MALDI-TOF/MS Analysis of Non-Invasive Human Urine and Saliva Samples for the Identification of New Cancer Biomarkers
Abstract
1. Introduction
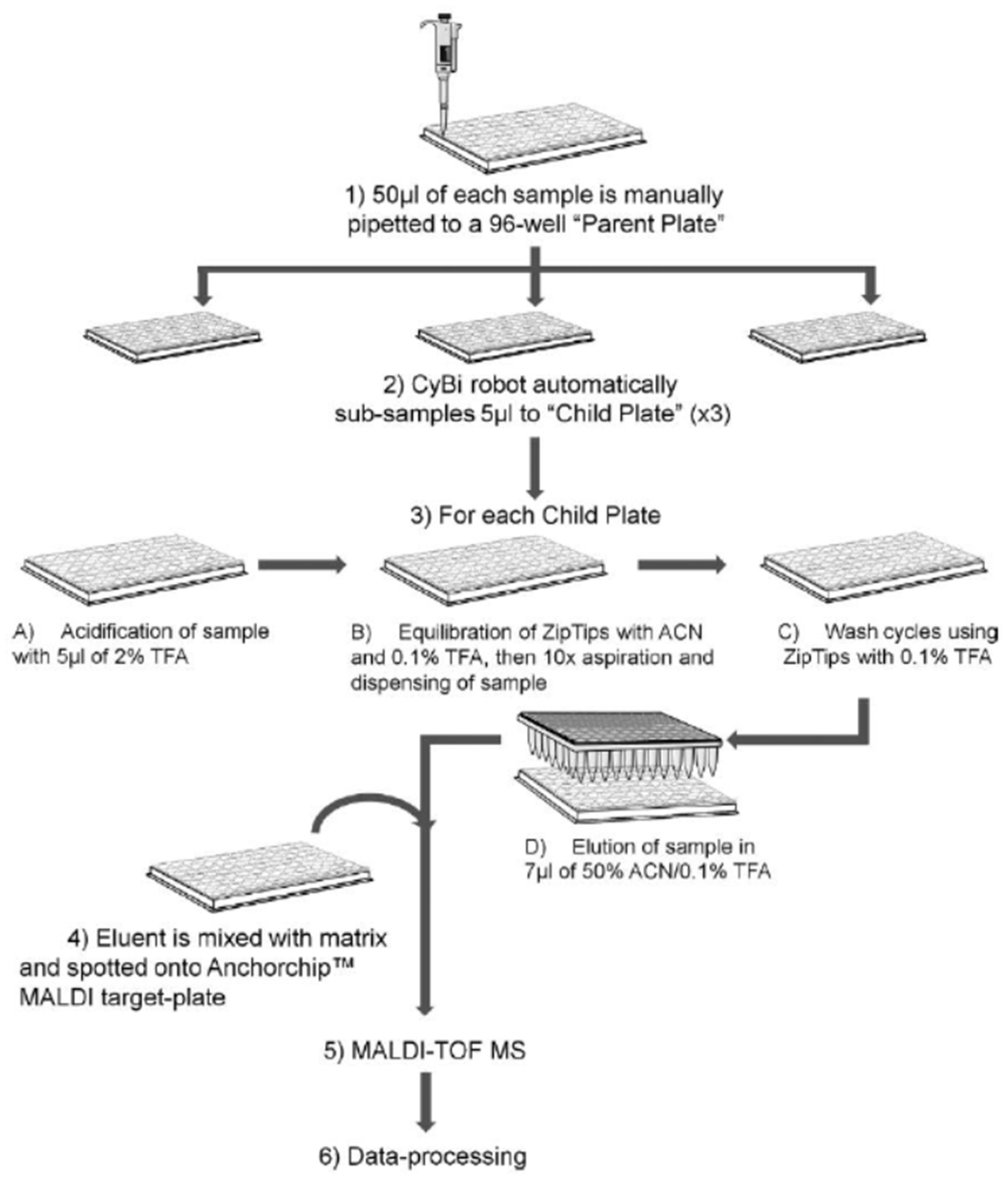
2. Urine Samples Analysis
2.1. Prostate Cancer
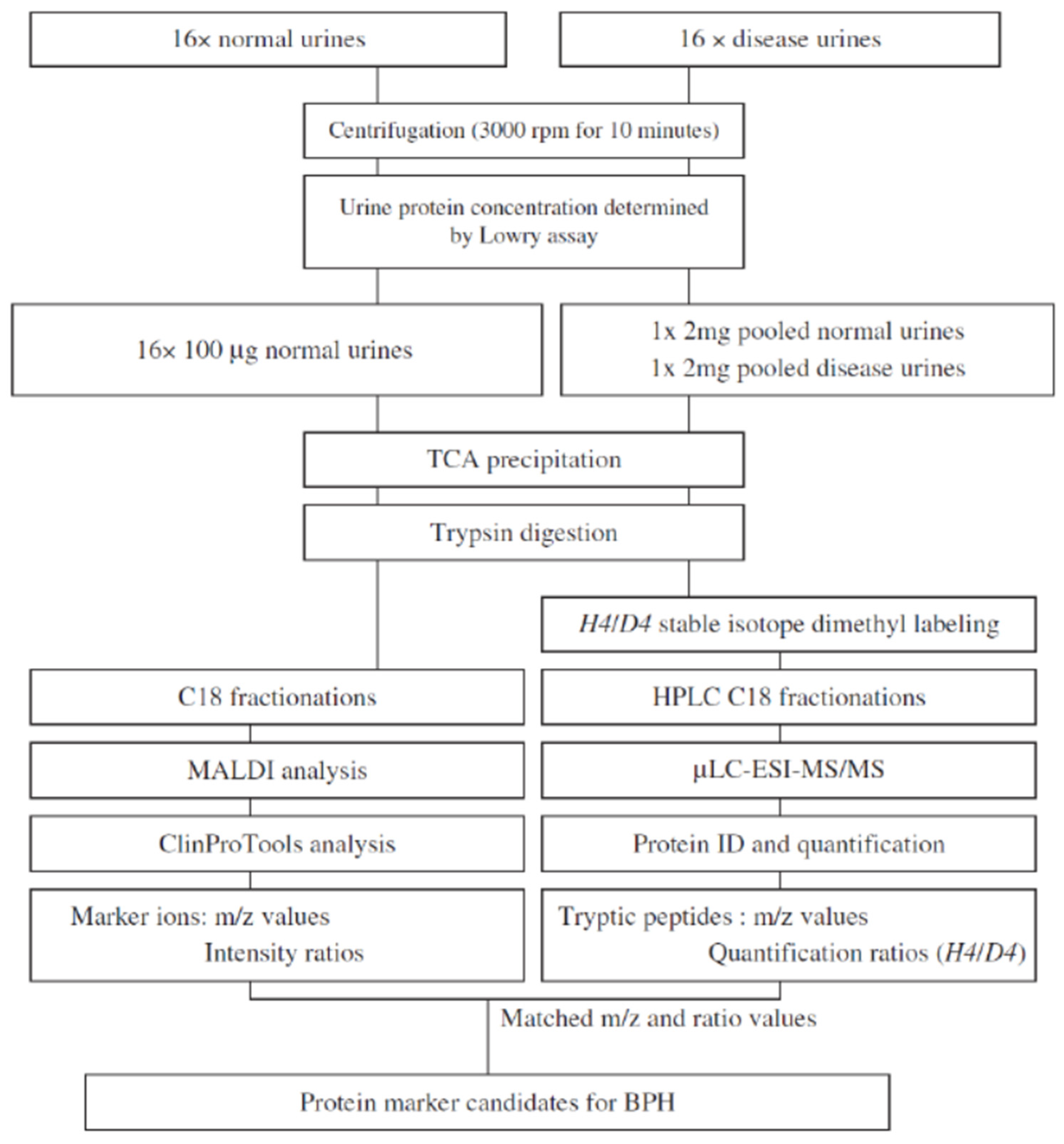
2.2. Bladder Cancer
2.3. Renal and Lung Cancer
2.4. Other Types of Cancer
3. Saliva Samples Analysis
3.1. Oral Cancer
3.2. Neck Cancer
3.3. Other Types of Cancer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lee, V.H.F.; Ng, M.K.; Yan, H.; Bijlsma, M.F. Molecular subtyping of cancer: Current status and moving toward clinical applications. Brief. Bioinform. 2019, 20, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Herceg, Z.; Hainaut, P. Genetic and epigenetic alterations as biomarkers for cancer detection, diagnosis and prognosis. Mol. Oncol. 2007, 1, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Ilango, S.; Paital, B.; Jayachandran, P.; Padma, P.R.; Nirmaladevi, R. Epigenetic alterations in cancer. Front. Biosci. Landmark 2020, 25, 1058–1109. [Google Scholar]
- World Health Organization. Guide to Early Cancer Diagnosis. 2017. Available online: www.who.int/publications/i/item/guide-to-cancer-early-diagnosis (accessed on 16 February 2017).
- Subramanian, S.; Klosterman, M.; Amonkar, M.M.; Hunt, T.L. Adherence with colorectal cancer screening guidelines: A review. Prev. Med. 2004, 38, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Pickhardt, P.J.; Hassan, C.; Halligan, S.; Marmo, R. Colorectal cancer: CT colonography and colonoscopy for detection—systematic review and metaanalysis. Radiology 2011, 259, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, P.F.; Prorok, P.C.; Kramer, B.S. Prostate cancer screening—A perspective on the current state of the evidence. N. Engl. J. Med. 2017, 376, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- van den Biggelaar, F.J.H.M.; Nelemans, P.J.; Flobbe, K. Performance of radiographers in mammogram interpretation: A systematic review. Breast 2008, 17, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Partridge, E.E.; Abu-Rustum, N.R.; Campos, S.M.; Fahey, P.J.; Farmer, M.; Garcia, R.L.; Giuliano, A.; Jones, H.W.; Lele, S.M.; Lieberman, R.W.; et al. Cervical cancer screening. J. Natl. Compr. Canc. Netw. 2010, 8, 1358–1386. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Vellekoop, A.; Ahmed, H.U.; Catto, J.; Emberton, M.; Nam, R.; Rosario, D.J.; Scattoni, V.; Lotan, Y. Systematic review of complications of prostate biopsy. Eur. Urol. 2013, 64, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; La Civita, E.; Liotti, A.; Cennamo, M.; Tortora, F.; Buonerba, C.; Crocetto, F.; Lucarelli, G.; Busetto, G.M.; Del Giudice, F.; et al. Liquid Biopsy Biomarkers in Urine: A Route towards Molecular Diagnosis and Personalized Medicine of Bladder Cancer. J. Pers. Med. 2021, 11, 237. [Google Scholar] [CrossRef] [PubMed]
- Vago, R.; Ravelli, A.; Bettiga, A.; Casati, S.; Lavorgna, G.; Benigni, F.; Salonia, A.; Montorsi, F.; Orioli, M.; Ciuffreda, P.; et al. Urine Endocannabinoids as Novel Non-Invasive Biomarkers for Bladder Cancer at Early Stage. Cancers 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Bax, C.; Taverna, G.; Eusebio, L.; Sironi, S.; Grizzi, F.; Guazzoni, G.; Capelli, L. Innovative diagnostic methods for early prostate cancer detection through urine analysis: A review. Cancers 2018, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Belskaya, L.V.; Sarf, E.A.; Kosenok, V.K.; Gundyrev, I.A. Biochemical Markers of Saliva in Lung Cancer: Diagnostic and Prognostic Perspectives. Diagnostics 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Romano, P.; Lenucci, M.S.; Civino, E.; Vergara, D.; Pitotti, E.; Neglia, C.; Distante, A.; Romano, G.D.; Di Renzo, N.; et al. Differential Glycosylation Levels in Saliva from Patients with Lung or Breast Cancer: A Preliminary Assessment for Early Diagnostic Purposes. Metabolites 2021, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Husi, H.; Fearon, K.C.; Ross, J.A. Can a simple proteomics urine test assist in the early diagnosis of early-stage cancer? Expert Rev. Proteom. 2011, 8, 555–557. [Google Scholar] [CrossRef][Green Version]
- Al-Tarawneh, S.K.; Border, M.B.; Dibble, C.F.; Bencharit, S. Defining salivary biomarkers using mass spectrometry-based proteomics: A systematic review. OMICS 2011, 15, 353–361. [Google Scholar] [CrossRef]
- Cho, Y.T.; Su, H.; Huang, T.L.; Chen, H.C.; Wu, W.J.; Wu, P.C.; Wu, D.C.; Shiea, J. Matrix-assisted laser desorption ionization/time-of-flight mass spectrometry for clinical diagnosis. Clin. Chim. Acta 2013, 415, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Marvin, L.F.; Roberts, M.A.; Fay, L.B. Matrix-assisted laser desorption/ionization time-of flight mass spectrometry in clinical chemistry. Clin. Chim. Acta 2003, 337, 11–21. [Google Scholar] [CrossRef]
- Pusch, W.; Kostrzewa, M. Application of MALDI-TOF mass spectrometry in screening and diagnostic research. Curr. Pharm. Des. 2005, 11, 2577–2591. [Google Scholar] [CrossRef]
- Merlos Rodrigo, M.A.; Zitka, O.; Krizkova, S.; Moulick, A.; Adam, V.; Kizek, R. MALDI-TOF MS as evolving cancer diagnostic tool: A review. J. Pharm. Biomed. Anal. 2014, 95, 245–255. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Sun, C.; Liu, J.; Pan, Y. MALDI-MSI analysis of cancer drugs: Significance, advances, and applications. Trends Anal. Chem. 2021, 136, 116183. [Google Scholar] [CrossRef]
- Lee, P.Y.; Yeoh, Y.; Omar, N.; Pung, Y.F.; Lim, L.C.; Low, T.Y. Molecular tissue profiling by MALDI imaging: Recent progress and applications in cancer research. Crit. Rev. Clin. Lab. Sci. 2021, 58, 513–529. [Google Scholar] [CrossRef]
- Berghmans, E.; Boonen, K.; Maes, E.; Mertens, I.; Pauwels, P.; Baggerman, G. Implementation of MALDI Mass Spectrometry Imaging in Cancer Proteomics Research: Applications and Challenges. J. Pers. Med. 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Pei, Y.; Wu, Y.; Guo, Y.; Cui, W. Performance of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) in diagnosis of ovarian cancer: A systematic review and meta-analysis. J. Ovarian Res. 2020, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, Y.; Jing, X.; Hu, C. Diagnostic accuracy of MALDI-TOF mass spectrometry for non-small cell lung cancer: A meta-analysis. Biomarkers 2018, 23, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Flatley, B.; Malone, P.; Cramer, R. MALDI mass spectrometry in prostate cancer biomarker discovery. Biochim. Biophys. Acta 2014, 1844, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.; Basuita, A.; Endersby, D.; Curtis, B.; Iacovidou, A.; Walker, M. A systematic review of the diagnostic accuracy of prostate specific antigen. BMC Urol. 2009, 9, 14–22. [Google Scholar] [CrossRef]
- Carter, H.B. Prostate cancers in men with low PSA levels—Must we find them? N. Engl. J. Med. 2004, 350, 2292–2294. [Google Scholar] [CrossRef]
- Calvano, C.D.; Aresta, A.; Iacovone, M.; De Benedetto, G.E.; Zambonin, C.G.; Battaglia, M.; Ditonno, P.; Rutigliano, M.; Bettocchi, C. Optimization of analytical and pre-analytical conditions for MALDI-TOF-MS human urine protein profiles. J. Pharm. Biomed. Anal. 2010, 51, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Basso, D.; Zambon, C.F.; Prayer-Galetti, T.; Arrigoni, G.; Bozzato, D.; Moz, S.; Zattoni, F.; Bellocco, R.; Plebani, M. MALDI-TOF peptidomic analysis of serum and post-prostatic massage urine specimens to identify prostate cancer biomarkers. Clin. Proteom. 2018, 15, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Huang, H.J.; Ou, B.Y.; Chow, N.H.; Chen, Y.W.; Tzai, T.S.; Wu, C.J.; Chen, S.H. Urinary CD14 as a potential biomarker for benign prostatic hyperplasia—Discovery by combining MALDI-TOF-based biostatistics and ESI-MS/MS-based stable-isotope labeling. Proteom. Clin. Appl. 2011, 5, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Inoue, T.; Sekiya, S.; Terada, N.; Miyazaki, Y.; Goto, T.; Kajihara, S.; Kawabata, S.I.; Iwamoto, S.; Ikawa, K.; et al. The C-Terminal Fragment of Prostate-Specific Antigen, a 2331 Da Peptide, as a New Urinary Pathognomonic Biomarker Candidate for Diagnosing Prostate Cancer. PLoS ONE 2014, 9, e107234. [Google Scholar]
- Flatley, B.; Wilmott, K.G.; Malone, P.; Cramer, R. MALDI MS Profiling of Post-DRE Urine Samples Highlights the Potentialof β-Microseminoprotein as a Marker for Prostatic Diseases. Prostate 2014, 74, 103–111. [Google Scholar] [CrossRef]
- Casanova-Salas, I.; Masia, E.; Arminan, A.; Calatrava, A.; Mancarella, C.; Rubio-Briones, J.; Scotlandi, K.; Vicent, M.J.; Lopez-Guerrero, J.A. MiR-187 Targets the Androgen-Regulated Gene ALDH1A3 in Prostate Cancer. PLoS ONE 2015, 10, e0125576. [Google Scholar] [CrossRef]
- Heger, Z.; Michalek, P.; Guran, R.; Cernei, N.; Duskova, K.; Vesely, S.; Anyz, J.; Stepankova, O.; Zitka, O.; Adam, V.; et al. Differences in urinary proteins related to surgical margin status after radical prostatectomy. Oncol. Rep. 2015, 34, 3247–3255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhudia, R.; Ahmad, A.; Akpenyi, O.; Whiley, A.; Wilks, M.; Oliver, T. Identification of low oxygentolerating bacteria in prostate secretions of cancer patients and discussion of possible aetiological significance. Sci. Rep. 2017, 7, 15164. [Google Scholar] [CrossRef]
- Blaschke, C.R.K.; Hartig, J.P.; Grimsley, G.; Liu, L.; Semmes, O.J.; Wu, J.D.; Ippolito, J.E.; Hughes-Halbert, C.; Nyalwidhe, J.O.; Drake, R.R. Direct N-Glycosylation Profiling of Urine and Prostatic Fluid Glycoproteins and Extracellular Vesicles. Front. Chem. 2021, 9, 734280. [Google Scholar] [CrossRef]
- Li, X.; Nakayama, K.; Goto, T.; Kimura, H.; Akamatsu, S.; Hayashi, Y.; Fujita, K.; Kobayashi, T.; Shimizu, K.; Nonomura, N.; et al. High level of phosphatidylcholines/lysophosphatidylcholine ratio in urine is associated with prostate cancer. Cancer Sci. 2021, 112, 4292–4302. [Google Scholar] [CrossRef]
- Buszewska-Forajta, M.; Pomastowski, P.; Monedeiro, F.; Krol-Gorniak, A.; Adamczyk, P.; Markuszewski, M.J.; Buszewski, B. New approach in determination of urinary diagnostic markers for prostate cancer by MALDI-TOF/MS. Talanta 2022, 236, 122843. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Yun, S.J.; Jeong, P.; Kim, I.Y.; Kim, W.J.; Park, S. Diagnosis of bladder cancer and prediction of survival by urinary metabolomics. Oncotarget 2014, 5, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Alberice, J.V.; Amaral, A.F.; Armitage, E.G.; Lorente, J.A.; Algaba, F.; Carrilho, E.; Marquez, M.; Garcia, A.; Malats, N.; Barbas, C. Searching for urine biomarkers of bladder cancer recurrence using a liquid chromatography-mass spectrometry and capillary electrophoresis-mass spectrometry metabolomics approach. J. Chromatogr. A 2013, 13, 163–170. [Google Scholar] [CrossRef]
- Welton, J.L.; Khanna, S.; Giles, P.J.; Brennan, P.; Brewis, I.A.; Staffurth, J.; Mason, M.D.; Clayton, A. Proteomics Analysis of Bladder Cancer Exosomes. Mol. Cell. Proteom. 2010, 9, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.T.; Wei, W.; Shimwell, N.J.; Collins, S.I.; Hussain, S.A.; Billingham, L.J.; Murray, P.G.; Deshmukh, N.; James, N.D.; Wallace, D.M.A.; et al. Assessment of high-throughput high-resolution MALDI-TOF-MS of urinary peptides for the detection of muscle-invasive bladder cancer. Proteom. Clin. Appl. 2011, 5, 493–503. [Google Scholar] [CrossRef]
- Li, F.; Chen, D.; He, C.; Zhou, Y.; Olkkonen, V.M.; He, N.; Chen, W.; Wan, P.; Chen, S.; Zhu, Y.; et al. Identification of urinary Gc-globulin as a novel biomarker for bladder cancer by two-dimensional fluorescent differential gel electrophoresis (2D-DIGE). J. Proteom. 2012, 77, 225–236. [Google Scholar] [CrossRef]
- Li, F.; Yu, Z.; Chen, P.; Lin, G.; Li, T.; Hou, L.; Du, Y.; Tan, W. The increased excretion of urinary orosomucoid 1 as a useful biomarker for bladder cancer. Am. J. Cancer Res. 2016, 6, 331–340. [Google Scholar]
- Halder, S.; Dey, R.K.; Chowdhury, A.R.; Bhattacharyya, P.; Chakrabarti, A. Differential regulation of urine proteins in urothelial neoplasm. J. Proteom. 2015, 127, 185–192. [Google Scholar] [CrossRef]
- Azevedo, R.; Soares, J.; Gaiteiro, C.; Peixoto, A.; Lima, L.; Ferreira, D.; Relvas-Santos, M.; Fernandes, E.; Tavares, A.; Cotton, S.; et al. Glycan affinity magnetic nanoplatforms for urinary glycobiomarkers discovery in bladder cancer. Talanta 2018, 184, 347–355. [Google Scholar] [CrossRef]
- Shpilsky, J.; Catalano, P.J.; McDermott, D.F. First-Line Immunotherapy Combinations in Advanced Renal Cell Carcinoma: A Rapid Review and Meta-Analysis. Kidney Cancer 2021, 5, 153–163. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.L.; Palleschi, G.; Silvestri, L.; Moschese, D.; Ricci, S.; Petrozza, V.; Carbone, A.; Di Carlo, A. Serum and urine biomarkers for human renal cell carcinoma. Dis. Markers 2015, 2015, 251403. [Google Scholar] [CrossRef] [PubMed]
- Chinello, C.; Cazzaniga, M.; De Sio, G.; James Smith, A.; Grasso, A.; Rocco, B.; Signorini, S.; Grasso, M.; Bosari, S.; Zoppis, I.; et al. Tumor size, stage and grade alterations of urinary peptidome in RCC. J. Transl. Med. 2015, 13, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Sandim, V.; de Abreu Pereira, D.; Kalume, D.E.; Oliveira-Carvalho, A.L.; Ornellas, A.A.; Soares, M.R.; Alves, G.; Zingali, R.B. Proteomic analysis reveals differentially secreted proteins in the urine from patients with clear cell renal cell carcinoma. Urol.Oncol. Semin. Orig. Investig. 2016, 34, 5.e11–5.e25. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Ji, Z.; Li, H.; Yan, W.; Zhang, Y. Preliminary Application of WCX Magnetic Bead-Based Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry in Analyzing the Urine of Renal Clear Cell Carcinoma. Chin. Med. Sci. J. 2017, 32, 248–252. [Google Scholar] [PubMed]
- Kandil, N.S.; Ghazala, R.A.; El Sharkawy, R.M.; Youssif, T.A.; Abouseda, N. Evaluation of Protein Profiling in a Cohort of Egyptian Population with Renal Cell Carcinoma and Benign Kidney Neoplasms. Asian Pac. J. Cancer Prev. 2019, 20, 2145–2152. [Google Scholar] [CrossRef]
- Dai, W.; Feng, J.; Hu, X.; Chen, Y.; Gu, Q.; Gong, W.; Feng, T.; Wu, J. SLC7A7 is a prognostic biomarker correlated with immune infiltrates in non-small cell lung cancer. Cancer Cell Int. 2021, 21, N106. [Google Scholar] [CrossRef]
- Jia, Z.; Patra, A.; Kutty, V.K.; Venkatesan, T. Critical Review of Volatile Organic Compound Analysis in Breath and In Vitro Cell Culture for Detection of Lung Cancer. Metabolites 2019, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Zhang, M. Identification of urine biomarkers associated with lung adenocarcinoma. Oncotarget 2017, 8, 38517–38529. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Liu, Z.; Xu, B.; Tang, C.; Li, X.; Quin, H.; Yang, S.; Gao, H.; He, K.; Liu, X. Exploratory study on application of MALDI-TOF-MS to detect serum and urine peptides related to small cell lung carcinoma. Mol. Med. Rep. 2020, 21, 51–60. [Google Scholar] [CrossRef]
- Starykovych, M.; Souchelnytskyi, S.; Fayura, O.; Abrahamovych, O.; Abrahamovych, M.; Lukavetskyy, N.; Stoika, R.; Kit, Y. Identification of cortactin molecular forms in human urine and their possible diagnostic value. Ukr. Biochem. J. 2021, 93, 103–110. [Google Scholar] [CrossRef]
- Rainczuk, A.; Condina, M.; Pelzing, M.; Dolman, S.; Rao, J.; Fairweather, N.; Jobling, T.; Stephens, A.N. The utility of isotope-coded protein labeling for prioritization of proteins found in ovarian cancer patient urine. J. Proteome Res. 2013, 12, 4074–4088. [Google Scholar] [CrossRef]
- Azzollini, J.; Pesenti, C.; Ferrari, L.; Fontana, L.; Calvello, M.; Peissel, B.; Portera, G.; Tabano, S.; Carcangiu, M.L.; Riva, P.; et al. Revertant mosaicism for family mutations is not observed in BRCA1/2 phenocopies. PLoS ONE 2017, 12, e0171663. [Google Scholar] [CrossRef]
- Zou, G.; Benktander, J.D.; Gizaw, S.T.; Gaunitz, S.; Novotny, M.V. Comprehensive Analytical Approach toward Glycomic Characterization and Profiling in Urinary Exosomes. Anal. Chem. 2017, 89, 5364–5372. [Google Scholar] [CrossRef]
- Li, X.; Nakayama, K.; Goto, T.; Akamatsu, S.; Shimizu, K.; Ogawa, O.; Inoue, T. Comparative evaluation of the extraction and analysis of urinary phospholipids and lysophospholipids using MALDI-TOF/MS. Chem. Phys. Lipids 2019, 223, 104787. [Google Scholar] [CrossRef]
- Banach, P.; Derezinski, P.; Matuszewska, E.; Matysiak, J.; Bochynski, H.; Kokot, Z.J.; Nowak-Markwitz, E. MALDI-TOF-MS Analysis in the Identification of Urine Proteomic Patterns of Gestational Trophoblastic Disease. Metabolites 2019, 9, 30. [Google Scholar] [CrossRef]
- Chang, Y.T.; Chu, L.J.; Liu, Y.C.; Chen, C.J.; Wu, S.F.; Chen, C.H.; Chang, I.Y.F.; Wang, J.S.; Wu, T.Y.; Dash, S.; et al. Verification of Saliva Matrix Metalloproteinase-1 as a Strong Diagnostic Marker of Oral Cavity Cancer. Cancers 2020, 12, 2273. [Google Scholar] [CrossRef]
- Jou, Y.J.; Lin, C.D.; Lai, C.H.; Chen, C.H.; Kao, J.Y.; Chen, S.Y.; Tsai, M.H.; Huang, S.H.; Lin, C.W. Proteomic identification of salivary transferrin as a biomarker for early detection of oral cancer. Anal. Chim. Acta 2010, 68, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.J.; Lin, C.D.; Lai, C.H.; Tang, C.H.; Huang, S.H.; Tsai, M.H.; Chen, S.Y.; Kao, J.Y.; Lin, C.W. Salivary zinc finger protein 510 peptide as a novel biomarker for detection of oral squamous cell carcinoma in early stages. Clin. Chim. Acta 2011, 412, 1357–1365. [Google Scholar] [CrossRef]
- Szanto, I.; Mark, L.; Bona, A.; Maasz, G.; Sandor, B.; Gelencser, G.; Turi, Z.; Gallyas, F. High-Throughput Screening of Saliva for Early Detection of Oral Cancer: A Pilot Study. Technol. Cancer Res. Treat. 2012, 11, 181–188. [Google Scholar] [CrossRef]
- Mu, A.K.W.; Chan, Y.S.; Kang, S.S.; Azman, S.N.; Zain, R.B.; Chai, W.L. Detection of host-specific immunogenic proteins in the saliva of patients with oral squamous cell carcinoma. J. Immunoass. Immunochem. 2014, 35, 183–193. [Google Scholar] [CrossRef]
- Jancsik, V.A.; Gelencser, G.; Maasz, G.; Schmidt, J.; Molnar, G.A.; Wittmann, I.; Olasz, L.; Mar, L. Salivary Proteomic Analysis of Diabetic Patients for Possible Oral Squamous Cell Carcinoma Biomarkers. Pathol. Oncol. Res. 2014, 20, 591–595. [Google Scholar] [CrossRef]
- Chaiyarit, P.; Taweechaisupapong, S.; Jaresitthikunchai, J.; Phaonakrop, N.; Roytrakul, S. Comparative evaluation of 5–15-kDa salivary proteins from patients with different oral diseases by MALDI-TOF/TOF mass spectrometry. Clin. Oral. Investig. 2015, 19, 729–737. [Google Scholar] [CrossRef]
- Jiang, W.P.; Wang, Z.; Xu, L.X.; Peng, X.; Chen, F. Diagnostic model of saliva peptide fingerprint analysis of oral squamous cell carcinoma patients using weak cation exchange magnetic beads. Biosci. Rep. 2015, 35, e00211. [Google Scholar] [CrossRef]
- Camisasca, D.R.; da Ros Goncalves, L.; Soares, M.R.; Sandim, V.; Nogueira, F.C.S.; Garcia, C.H.S.; Santana, R.; de Oliveira, S.P.; Buexme, L.A.; de Faria, P.A.S.; et al. A proteomic approach to compare saliva from individuals with and without oral leukoplakia. J. Proteom. 2017, 151, 43–52. [Google Scholar] [CrossRef]
- Hsiao, Y.C.; Lin, S.Y.; Chien, K.Y.; Chen, S.F.; Wu, C.C.; Chang, Y.T.; Chi, L.M.; Chu, L.J.; Chiang, W.F.; Chien, C.Y.; et al. An immuno-MALDI mass spectrometry assay for the oral cancer biomarker, matrix metalloproteinase-1, in dried saliva spot samples. Anal. Chim. Acta 2020, 1100, 118–130. [Google Scholar] [CrossRef]
- Kabzinski, J.; Maczynska, M.; Majsterek, I. MicroRNA as a Novel Biomarker in the Diagnosis of Head and Neck Cancer. Biomolecules 2021, 11, 844. [Google Scholar] [CrossRef]
- Vitorino, R.; Alves, R.; Barros, A.; Caseiro, A.; Ferreira, R.; Calheiros Lobo, M.; Bastos, A.; Duarte, J.; Carvalho, D.; Santos, L.L.; et al. Finding new posttranslational modifications in salivary proline-rich proteins. Proteomics 2010, 10, 3732–3742. [Google Scholar] [CrossRef]
- Jarai, T.; Maasz, G.; Burian, A.; Bona, A.; Jambor, E.; Gerlinger, I.; Mark, L. Mass Spectrometry-Based Salivary Proteomics for the Discovery of Head and Neck Squamous Cell Carcinoma. Pathol. Oncol. Res. 2012, 18, 623–628. [Google Scholar] [CrossRef]
- Laus, A.C.; Guerreiro da Silva, I.D.C.; Bertuccez Cordeiro, F.; Lo Turco, E.G.; de Souza Viana, L.; Lopes Carvalho, A. Is Lipidomic the Answer to the Search of a Biomarker for Organ Preservation Protocol in Head and Neck Squamous Cell Carcinoma? Pathol. Oncol. Res. 2018, 24, 931–935. [Google Scholar] [CrossRef]
- Baldini, C.; Giusti, L.; Ciregia, F.; Da Valle, Y.; Giacomelli, C.; Donadio, E.; Ferro, F.; Galimberti, S.; Donati, V.; Bazzichi, L.; et al. Correspondence between salivary proteomic pattern and clinical course in primary Sjögren syndrome and non-Hodgkin’s lymphoma: A case report. J. Transl. Med. 2011, 9, 1–8. [Google Scholar] [CrossRef]
- Qin, Y.; Zhong, Y.; Ma, T.; Zhang, J.; Yang, G.; Guan, F.; Li, Z.; Li, B. A pilot study of salivary N-glycome in HBV-induced chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Glycoconj. J. 2017, 34, 523–535. [Google Scholar] [CrossRef]
- Jian, S.; Hanjie, Y.; Haoqi, D.; Jiaxu, Z.; Kun, Z.; Xiaojie, L.; Hailong, X.; Zheng, L. Identification of N- and O-linked glycans recognized by AAL in saliva of patients with atrophic gastritis and gastric cancer. Cancer Biomark. 2018, 22, 669–681. [Google Scholar]
- Tajmul, M.; Parween, F.; Singh, L.; Mathur, S.R.; Sharma, J.B.; Kumar, S.; Sharma, D.N.; Yadav, S. Identification and validation of salivary proteomic signatures for non-invasive detection of ovarian cancer. Int. J. Biol. Macromol. 2018, 108, 503–514. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Shu, J.; Hou, Y.; Chen, M.; Yu, H.; Ma, T.; Du, H.; Zhang, J.; Qiao, Y.; et al. Abnormal Galactosylated–Glycans recognized by Bandeiraea Simplicifolia Lectin I in saliva of patients with breast Cancer. Glycoconj. J. 2020, 37, 373–394. [Google Scholar] [CrossRef]
- Yang, J.; Ma, T.; Yu, H.; Yin, M.; Qiao, Y.; Niu, L.; Yang, F.; He, J.; Li, Z. Alternations of N-glycans recognized by Phaseolus vulgaris leucoagglutinin in the saliva of patients with breast cancer. Neoplasma 2021, 68, 994–1004. [Google Scholar] [CrossRef]
- Morsi, H.; Golizeh, M.; Brosseau, N.; Janat, A.I.; Emami, E.; Ndao, M.; Tran, S.D. Detection of Fusobacterium nucleatum subspecies in the saliva of pre-colorectal cancer patients, using tandem mass spectrometry. Arch. Oral Biol. 2022, 134, 105337–105345. [Google Scholar] [CrossRef]
- Xie, Z.; Yan, Y.; Tang, K.; Ding, C.F. Post-synthesis modification of covalent organic frameworks for ultrahigh enrichment of low-abundance glycopeptides from human saliva and serum. Talanta 2022, 236, 122831–122839. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zambonin, C.; Aresta, A. MALDI-TOF/MS Analysis of Non-Invasive Human Urine and Saliva Samples for the Identification of New Cancer Biomarkers. Molecules 2022, 27, 1925. https://doi.org/10.3390/molecules27061925
Zambonin C, Aresta A. MALDI-TOF/MS Analysis of Non-Invasive Human Urine and Saliva Samples for the Identification of New Cancer Biomarkers. Molecules. 2022; 27(6):1925. https://doi.org/10.3390/molecules27061925
Chicago/Turabian StyleZambonin, Carlo, and Antonella Aresta. 2022. "MALDI-TOF/MS Analysis of Non-Invasive Human Urine and Saliva Samples for the Identification of New Cancer Biomarkers" Molecules 27, no. 6: 1925. https://doi.org/10.3390/molecules27061925
APA StyleZambonin, C., & Aresta, A. (2022). MALDI-TOF/MS Analysis of Non-Invasive Human Urine and Saliva Samples for the Identification of New Cancer Biomarkers. Molecules, 27(6), 1925. https://doi.org/10.3390/molecules27061925