Abstract
In this study, the in vitro antimicrobial, antiparasitic, antiproliferative and cytotoxic activities of essential oil from Baccharis parvidentata Malag. (EO-Bp) and Lippia origanoides Kunth (EO-Lo) were explored. The relevant effects were observed against the parasitic protozoans Plasmodium falciparum, Trypanosoma cruzi, Trypanosoma brucei and Leishmania amazonensis (ranging 0.6 to 39.7 µg/mL) and malignant MCF-7, MCF-7/HT, 22Rv1, and A431 cell lines (ranging 6.1 to 31.5 µg/mL). In parallel, EO-Bp showed better selective indexes in comparison with EO-Lo against peritoneal macrophages from BALB/c mice and MRC-5 cell line. In conclusion, EO-Lo is known to show a wide range of health benefits that could be added as another potential use of this oil with the current study. In the case of EO-Bp, the wide spectrum of its activities against protozoal parasites and malignant cells, as well as its selectivity in comparison with non-malignant cells, could suggest an interesting candidate for further tests as a new therapeutic alternative.
1. Introduction
The search for biologically active compounds is currently a subject of interest in the food, cosmetic and pharmaceutical industries, among others [1]. Plant-based natural products are a potential source of new agents and/or drugs [2]. The ability of plant species to biosynthesize a broad variety of complex and innovative scaffolds is outstanding and can be utilized or further developed as a major source of leads for new drugs if appropriate in vitro and in vivo assays are established. Numerous plant extracts and pure natural compounds have been discovered [3,4]. Among those, essential oils (EOs) have been shown to display a wide range of therapeutic properties [5,6]. EOs are a mixture of volatile compounds that have been found in different plant organs from several botanical families [5], including Asteraceae [7] and Verbenaceae [8]. In our previous study, the chemical and biological properties of the EOs from Baccharis parvidentata Malag. (Asteraceae) and Lippia origanoides Kunth (Verbenaceae) from high-altitude locations in Brazil were assessed [9].
Several of the curative properties found in the genus Baccharis have been ascribed to their EOs, which have been reported in the scientific literature to exhibit a wide range of biological properties [9,10,11]. However, the studies of B. parvidentata of the southeast of Minas Gerais and Rio de Janeiro States, Brazil, are scarce. The composition of the EOs from the aerial parts was recently reported, and sabinene (15.2%), himachalol (10.3%) and β-pinene (9.2%) were identified as the major volatile compounds [9].
On the other hand, the pharmacology of the genus Lippia has been widely examined. The content of EOs from the species of the genus Lippia is frequently variable [12,13] and exhibit numerous biological activities. The bioactivity of the EOs produced from L. origanoides has also been widely evaluated and exhibits numerous biological activities and has been summarized [14]. The EO composition of L. origanoides from Petropolis, Brazil, showed methyl (E)-cinnamate (40.0%), hedycaryol (8%), α-eudesmol (7.6%) and β-eudesmol (7.3%) as the most noteworthy compounds [9]. The high content of methyl (E)-cinnamate in the EO of L. origanoides herein evaluated, matched somewhat with the chemotype E previously described [14], which to the best of our knowledge has not been fully explored.
The therapeutic potential of the EOs has not been fully investigated; although a preliminary antimicrobial activity has been assayed that documented the minimal inhibitory concentrations (MIC) against some bacteria and fungi [9]. In this context, the purpose of this study was to explore in vitro the pharmacological properties of EOs from B. parvidentata (EO-Bp) and L. origanoides (EO-Lo), including antimicrobial, antiparasitic, antiproliferative, and cytotoxic activities.
2. Results and Discussion
The EOs were evaluated against a wide panel of microorganisms: Gram-negative Escherichia coli; Gram-positive Staphylococcus aureus; yeast Candida albicans; and protozoa Plasmodium falciparum, Trypanosoma cruzi, T. brucei brucei, Leishmania amazonensis, and L. infantum; malignant: MCF-7 (human breast cancer), MCF-7/HT (4-hydroxytamoxifen-resistant MCF-7/HT subline), 22Rv1 (human prostate carcinoma), and A431 (human epidermoid carcinoma); and non-malignant cells: MRC-5 (human fetal lung fibroblast), MCF-10A (normal breast cells), and peritoneal macrophage from BALB/c mice (PMM).
In general, the EOs showed low antimicrobial activity except for the EO-Bp that caused relevant growth inhibition of S. aureus with a median inhibitory concentration (IC50) value < 10 µg/mL (Table 1). Both EOs inhibited all protozoal parasites. EO-Bp displayed the best activity, T. brucei and P. falciparum were the most susceptible to its effect (Table 2). The protozoal parasites were also susceptible to EO-Lo, although with higher values of IC50, except for L. amazonensis equally susceptible to EO-Lo and EO-Bp and for L. infantum, which showed IC50 > 64 µg/mL. Moreover, both EOs showed antiproliferative activity (Table 3), but EO-Bp, with IC50 ranging from 6.1 to 15.1 µg/mL, was a better inhibitor of all malignant cell growth than EO-Lo with IC50 from 9.1 to 31.5 µg/mL.

Table 1.
Antimicrobial and antiparasitic activity of essential oils from Baccharis parvidentata and Lippia origanoides growing in Brazil.

Table 2.
Antiproliferative activity of essential oils from Baccharis parvidentata and Lippia origanoides growing in Brazil.

Table 3.
Cytotoxic in vitro effect of essential oils from Baccharis parvidentata and Lippia origanoides from Brazil.
In the same way, EO-Bp showed higher cytotoxic effects than EO-Lo in the used models (Table 3), according to the obtained median cytotoxic concentration (CC50). However, when the selectivity indices (SI) were obtained, the most promising activity was achieved for EO-Bp in PMM and MRC-5 models with respect to antiprotozoal activities (Figure 1A,C). In contrast, for the antiproliferative effect, EO-Bp showed better results in the PMM model, while for EO-Lo, better indexes were obtained with respect to the MRC-5 model (Figure 1B,D). In particular, with regards to the SI values calculated with respect to MCF-10A, both EOs showed unspecific activity in comparison with a malignant sensitive cell line (MCF-7) and a hormone-resistant cell subline (MCF-7/HT) with values around unity.
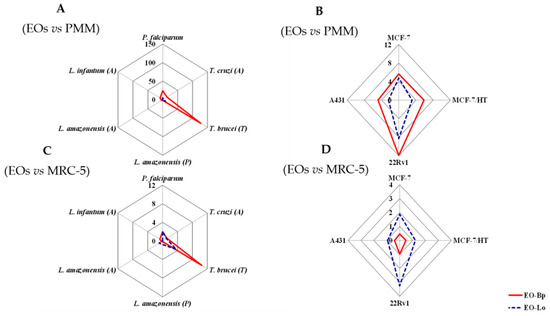
Figure 1.
Selective indexes of essential oils from Baccharis parvidentata and Lippia origanoides from Brazil. (A) Antiprotozoal activity of EOs in comparison with cytotoxicity against PMM; (B) antiproliferative activity against malignant cells by EOs in comparison with cytotoxicity against PMM; (C) antiprotozoal activity of EOs in comparison with cytotoxicity against MRC-5; (D) antiproliferative activity against malignant cells of EOs in comparison with cytotoxicity against MRC-5. EOs: essential oils from Baccharis parvidentata and Lippia origanoides; EO-Bp: essential oil from Baccharis parvidentata; EO-Lo: essential oil from Lippia origanoides; PMM: peritoneal macrophage from BALB/c mice; MRC-5: human fetal lung fibroblast cells line.
Brazil is reputed for its floristic diversity; hence its flora should be considered an important reservoir of active molecules with potential industrial applications [15]. Plant species from several botanical families have been described for their EO content and their antimicrobial [16], antiparasitic [17], anticancer [18], anti-oxidant [19], and insecticidal [20] activities. In the present study, the antiproliferative properties of EO from B. parvidentata and L. origanoides, collected in Brazil, against bacteria, fungi, protozoal parasites, malignant and non-malignant cells were investigated.
Although MIC values of these EOs: 625 to 1250 µg/mL against bacteria (E. coli and S. aureus), 156 to 2500 µg/mL against fungi (Aspergillus niger, Fonsecaea pedrosoi, and Trycophyton rubrum) and 78 to 2500 µg/mL against yeast (C. albicans and Cryptococcus neoformans) have been reported [9], our results confirm that no relevant activity was observed at 64 µg/mL, in both studied EOs except EO-Bp against S. aureus. It is known that S. aureus is a Gram-positive bacterium frequently found in the upper respiratory tract and on the skin as an opportunistic pathogen, being a common cause of sinusitis, abscesses, and food poisoning [21,22]. In this sense, EOs of other Baccharis species have been documented due to their potentialities against S. aureus, such as B. dracunculifolia DC [23] and B. oreophila Malme [24].
A wide inhibitory spectrum was appreciated against protozoal parasites of medical importance and malignant cells. Therefore, our screening strategy suggests the activity of the studied EOs against eukaryotic cells. The mechanisms underlying the antiprotozoal and antiproliferative actions of the tested EOs were not studied. Nevertheless, during the last decade, it has been postulated that the mechanism of action for oils and their constituents is rather complex. Several of these effects are attributable to the lipophilic nature and low molecular weight of the main components that comprise the EOs, which allow them to cross cell membranes, alter membrane composition, and increase membrane fluidity, leading to the leakage of ions and cytoplasmic molecules. Altering membranes leads to reduced ATP production, alteration of the pH gradient, and loss of mitochondrial potential, which can result in cell death. In addition, the induction of cell death by the activation of apoptotic and/or necrotic processes, cell cycle arrest, and loss of function of essential organelles has been observed. In parallel, some EOs may also act as pro-oxidant elements, which can alter the cellular redox state and compromise cellular survival. Nevertheless, the activities of EOs generally result from complex interactions between the different classes of compounds that can result in a great diversity of mechanisms of action and molecular targets [5].
The most sensitive parasite was T. brucei, which is responsible for African trypanosomiasis or sleeping sickness, mostly found in equatorial Africa. Human African trypanosomiasis takes two forms depending on the parasite involved, which are both transmitted by tsetse flies (Glossina spp.). Sleeping sickness in eastern and southern sub-Saharan Africa is an acute form caused by the subspecies T. brucei rhodesiense. Trypanosomiasis in the central and western regions of Africa is a slow-progressing form caused by T. brucei gambiense. Both trypanosomes invade the brain, causing mental deterioration, coma and death if left untreated [25,26].
The studied EOs displayed an important activity against 22Rv1 malignant cells, which are derived from human prostate carcinoma. In men, prostate cancer is the most commonly identified cancer and one of the most important causes of cancer-related deaths; inflammation is also associated with the pathogenesis of this disease [27,28]. The discovery and development of chemotherapeutic agents that can bind specifically prostate tumor cells is crucial to improve the treatment effectiveness and eventually avoid castration in affected patients [28,29].
Another point to consider is that EO-Bp showed the same IC50 value (p > 0.05) against susceptible MCF-7 and hormone-resistant MCF-7/HT (12.9 and 12.4 μg/mL, respectively). It is known that one of the hallmarks of cancer therapy resides in the ability of malignant cells to accumulate genetic/epigenetic changes until they achieve self-renewal, produce differentiated progeny, and develop resistance to therapy [30]. Thus, these results could represent a potential treatment opportunity for cancers that develop hormone resistance.
Moreover, toxicity on non-malignant cells is an important criterion in the drug development process. In that regard, EOs showed certain cytotoxicity, with CC50 values < 100 μg/mL. However, EO-Bp exhibited a selective antiparasitic effect on T. brucei, T. cruzi and P. falciparum, with SI values ranging from 14 to 128. Thus, these EOs should be considered for further exploration in animal models.
Another interesting result was that, in general, higher biological activities were observed for EO-Bp in comparison with EO-Lo (Table 1, Table 2 and Table 3), including: (i) number of susceptible organisms or cells: 7 vs. 5; (ii) average of all activities: 11.8 μg/mL vs. 22.5 μg/mL; (iii) range of activity against parasite: 0.6–39.7 μg/mL vs. 8.1–37.8 μg/mL, as well as malignant cell lines: 6.1–15.1 μg/mL vs. 9.1–31.5 μg/mL, and (iv) better SI with respect to parasites: 1–128 vs. 0–9 and malignant cells: 1–12 vs. 0–8, respectively. In addition, to the best of our knowledge, EO-Bp was analyzed in our study for the first time for its antiparasitic and antiproliferative potential; while EO-Lo was previously studied against protozoal parasites T. evansi [31], T. cruzi [32], and L. chagasi [33], and malignant cell line MDA-MB-231 [34]. Therefore, the higher potential activity of EO-Bp opens the possibility for further studies as a new therapeutic agent.
In Brazil, there are currently 167 species in the Baccharis genus; the country is one of the main centers of diversity of this genus [35,36]. The species of this genus are very relevant in folk medicine, with several species being used for the control and treatment of diseases, beyond the economic and environmental aspects. In this regard, a previous review highlights the antimicrobial and antiprotozoal activity of Baccharis species, which was considered a promising source of biologically active compounds [10]. In particular, the activity of EO-Bp have been scarcely explored; however, knowledge of the chemical structures may explain the observed biological activity and could serve as scaffolds for rational drug design, suggesting chemical modifications to increase activity, bioavailability, and toxicity, among other characteristics, allowing the design of new and more active compounds [17]. Then, to have a general overview of probable bioactive compounds of EO-Bp, reports retrieved from scientific literature related to the assayed pharmacological activities of identified major compounds are summarized in Table 4 [9]. In this sense, reports about the antimicrobial, antiparasitic and antiproliferative activities of the main compounds from EO-Bp were found. In some instances, although different cell targets in comparison with our study have been assayed, the versatility of activities is demonstrated and supports our results. Among these compounds, reports suggest that sabinene demonstrates a wide spectrum of antimicrobial and antiparasitic activities, while himachalol displayed antimicrobial and anticancer actions. In contrast, β-pinene was the most versatile compound and displayed antimicrobial, antiparasitic and anticancer activities.

Table 4.
In vitro pharmacological activities retrieved from scientific literature on major compounds identified in the essential oils from Baccharis parvidentata growing in Brazil.
3. Materials and Methods
3.1. Essential Oils
EO-Bp and EO-Lo were obtained previously by Perera et al. [9] and were obtained by common hydrodistillation in a Clevenger apparatus for 3 h and dried with anhydrous sodium sulfate. The EOs were chemically characterized using gas chromatography–mass spectrometry (GC–MS) and gas chromatography coupled to flame ionization detection (GC-FID) analyses (Supplemental Materials; Table S1). For biological assays, each EO was diluted in dimethyl sulfoxide (DMSO) at 20 mg/mL. This work is registered in SISGEN (Sistema Nacional de Gestão do Patrimonio Genetico, Brazil) under the access authorization number AC7DDF5.
3.2. Microorganisms and Cells
The reference strain of Gram-negative E. coli ATCC8739, Gram-positive S. aureus ATCC6538, yeast C. albicans B59630, and protozoa P. falciparum Ghana, T cruzi Tulahuen CL2, T. brucei brucei Squib-427, L. amazonensis MHOM/77BR/LTB0016, and L. infantum MHOM/MA(BE)/67 were used for assessing the EOs against infectious agents. In addition, four malignant cell lines were included: MCF-7 (ATCC® HTB-22), MCF-7/HT (established in the laboratory), 22Rv1 (ATCC® CRL-2505TM), and A431 (CRL-1555TM); three non-malignant cells were included MRC-5 (CCL-171™), MCF-10A (ATCC® CRL-10317) breast cells, and PMM isolated at the moment of use from healthy animals (approved protocol number: CEI-IPK-68-20).
3.3. Antimicrobial Screening
The integrated antimicrobial screening in 96-well plates was adopted from Cos et al. [45]. Serial dilutions of the EOs were prepared from the DMSO stock solutions in sterile demineralized water in 96-well plates using an automated liquid-handling workstation (Beckman Coulter Biomek 3000), in which the final DMSO concentration was <1%. In all cases, negative (100% growing) and positive (a reference drug) control were included.
Antibacterial and antifungal assays were performed with 5 × 103 CFU/well of E. coli, S. aureus (cultured in Mueller Hinton Broth medium (MHB) from Sigma-Aldrich, St. Louis, MO, USA) or C. albicans (cultured in RPMI medium from Sigma-Aldrich, St. Louis, MO, USA). EOs were added at concentrations ranging from 0.25 to 64 µg/mL. Then, plates were incubated 17 h at 37 °C, and viability was determined fluorimetrically by the addition of resazurin (Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37 °C or 4 h at 37 °C to bacteria and fungi cultures, respectively [46]. Finally, fluorescence was measured at 530 nm excitation and emission of 590 nm using a fluorimeter (Tecan Group, Maennedorf, Switzerland). Reference drugs were chloramphenicol (Sigma-Aldrich, Bornem, Belgium), erythromycin (Sigma-Aldrich, Bornem, Belgium) and miconazole (Janssen Pharmaceuticals, Beerse, Belgium) for E. coli, S. aureus and C. albicans, respectively.
The antiprotozoal assessment was carried out using different models, and parasite forms as will be described below. Activity against P. falciparum was measured with parasites cultured in human erythrocytes A+ (with 1% parasitemia and 2% hematocrit) in RPMI medium (with 0.5 (g/v)% AlbumaxTM) at 37 °C in an atmosphere of 3% O2, 4% CO2, and 93% N2 [47]. Suspensions of cells were distributed in a 96-well plate, treated with different concentrations of EOs and incubated for 72 h under the same conditions. Then, the plate was frozen at −20 °C, and parasite multiplication was measured after mixing 20 μL of the hemolyzed parasite suspension with 100 µL of MalstatTM (Flow Inc., Portland, OR, USA) reagent in a new plate. After 15 min of incubation at room temperature, 20 μL of nitro blue tetrazolium chloride (NBT; Sigma Aldrich, St. Louis, MO, USA), and 2 mg/mL/phenazine ethosulfate (Sigma Aldrich, St. Louis, MO, USA) solution was added. The plate was incubated again for 2 h at room temperature in the dark, and the absorbance was read in a Biorad 3550-UV microplate reader at 655 nm. Antitrypanosomal activity on amastigotes of T. cruzi was evaluated, using 4 × 104 amastigotes in 4 × 103 MRC-5 cells maintained in minimal essential medium (MEM; Life Technologies, Carlsbad, CA, USA) supplemented with 20 mM L-glutamine, 16.5 mM sodium bicarbonate and 5% of inactivated fetal calf serum. Then, EOs in tested concentrations were added and incubated for 7 days in the previous conditions. Parasite growth was assessed by adding the β-galactosidase substrate chlorophenol red β-D-galactopyranoside (Sigma Aldrich, St. Louis, MO, USA) subsequent to an additional incubation for 4 h at 37 °C. The absorbance was then read at 540 nm [48]. In the case of trypomastigotes of T. brucei, the assessment of activity was performed in Hirumi-9 medium supplemented with 10% inactivated fetal calf serum (FCSi) at 37 °C and 5% CO2 [49]. Assays were performed by adding 1.5 × 104 trypomastigotes/well to the EOs at different concentrations and incubating them for 72 h under the same conditions. The fluorimetric resazurin method, with an additional incubation for 24 h at 37 °C, was used to measure parasite growth. Evaluation of activity against L. amazonensis promastigotes was carried out in a 96-well plate with 2 × 105 parasites/mL and with the different concentrations of EOs. The plates were sealed with Parafilm and incubated at 26 °C for 72 h. Afterward, 20 μL of a solution (5 mg/mL) of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, St. Louis, MO, USA) was added to each well and incubated for an additional 4 h. The supernatant was removed, and the formazan crystals were dissolved with 100 μL DMSO. The absorbance of each well was determined with a plate reader (Molecular Devices, Sunnyvale, CA, USA) with a test wavelength of 560 nm and a reference wavelength of 630 nm [50]. On the other hand, the evaluation of antileishmanial activity against the intracellular amastigote form of L. amazonensis and L. infantum was also performed. In the model of L. amazonensis, a PMM monolayer was used (plated at 106/mL in 24-well plates) and infected with stationary-phase promastigotes at a 4:1 parasite/macrophage ratio for 4 h at 5% CO2 and 37 °C. The cells were washed to remove free parasites. EOs were added at different concentrations, and the plates were further incubated under the same conditions for 48 h [51]. For L. infantum, 3 × 104 PMM were infected with amastigotes obtained from an infected hamster at a ratio of 15 parasites per cell. The plate was incubated for 48 h at 37 °C and 5% CO2, and different concentrations of EOs were added. Then, the plate was incubated under the same conditions for 120 h. In both parasite species, the supernatant was discarded after the incubation period with the products; cells were fixed with methanol, stained with 10% Giemsa and microscopically examined (Motic, Japan) under immersion oil. Total parasite burden was determined by the number of infected macrophages and the number of amastigotes inside the macrophages. In the antiprotozoal assays, chloroquine, enznidazole, suramine and miltefosine (donated by Special Programme for Research and Training in Tropical Diseases from the World Health Organization (WHO-TDR)) were used as reference drugs for P. falciparum, T. cruzi, T. brucei, and L. infantum, respectively, while pentamidine (Richet, Buenos Aires, Argentina) was used for L. amazonensis.
3.4. Antiproliferative Assay on Malignant Cells
MCF-7, MCF-7/HT, 22Rv1 and A431 cancer cells were cultured in standard 4.5 g/L glucose DMEM medium (Gibco-Life Technologies, Paisley, UK), when 22Rv1 were cultivated in RPMI-1640 medium (Gibco-Life Technologies, Paisley, UK) supplemented with RPMI-1640 Vitamins (PanEco, Moscow, Russia). In all cases, the culture was supplemented with 10% fetal calf serum (FCS), antibiotics (50 μg of streptomycin/mL and 50 U of penicillin/mL) and 0.1 mg/mL sodium pyruvate (Santa Cruz Biotechnology, Dallas, TX, USA) and maintained in a NuAir incubator (NuAir, Plymouth, MN, USA) at 37 °C, 5% CO2 and 80–85% humidity. Then, 100 × 103 22Rv1 cells/well, 40 × 103 MCF-7 or MCF-7/HT cells/well and 35 × 103 A431 cells/well, were seeded into 24-well plates in 900 μL of the medium, and the plates were incubated for 24 h at 37 °C and 5% CO2. Subsequently, EOs or reference drug were added at different concentrations and the plates were incubated for 72 h under the same conditions. Cell viability was assessed using MTT at 0.2 mg/mL per well [52]. After additional incubation for 2 h, the supernatant was discarded, the MTT formazan purple crystals were dissolved in DMSO (350 μL per well) and the absorbance was measured at 571 nm and 630 nm as a reference in a MultiScan reader (ThermoFisher, Waltham, MA, USA) after the plates were gently shaken. Cisplatin (Teva Pharmaceutical Industries, Petah Tikva, Israel) was used as a reference drug.
3.5. Cytotoxicity Test on Non-Malignant Cells
The cytotoxicity analysis of EOs on 104 MRC-5 cells/well was performed in MEM-supplemented medium at 37 °C and 5% CO2 seeded onto the test plates for 72 h. Cell viability was assessed fluorometrically after the addition of resazurin as described above. Cytotoxicity was also studied using MCF-10A cells cultured in medium supplemented with 5% donor horse serum (BioSera, Nuaille, France), 20 ng/mL epidermal growth factor (PanEco, Moscow, Russia), 0.5 µg/mL hydrocortisone (ChemCruz, Dallas, TX, USA), and 10 µg/mL insulin (PanEco) at 37 °C, 5% CO2 and 80–85% humidity). Briefly, 60 × 103 MCF-10A cells were seeded into 24-well plates in 900 μL of the medium, and then, plates were incubated for 24 h at 37 °C and 5% CO2. Then, different concentrations of EOs were added, and the plates were incubated for 48 h under the same conditions. Cell viability was then measured by the MTT method as described above for cancer cell lines. Finally, in the model of PMM, 3 × 105 cells/mL was treated with different concentrations of EOs over 48 h in the same conditions. Then, 15 μL of MTT solutions were added to each well, and, after 4 h of additional incubation in the same conditions, the supernatant was discarded, formazan crystals were dissolved with 100 μL of DMSO and the absorbance was obtained as described above.
3.6. Statistical Analysis
In each model included in this assessment, three experiments were performed and percentage growth inhibition for each product concentration was calculated compared to the untreated cultures (negative control). The IC50 for antibacterial, antifungal and antiprotozoal assays were determined, while CC50 was obtained in mammalian cell assays. In both cases, values were obtained from dose–response curves, and results were expressed as mean with a 95% confidence interval. Finally, SI was calculated as the ratio of the CC50 for MRC-5/PMM cells and the IC50 for microorganism or malignant cells.
4. Conclusions
In conclusion, EO-Lo is known to show a wide range of health benefits. The current study added another potential use of this oil in the area of infectious parasitic and malignant diseases. EO-Bp had antiparasitic and antiproliferative activity with a wide spectrum and selectivity in comparison with non-malignant eukaryotic cells. Thus, these products from a plant that grows in Brazilian regions can be considered interesting candidates for further tests as new therapeutic alternatives.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27061926/s1, Table S1: Chemical composition of the essential oils from Baccharis parvidentata (EO-Bp) and Lippia origanoides (EO-Lo), growing in Brazil.
Author Contributions
Conceptualization, W.H.P. and L.M.; methodology, A.M.S., G.I.B., E.I.M., P.C. and L.M.; formal analysis, S.G.L., A.E.S. and W.N.S.; investigation, all authors; resources, W.H.P. and S.G.L.; data curation, A.M.S., P.C. and L.M.; writing—original draft preparation, W.H.P., A.M.S. and L.M.; writing—review and editing, all authors; project administration, L.M. and A.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was partly supported by the Russian Foundation for Basic Research (RFBR No. 18-53-34005), the Ministry of Science, Technology and Environment of the Republic of Cuba, and by FAPERJ and CNPq (Brazil).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the Institute of Tropical Medicine “Pedro Kouri” (CEI-IPK-68-20).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in the article and the Supplementary Material.
Acknowledgments
This work represents a contribution of members of the Research Network Natural Products against Neglected Diseases (ResNetNPND, http://www.resnetnpnd.org/Start/, accessed on 9 February 2022) and the Aromatic Plant Research Center (APRC, https://aromaticplant.org/, accessed on 9 February 2022).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
There are no samples available.
References
- Ekiert, H.M.; Szopa, A. Biological activities of natural products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.T.; Ferreira, S.; Duarte, A.P. Genus Ruta: A natural source of high value products with biological and pharmacological properties. J. Ethnopharmacol. 2020, 260, 113076. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.M.; Sun, S.; Kim, M.J.; Phan, N.D.; Tawila, A.M.; Awale, S. Benzophenones from Betula alnoides with antiausterity activities against the PANC-1 human pancreatic cancer cell line. J. Nat. Prod. 2021, 84, 1607–1616. [Google Scholar] [CrossRef]
- Salam, S.; Harneti, D.; Maharani, R.; Nurlelasari; Safari, A.; Hidayat, A.T.; Lesmana, R.; Nafiah, M.A.; Supratman, U.; Prescott, T.A.K.; et al. Cytotoxic triterpenoids from Chisocheton pentandrus. Phytochemistry 2021, 187, 112759. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Edris, A.; Kot, A.M.; Piwowarek, K. Biological activity of some aromatic plants and their metabolites, with an emphasis on health-promoting properties. Molecules 2020, 25, 2478. [Google Scholar] [CrossRef]
- Küçükbay, F.Z.; Kuyumcu, E.; Bilenler, T.; Yıldız, B. Chemical composition and antimicrobial activity of essential oil of Achillea cretica L. (Asteraceae) from Turkey. Nat. Prod. Res. 2012, 26, 1668–1675. [Google Scholar] [CrossRef]
- Pérez Zamora, C.M.; Torres, C.A.; Nuñez, M.B. Antimicrobial activity and chemical composition of essential oils from Verbenaceae species growing in South America. Molecules 2018, 23, 544. [Google Scholar] [CrossRef] [Green Version]
- Perera, W.H.; Bizzo, H.R.; Gama, P.E.; Alviano, C.S.; Salimena, F.R.G.; Alviano, D.S.; Leitão, S.G. Essential oil constituents from high altitude Brazilian species with antimicrobial activity: Baccharis parvidentata Malag., Hyptis monticola Mart. ex Benth. and Lippia origanoides Kunth. J. Essent. Oil Res. 2017, 29, 109–116. [Google Scholar] [CrossRef]
- Ramos Campos, F.; Bressan, J.; Godoy Jasinski, V.C.; Zuccolotto, T.; da Silva, L.E.; Bonancio Cerqueira, L. Baccharis (Asteraceae): Chemical constituents and biological activities. Chem. Biodivers. 2016, 13, 1–17. [Google Scholar] [CrossRef]
- Budel, J.M.; Wang, M.; Raman, V.; Zhao, J.; Khan, S.I.; Rehman, J.U.; Techen, N.; Tekwani, B.; Monteiro, L.M.; Heiden, G.; et al. Essential oils of five Baccharis species: Investigations on the chemical composition and biological activities. Molecules 2018, 23, 2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terblanché, F.C.; Kornelius, G. Essential oil constituents of the genus Lippia (Verbenaceae)—A literature review. J. Essent. Oil Res. 1996, 8, 471–485. [Google Scholar] [CrossRef]
- Pascual, M.E.; Slowing, K.; Carretero, E.; Sánchez Mata, D.; Villar, A. Lippia: Traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 2001, 76, 201–214. [Google Scholar] [CrossRef]
- Ribeiro, A.F.; Andrade, E.H.A.; Salimena, F.R.G.; Maia, J.G.S. Circadian and seasonal study of the cinnamate chemotype from Lippia origanoides Kunth. Biochem. Syst. Ecol. 2014, 55, 249–259. [Google Scholar] [CrossRef]
- Valli, M.; Pivatto, M.; Danuello, A.; Castro-Gamboa, I.; Silva, D.H.S.; Cavalheiro, A.J.; Araujo, A.R.; Furlan, M.; Lopes, M.N.; Bolzani, V.D.S. Tropical biodiversity: Has it been a potential source of secondary metabolites useful for medicinal chemistry? Química Nova 2012, 35, 2278–2287. [Google Scholar] [CrossRef]
- D’agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential oils and their natural active compounds presenting antifungal properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef] [Green Version]
- Luna, E.C.; Silva Luna, I.; Scotti, L.; Monteiro, A.F.M.; Scotti, M.T.; de Moura, R.O.; de Araújo, R.S.A.; Monteiro, K.L.C.; de Aquino, T.M.; Ribeiro, F.F.; et al. Active essential oils and their components in use against neglected diseases and arboviruses. Oxidative Med. Cell. Longev. 2019, 2019, 6587150. [Google Scholar] [CrossRef] [Green Version]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer properties of essential oils and other natural products. Evid. Based Complement. Alternat. Med. 2018, 2018, e3149362. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: Implications in colonic pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef]
- de Lima, G.P.G.; de Souza, T.M.; de Paula Freire, G.; Farias, D.F.; Cunha, A.P.; Ricardo, N.M.P.S.; de Morais, S.M.; Carvalho, A.F.U. Further insecticidal activities of essential oils from Lippia sidoides and Croton species against Aedes aegypti L. Parasitol. Res. 2013, 112, 1953–1958. [Google Scholar] [CrossRef]
- Masalha, M.; Borovok, I.; Schreiber, R.; Aharonowitz, Y.; Cohen, G. Analysis of transcription of the Staphylococcus aureus aerobic class Ib and anaerobic class III ribonucleotide reductase genes in response to oxygen. J. Bacteriol. 2001, 183, 7260–7272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timbe, P.P.R.; de Souza da Motta, A.; Stincone, P.; Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of Baccharis dracunculifolia DC and its synergistic interaction with nisin against food-related bacteria. J. Food Sci. Technol. 2021, 58, 3010–3018. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.T.; Maia, B.H.L.D.N.S.; Ferriani, A.P.; Santos, V.A.Q.; Da Cunha, M.A.A.; Teixeira, S.D. Chemical Characterization, Antioxidant Capacity and Antimicrobial Potential of Essential Oil from the Leaves of Baccharis oreophila Malme. Chem. Biodivers. 2019, 16, e1800372. [Google Scholar] [CrossRef]
- Bottieau, E.; Clerinx, J. Human African trypanosomiasis: Progress and stagnation. Infect. Dis. Clin. N. Am. 2019, 33, 61–77. [Google Scholar] [CrossRef]
- Simarro, P.P.; Cecchi, G.; Paone, M.; Franco, J.R.; Diarra, A.; Ruiz, J.A.; Fèvre, E.M.; Courtin, F.; Mattioli, R.C.; Jannin, J.G. The Atlas of Human African trypanosomiasis: A contribution to global mapping of neglected tropical diseases. Int. J. Health Geogr. 2010, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Kwon, O.-J.; Zhang, L.; Ittmann, M.M.; Xin, L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc. Natl. Acad. Sci. USA 2014, 111, E592–E600. [Google Scholar] [CrossRef] [Green Version]
- Juan-Rivera, M.C.; Martínez-Ferrer, M. Integrin inhibitors in prostate cancer. Cancers 2018, 10, 44. [Google Scholar] [CrossRef] [Green Version]
- Roubaud, G.; Liaw, B.C.; Oh, W.K.; Mulholland, D.J. Strategies to avoid treatment-induced lineage crisis in advanced prostate cancer. Nat. Rev. Clin. Oncol. 2017, 14, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Dembic, Z. Antitumor drugs and their targets. Molecules 2020, 25, 5776. [Google Scholar] [CrossRef]
- Baldissera, M.D.; de Freitas Souza, C.; Mourão, R.H.V.; da Silva, L.V.F.; Monteiro, S.G. Trypanocidal action of Lippia alba and Lippia origanoides essential oils against Trypanosoma evansi in vitro and in vivo used mice as experimental model. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2017, 41, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.R.; Aires, J.R.D.A.; Higino, T.M.M.; Medeiros, M.D.G.F.D.; Citó, A.M.D.G.L.; Lopes, J.A.D.; de Figueiredo, R.C.B.Q. Trypanocidal and cytotoxic activities of essential oils from medicinal plants of Northeast of Brazil. Exp. Parasitol. 2012, 132, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Escobar, P.; Milena Leal, S.; Herrera, L.V.; Martinez, J.R.; Stashenko, E. Chemical composition and antiprotozoal activities of Colombian Lippia spp. essential oils and their major components. Mem. Inst. Oswaldo Cruz 2010, 105, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Fuentes Lorenzo, J.L.; Stashenko, E.E.; Levy, M.; Levy, M.M.; Camarillo, I.G. Lippia origanoides extract induces cell cycle arrest and apoptosis and suppresses NF-ΚB signaling in triple-negative breast cancer cells. Int. J. Oncol. 2017, 51, 1801–1808. [Google Scholar] [CrossRef] [Green Version]
- Heiden, G.; Ribas, O.D.S. Baccharis umbellata (Asteraceae, Astereae): A new species endemic to the highest summits of Paraná, Southern Brazil. Phytotaxa 2012, 49, 23. [Google Scholar] [CrossRef]
- Heiden, G.; Leoni, L.; Nakajima, J. Baccharis magnifica (Asteraceae, Astereae): A striking new species endemic to the summits of Serra Do Caparaó, southeastern Brazil. Phytotaxa 2014, 162, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Sieniawska, E.; Sawicki, R.; Swatko-Ossor, M.; Napiorkowska, A.; Przekora, A.; Ginalska, G.; Augustynowicz-Kopec, E. The effect of combining natural terpenes and antituberculous agents against reference and clinical Mycobacterium tuberculosis strains. Molecules 2018, 23, 176. [Google Scholar] [CrossRef] [Green Version]
- Vimal, A.; Pal, D.; Tripathi, T.; Kumar, A. Eucalyptol, sabinene and cinnamaldehyde: Potent inhibitors of Salmonella yarget protein l-asparaginase. 3 Biotech 2017, 7, 258. [Google Scholar] [CrossRef]
- Mikus, J.; Harkenthal, M.; Steverding, D.; Reichling, J. In vitro effect of essential oils and isolated mono- and sesquiterpenes on Leishmania major and Trypanosoma brucei. Planta Med. 2000, 66, 366–368. [Google Scholar] [CrossRef]
- Chowdhry, L.; Khan, Z.K.; Kulshrestha, D.K. Evaluation of himachalol in murine invasive aspergillosis. Indian J. Exp. Biol. 1996, 39, 449–452. [Google Scholar] [CrossRef]
- Shebaby, W.; Elias, A.; Mroueh, M.; Nehme, B.; El Jalbout, N.D.; Iskandar, R.; Daher, J.C.; Zgheib, M.; Ibrahim, P.; Dwairi, V.; et al. Himachalol induces apoptosis in B16-F10 murine melanoma cells and protects against skin carcinogenesis. J. Ethnopharmacol. 2020, 253, 112545. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.C.R.; Lopes, P.M.; de Azevedo, M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardo, N.I.; Sainz, P.; González-Coloma, A.; Burillo, J.; Martinez-Díaz, R.A. Trypanocidal effects of essential oils from selected medicinal plants. Synergy among the main components. Nat. Prod. Commun. 2017, 12, 709–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, T.Q.; Felisberto, J.R.S.; Guimarães, E.F.; de Queiroz, G.A.; da Fonseca, A.C.C.; Ramos, Y.J.; Marques, A.M.; Moreira, D.D.L.; Robbs, B.K. Apoptotic effect of β-pinene on oral squamous cell carcinoma as one of the major compounds from essential oil of medicinal plant Piper rivinoides Kunth. Nat. Prod. Res. 2021, 36, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Vanden, D.; Maesa, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Räz, B.; Iten, M.; Grether-Bühler, Y.; Kaminsky, R.; Brun, R. The alamar blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. J. Parasitol. 2005, 91, 484–486. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef] [Green Version]
- Sladowski, D.; Steer, S.J.; Clothier, R.H.; Balls, M. An improved MTT assay. J. Immunol. Methods 1993, 157, 203–207. [Google Scholar] [CrossRef]
- Torres-Santos, E.C.; Moreira, D.L.; Kaplan, M.A.; Meirelles, M.N.; Rossi-Bergmann, B. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 1999, 43, 1234–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komendantova, A.S.; Scherbakov, A.M.; Komkov, A.V.; Chertkova, V.V.; Gudovanniy, A.O.; Chernoburova, E.I.; Sorokin, D.V.; Dzichenka, Y.U.; Shirinian, V.Z.; Volkova, Y.A.; et al. Novel steroidal 1,3,4-thiadiazines: Synthesis and biological evaluation in androgen receptor-positive prostate cancer 22Rv1 Cells. Bioorg. Chem. 2019, 91, 103142. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).