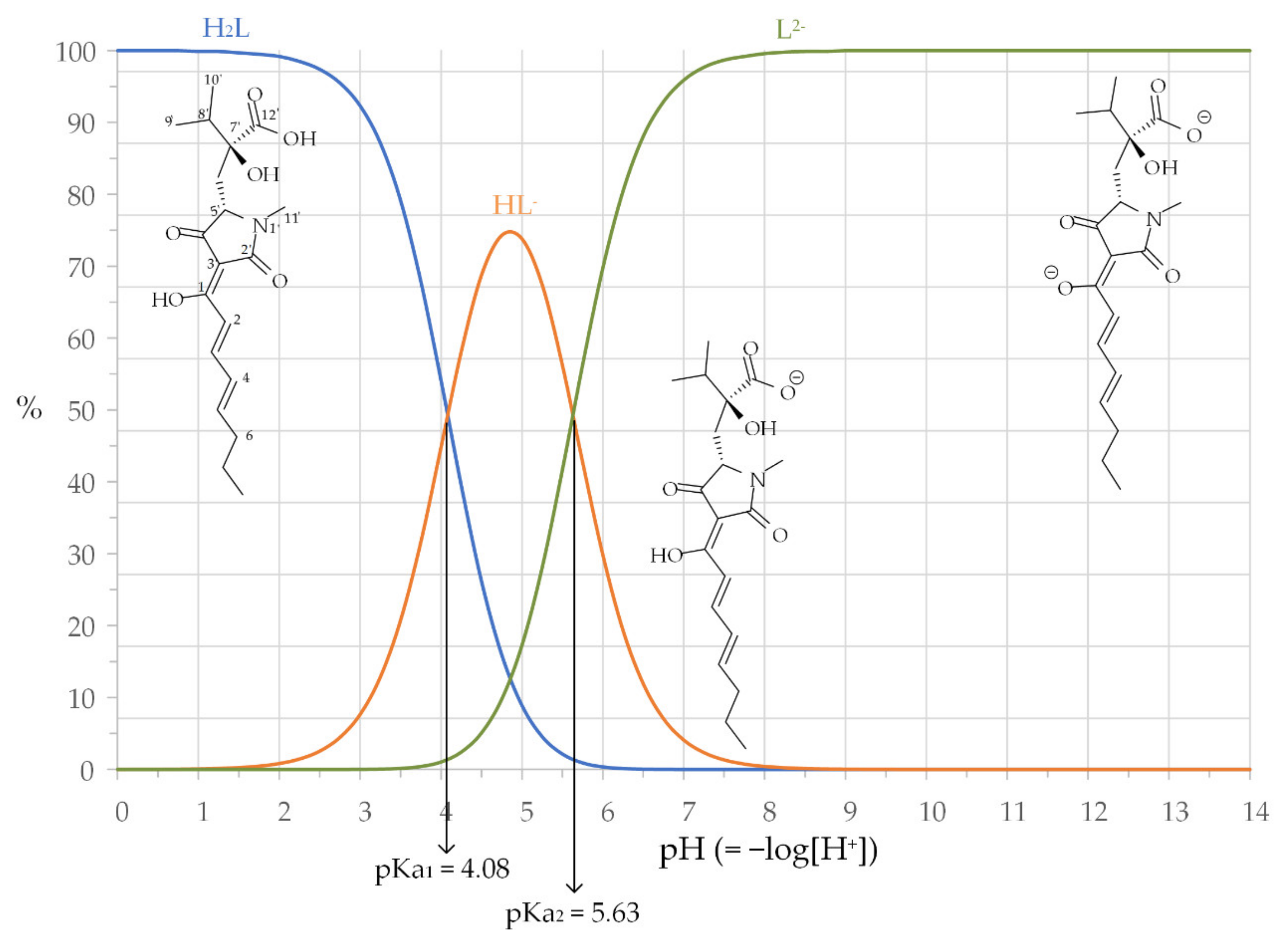
2.1. Harzianic Acid
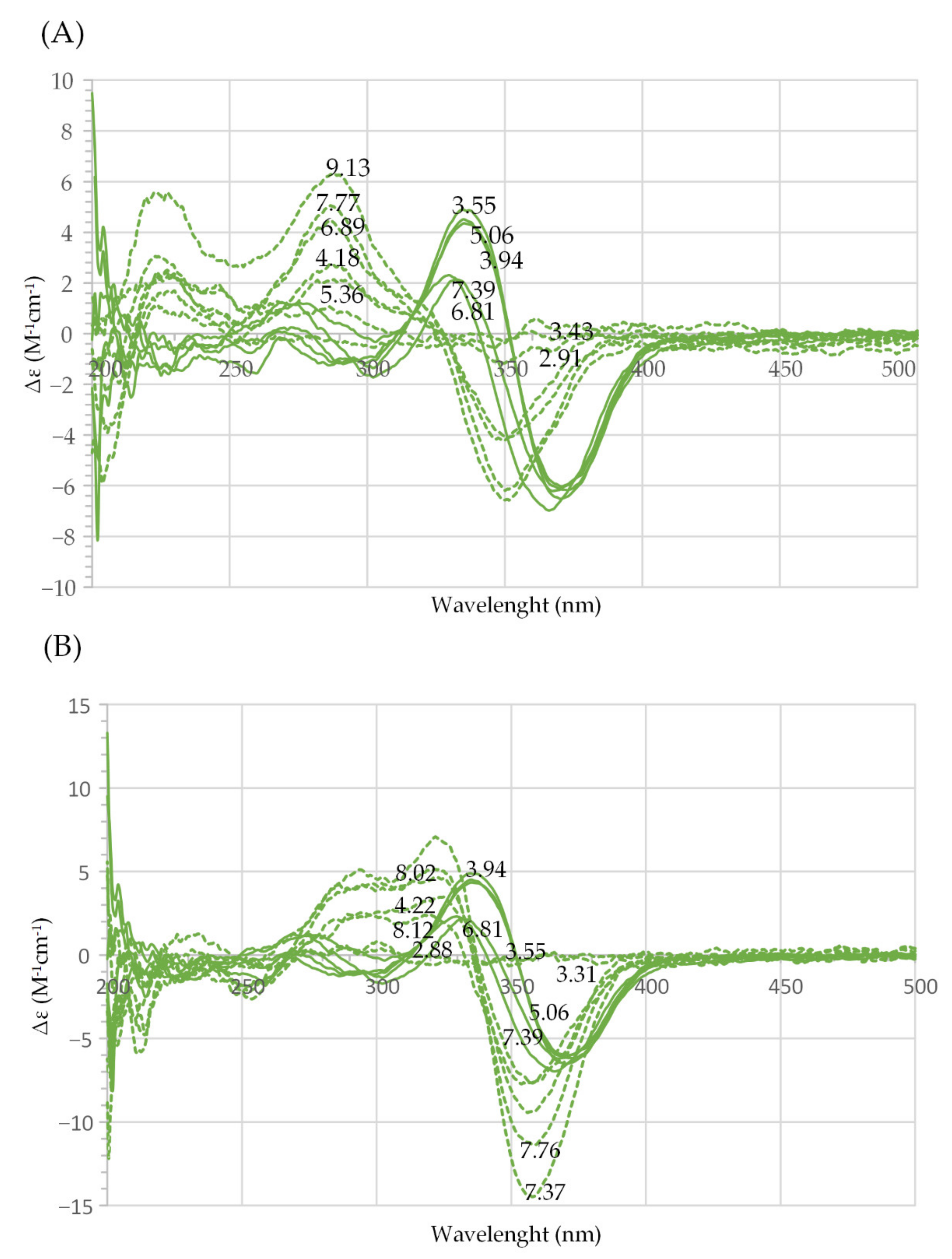
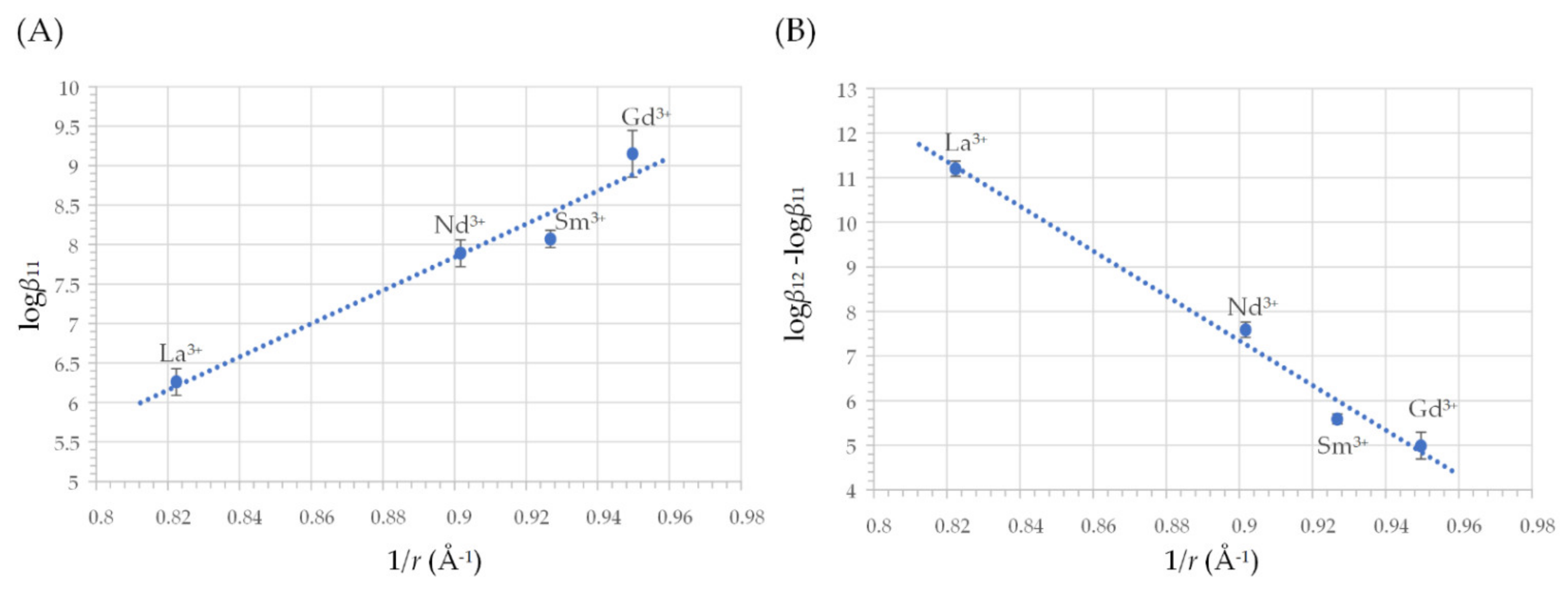
2.2. Coordination Properties of Harzianic Acid toward La3+, Nd3+, Sm3+ and Gd3+
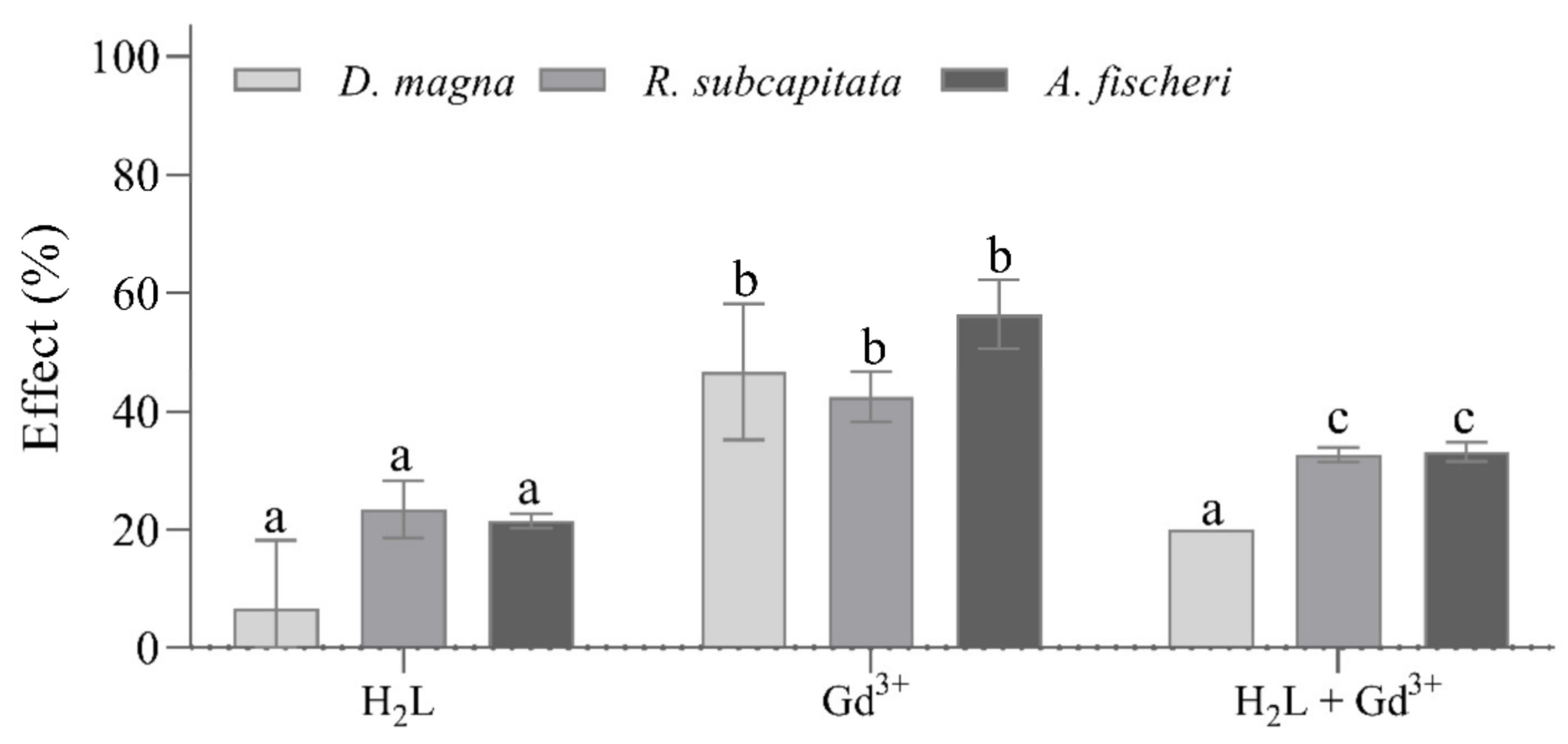
2.3. Ecotoxicity Tests on Raphidocelis subcapitata, Daphnia magna, and Aliivibrio fischeri
3. Materials and Methods
3.1. Reagents and Their Analysis
3.2. HPLC–MS Q-TOF Analyses
3.3. Preparation of Test Solutions for CD and UV–Vis Spectrophotometric Measurements
3.4. Ecotoxicity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bills, G.F.; Gloer, J.B. Biologically active secondary metabolites from the fungi. Fungal Kingd. 2017, 4, 1087–1119. [Google Scholar]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and role of fungal secondary metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.M.; Nicoletti, R.; Salvatore, F.; Naviglio, D.; Andolfi, A. GC–MS approaches for the screening of metabolites produced by marine-derived Aspergillus. Mar. Chem. 2018, 206, 19–33. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Alves, A.; Andolfi, A. Secondary metabolites of Lasiodiplodia theobromae: Distribution, chemical diversity, bioactivity, and implications of their occurrence. Toxins 2020, 12, 457. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The genus Cladosporium: A rich source of diverse and bioactive natural compounds. Molecules 2021, 26, 3959. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Vinale, F. Bioactive compounds from marine-derived Aspergillus, Penicillium, Talaromyces and Trichoderma Species. Mar. Drugs 2018, 16, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chepkirui, C.; Stadler, M. The genus Diaporthe: A rich source of diverse and bioactive metabolites. Mycol. Prog. 2017, 16, 477–494. [Google Scholar] [CrossRef]
- Toghueo, R.M.K. Bioprospecting endophytic fungi from Fusarium genus as sources of bioactive metabolites. Mycology 2020, 11, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Mo, X.; Li, Q.; Ju, J. Naturally occurring tetramic acid products: Isolation, structure elucidation and biological activity. RSC Adv. 2014, 4, 50566–50593. [Google Scholar] [CrossRef]
- Sawa, R.; Mori, Y.; Iinuma, H.; Naganawa, H.; Hamada, M.; Yoshida, S.; Furutani, H.; Kajimura, Y.; Fuwa, T.; Takeuchi, T. Harzianic acid, a new antimicrobial antibiotic from a fungus. J. Antibiot. 1994, 47, 731–732. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.; Kim, J.; Choi, J.N.; Liu, K.H.; Lee, C.H. Chemotaxonomy of Trichoderma spp. using mass spectrometry-based metabolite profiling. J. Microbiol. Biotechnol. 2011, 21, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Manganiello, G.; Sacco, A.; Ercolano, M.R.; Vinale, F.; Lanzuise, S.; Pascale, A.; Napolitano, M.; Lombardi, N.; Lorito, M.; Woo, S.L. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol. 2018, 9, 1966. [Google Scholar] [CrossRef]
- De Filippis, A.; Nocera, F.P.; Tafuri, S.; Ciani, F.; Staropoli, A.; Comite, E.; Bottiglieri, A.; Gioia, L.; Lorito, M.; Woo, S.L.; et al. Antimicrobial activity of harzianic acid against Staphylococcus pseudintermedius. Nat. Prod. Res. 2021, 35, 5440–5445. [Google Scholar] [CrossRef]
- De Tommaso, G.; Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Vinale, F.; Bottiglieri, A.; Staropoli, A.; Salvatore, F.; Lorito, M.; Iuliano, M.; et al. Bivalent metal-chelating properties of harzianic acid produced by Trichoderma pleuroticola associated to the gastropod Melarhaphe neritoides. Molecules 2020, 25, 2147. [Google Scholar] [CrossRef]
- De Tommaso, G.; Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Vinale, F.; Staropoli, A.; Salvatore, F.; Lorito, M.; Iuliano, M.; Andolfi, A. Coordination properties of the fungal metabolite harzianic acid toward toxic heavy metals. Toxics 2021, 9, 19. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare earth elements: Industrial applications and economic dependency of Europe. Procedia Econ. Financ. 2015, 24, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef] [Green Version]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Chao, Y.; Liu, W.; Chen, Y.; Chen, W.; Zhao, L.; Ding, Q.; Wang, S.; Tang, Y.T.; Zhang, T.; Qiu, R.L. Structure, variation, and co-occurrence of soil microbial communities in abandoned sites of a rare earth elements mine. Environ. Sci. Technol. 2016, 50, 11481–11490. [Google Scholar] [CrossRef]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, Z.; Li, X.; Guan, Z.; Cai, Y.; Liao, X. The effects of phytoremediation on soil bacterial communities in an abandoned mine site of rare earth elements. Sci. Total Environ. 2019, 670, 950–960. [Google Scholar] [CrossRef]
- D’Aquino, L.; Morgana, M.; Carboni, M.A.; Staiano, M.; Antisari, M.V.; Re, M.; Lorito, M.; Vinale, F.; Abadi, K.M.; Woo, S.L. Effect of some rare earth elements on the growth and lanthanide accumulation in different Trichoderma strains. Soil Biol. Biochem. 2009, 41, 2406–2413. [Google Scholar] [CrossRef]
- Kostova, I.; Valcheva-Traykova, M. New samarium(III) complex of 5-aminoorotic acid with antioxidant activity. Appl. Organomet. Chem. 2015, 29, 815–824. [Google Scholar] [CrossRef]
- Ajlouni, A.M.; Abu-Salem, Q.; Taha, Z.A.; Hijazi, A.K.; Al Momani, W. Synthesis, characterization, biological activities and luminescent properties of lanthanide complexes with [2-thiophenecarboxylic acid, 2-(2-pyridinylmethylene)hydrazide] Schiff bases ligand. J. Rare Earths 2016, 34, 986–993. [Google Scholar] [CrossRef]
- Cota, I.; Marturano, V.; Tylkowski, B. Ln complexes as double faced agents: Study of antibacterial and antifungal activity. Coord. Chem. Rev. 2019, 396, 49–71. [Google Scholar] [CrossRef]
- Arciszewska, Ż.; Gama, S.; Kalinowska, M.; Świderski, G.; Świsłocka, R.; Gołębiewska, E.; Naumowicz, M.; Worobiczuk, M.; Cudowski, A.; Pietryczuk, A.; et al. Caffeic Acid/Eu (III) complexes: Solution equilibrium studies, structure characterization and biological activity. Int. J. Mol. Sci. 2022, 23, 888. [Google Scholar] [CrossRef]
- Hermann, P.; Kotek, J.; Kubíček, V.; Lukeš, I. Gadolinium(III) complexes as MRI contrast agents: Ligand design and properties of the complexes. Dalt. Trans. 2008, 9226, 3027–3047. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, M.N.; Podberesky, D.J. Gadolinium-based contrast agents in children. Pediatr. Radiol. 2018, 48, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Runge, V.M. Safety of the gadolinium-based contrast agents for magnetic resonance imaging, focusing in part on their accumulation in the brain and especially the dentate nucleus. Investig. Radiol. 2016, 51, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Fur, M.; Caravan, P. The biological fate of gadolinium-based MRI contrast agents: A call to action for bioinorganic chemists. Metallomics 2019, 11, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Port, M.; Idée, J.M.; Medina, C.; Robic, C.; Sabatou, M.; Corot, C. Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: A critical review. BioMetals 2008, 21, 469–490. [Google Scholar] [CrossRef]
- Joonas, E.; Aruoja, V.; Olli, K.; Syvertsen-Wiig, G.; Vija, H.; Kahru, A. Potency of (doped) rare earth oxide particles and their constituent metals to inhibit algal growth and induce direct toxic effects. Sci. Total Environ. 2017, 593, 478–486. [Google Scholar] [CrossRef]
- Herrmann, H.; Nolde, J.; Berger, S.; Heise, S. Aquatic ecotoxicity of lanthanum—A review and an attempt to derive water and sediment quality criteria. Ecotoxicol. Environ. Saf. 2016, 124, 213–238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.Y.; Shen, X.Y.; Ruan, Q.; Xu, X.L.; Yang, S.P.; Lu, Y.; Xu, H.Y.; Hao, F.L. Effects of subchronic samarium exposure on the histopathological structure and apoptosis regulation in mouse testis. Environ. Toxicol. Pharmacol. 2014, 37, 505–512. [Google Scholar] [CrossRef]
- Healy, A.R.; Vinale, F.; Lorito, M.; Westwood, N.J. Total synthesis and biological evaluation of the tetramic acid based natural product harzianic acid and its stereoisomers. Org. Lett. 2015, 17, 692–695. [Google Scholar] [CrossRef] [Green Version]
- Hyperquad. Available online: http://www.hyperquad.co.uk/ (accessed on 14 February 2022).
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Ohtaki, H.; Tanaka, N. Ionic equilibria in mixed solvents. VI. Dissociation constants of aliphatic diamines in water-methanol solutions. J. Phys. Chem. 1971, 75, 90–92. [Google Scholar] [CrossRef]
- Brown, P.L.; Ekberg, C. Hydrolysis of Metal Ions; John Wiley & Sons: Wienheim, Germany, 2016; Volume 1, ISBN 978-3-527-33010-2. [Google Scholar]
- Kaufmann, G.F.; Sartorio, R.; Lee, S.H.; Rogers, C.J.; Meijler, M.M.; Moss, J.A.; Clapham, B.; Brogan, A.P.; Dickerson, T.J.; Janda, K.D. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. USA 2005, 102, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martell, A.; Smith, R.M.M. Critical Stability Constants; Springer: New York, NY, USA, 1989; ISBN 978-1-4615-6766-0. [Google Scholar]
- Siciliano, A.; Guida, M.; Serafini, S.; Micillo, M.; Galdiero, E.; Carfagna, S.; Salbitani, G.; Tommasi, F.; Lofrano, G.; Padilla Suarez, E.G.; et al. Long-term multi-endpoint exposure of the microalga Raphidocelis subcapitata to lanthanum and cerium. Sci. Total Environ. 2021, 790, 148229. [Google Scholar] [CrossRef]
- Tsiridis, V.; Petala, M.; Samaras, P.; Hadjispyrou, S.; Sakellaropoulos, G.; Kungolos, A. Interactive toxic effects of heavy metals and humic acids on Vibrio fischeri. Ecotoxicol. Environ. Saf. 2006, 63, 158–167. [Google Scholar] [CrossRef]
- González, V.; Vignati, D.A.L.; Pons, M.N.; Montarges-Pelletier, E.; Bojic, C.; Giamberini, L. Lanthanide ecotoxicity: First attempt to measure environmental risk for aquatic organisms. Environ. Pollut. 2015, 199, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kolthoff, I.M.; Elving, P.J.; Meehan, E.J. Treatise on Analytical Chemistry; Wiley: Hoboken, NJ, USA, 1978; ISBN 978-0-471-80647-9. [Google Scholar]
- ISO 6341:2012; Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna straus (Cladocera, Crustacea)—Acute Toxicity Test. ISO: Geneve, Switzerland, 2012.
- DIN ISO 11348-3:2007; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria. ISO: Geneve, Switzerland, 2007.
- BS EN ISO 8692:2012; Water Quality—Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. ISO: Geneve, Switzerland, 2012.



| Ion | Observed m/z of Main Isotopic Peak | Formula | Exact Mass |
|---|---|---|---|
| Harzianic acid + La(ClO4)3 | |||
| [H2L + H]+ | 366.1921 | C19H28NO6 | 366.1917 |
| [H2L + Na]+ | 388.1730 | C19H27NO6Na | 388.1736 |
| [H2L − H + La + ClO4]+ | 602.0315 | C19H26NO6LaClO4 | 602.0309 |
| [H2L + La + 2ClO4]+ | 701.9867 | C19H27NO6La(ClO4)2 | 701.9872 |
| [2H2L − 2H + La]+ | 867.2592 | C38H52N2O12La | 867.2584 |
| Harzianic acid + NdCl3 | |||
| [H2L + H]+ | 366.1908 | C19H28NO6 | 366.1917 |
| [H2L + Na]+ | 388.1728 | C19H27NO6Na | 388.1736 |
| [2H2L − 2H + Nd]+ | 870.2597 | C38H52N2O12Nd | 870.2602 |
| Harzianic acid + Sm(ClO4)3 | |||
| [H2L + H]+ | 366.1926 | C19H28NO6 | 366.1917 |
| [H2L + Na]+ | 388.1741 | C19H27NO6Na | 388.1736 |
| [2H2L − 2H + Sm]+ | 880.2716 | C38H52N2O12Sm | 880.2718 |
| Harzianic acid + GdCl3 | |||
| [H2L + H]+ | 366.1921 | C19H28NO6 | 366.1917 |
| [H2L + Na]+ | 388.1730 | C19H27NO6Na | 388.1736 |
| [2H2L − 2H + Gd]+ | 886.2765 | C38H52N2O12Gd | 886.2761 |
| Element | O.P., cm | Dataset | ||
|---|---|---|---|---|
| La | 0.995 | 3.82(2.88); 3.64(3.31); 3.55(4.22); 3.49(7.37); 3.45(7.76); 3.42(8.00); 3.38(8.12) | 1 | Figure 3A (orange) |
| 1.992 | 3.99(2.88); 3.86(3.20); 3.76(3.83); 3.74(4.62); 3.71(6.48); 3.68(7.34) | 1 | Figure 3A (blue) | |
| Nd | 1.000 | 23.3(2.84); 21.5(4.44); 21.2(6.36); 20.8(7.31); 20.5(7.79); 20.1(8.11); 19.7(8.59) | 0.2 | Figure 3B (orange) |
| 1.935 | 8.26(2.76); 7.78(3.26); 7.60(3.81); 7.54(4.57); 7.46(6.90); 7.42(7.32); 7.37(7.62); 7.04(9.71) | 0.2 | Figure 3B (blue) | |
| Sm | 1.051 | 3.01(2.91); 2.85(3.76); 2.81(5.59); 2.80(6.30); 2.79(6.81); 2.78(7.29); 2.74(7.86); 2.69(8.24) | 1 | Figure 3C (orange) |
| 2.02 | 8.04(2.94); 7.85(3.17); 7.65(3.71); 7.59(4.12); 7.55 (4.83) | 1 | Figure 3C (blue) | |
| Gd | 1.00 | 6.44(3.55); 6.36(3.94); 6.32(5.06); 6.26 (6.81); 6.23(7.39) | 1 | Figure 3D (orange) |
| 1.99 | 4.57(3.18); 4.41(4.23); 4.35(7.39); 4.25(9.62) | 1 | Figure 3D (blue) |
| Ln3+ | Reaction | log (Formation Constant), log βpq ± σ |
|---|---|---|
| La3+ | La3+ + L2− ⇌ LaL+ La3+ + 2 L2− ⇌ LaL2− | 6.26 ± 0.04 17.46 ± 0.04 |
| Nd3+ | Nd3+ + L2− ⇌ NdL+ Nd3+ + 2 L2− ⇌ NdL2− | 7.89 ± 0.04 15.48 ± 0.04 |
| Sm3+ | Sm3+ + L2− ⇌ SmL+ Sm3+ + 2 L2− ⇌ SmL2− | 8.07 ± 0.02 13.66 ± 0.03 |
| Gd3+ | Gd3+ + L2− ⇌ GdL+ Gd3+ + 2 L2− ⇌ GdL2− | 9.15 ± 0.07 14.14 ± 0.07 |
| Organism | Compound | EC10 (µM) | EC50 (µM) |
|---|---|---|---|
| A. fischeri | Harzianic acid | >3.5 | >3.5 |
| Gadolinium | 1.5 (0.7–2.6) | 2.6 (1.4–4.0) | |
| R. subcapitata | Harzianic acid | 0.7 (0.4–0.9) | 1.2 (0.8–1.9) |
| Gadolinium | 0.0 (0.0–0.1) | 3.5 (1.4–8.3) | |
| D. magna | Harzianic acid | 0.7 (0.3–1.1) | 1.2 (0.7–2.2) |
| Gadolinium | 0.0 (0.0–1.3) | 1.1 (0.0–14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Tommaso, G.; Salvatore, M.M.; Siciliano, A.; Staropoli, A.; Vinale, F.; Nicoletti, R.; DellaGreca, M.; Guida, M.; Salvatore, F.; Iuliano, M.; et al. Interaction of the Fungal Metabolite Harzianic Acid with Rare-Earth Cations (La3+, Nd3+, Sm3+, Gd3+). Molecules 2022, 27, 1959. https://doi.org/10.3390/molecules27061959
De Tommaso G, Salvatore MM, Siciliano A, Staropoli A, Vinale F, Nicoletti R, DellaGreca M, Guida M, Salvatore F, Iuliano M, et al. Interaction of the Fungal Metabolite Harzianic Acid with Rare-Earth Cations (La3+, Nd3+, Sm3+, Gd3+). Molecules. 2022; 27(6):1959. https://doi.org/10.3390/molecules27061959
Chicago/Turabian StyleDe Tommaso, Gaetano, Maria Michela Salvatore, Antonietta Siciliano, Alessia Staropoli, Francesco Vinale, Rosario Nicoletti, Marina DellaGreca, Marco Guida, Francesco Salvatore, Mauro Iuliano, and et al. 2022. "Interaction of the Fungal Metabolite Harzianic Acid with Rare-Earth Cations (La3+, Nd3+, Sm3+, Gd3+)" Molecules 27, no. 6: 1959. https://doi.org/10.3390/molecules27061959
APA StyleDe Tommaso, G., Salvatore, M. M., Siciliano, A., Staropoli, A., Vinale, F., Nicoletti, R., DellaGreca, M., Guida, M., Salvatore, F., Iuliano, M., & Andolfi, A. (2022). Interaction of the Fungal Metabolite Harzianic Acid with Rare-Earth Cations (La3+, Nd3+, Sm3+, Gd3+). Molecules, 27(6), 1959. https://doi.org/10.3390/molecules27061959













