MXene–AuNP-Based Electrochemical Aptasensor for Ultra-Sensitive Detection of Chloramphenicol in Honey
Abstract
:1. Introduction
2. Results and Discussion
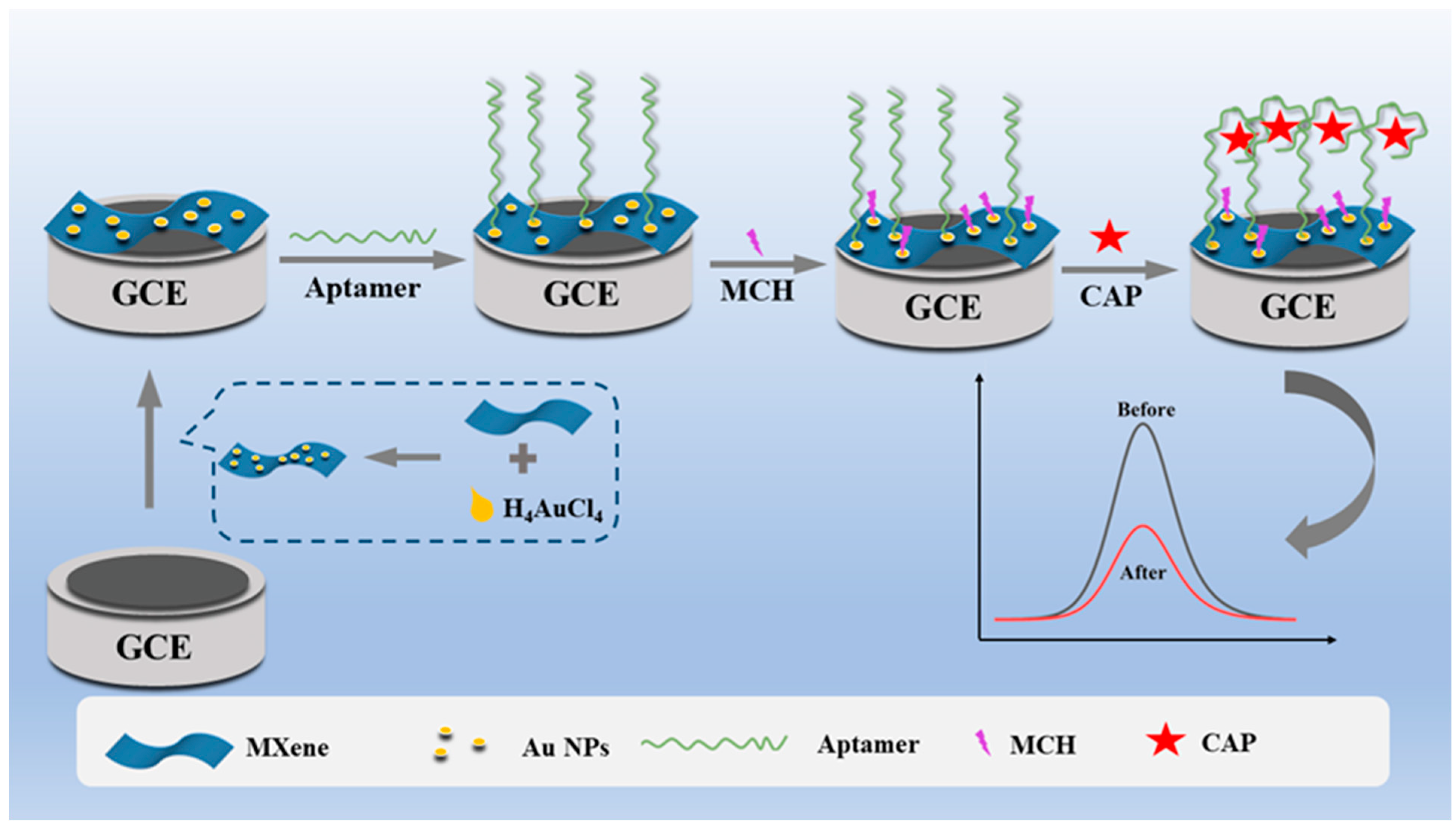
2.1. Sensing Mechanism of the CAP Electrochemical Aptasensor
2.2. Material Characterization
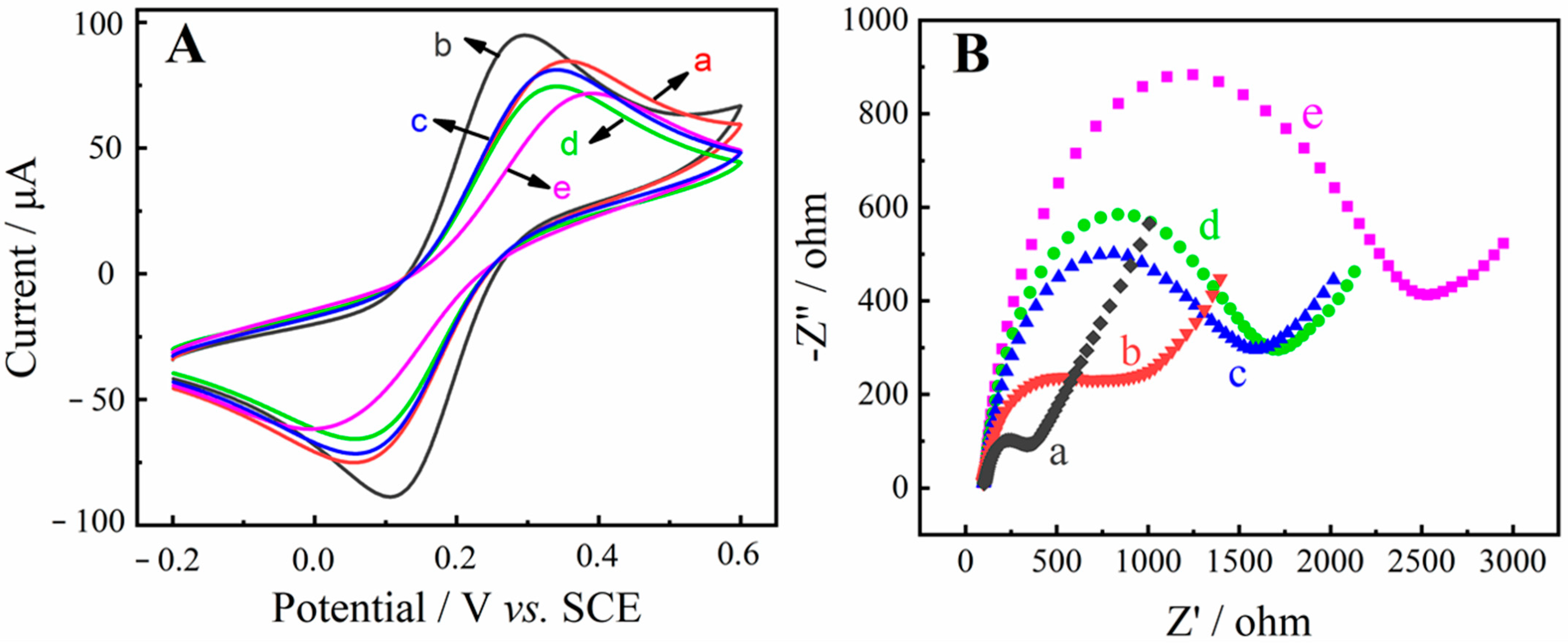
2.3. Electrochemical Characterization of the Aptasensor
2.4. Optimization of Experimental Conditions
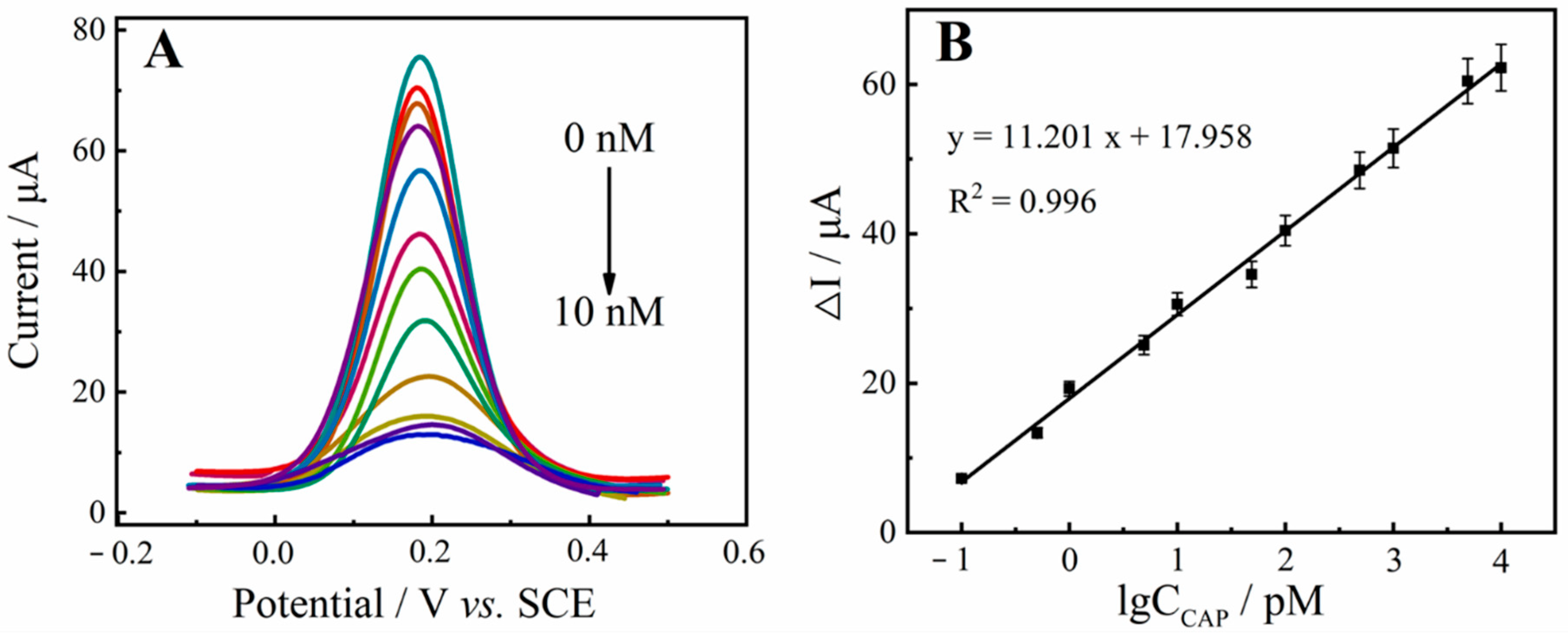
2.5. Analytical Performance of the Aptasensor
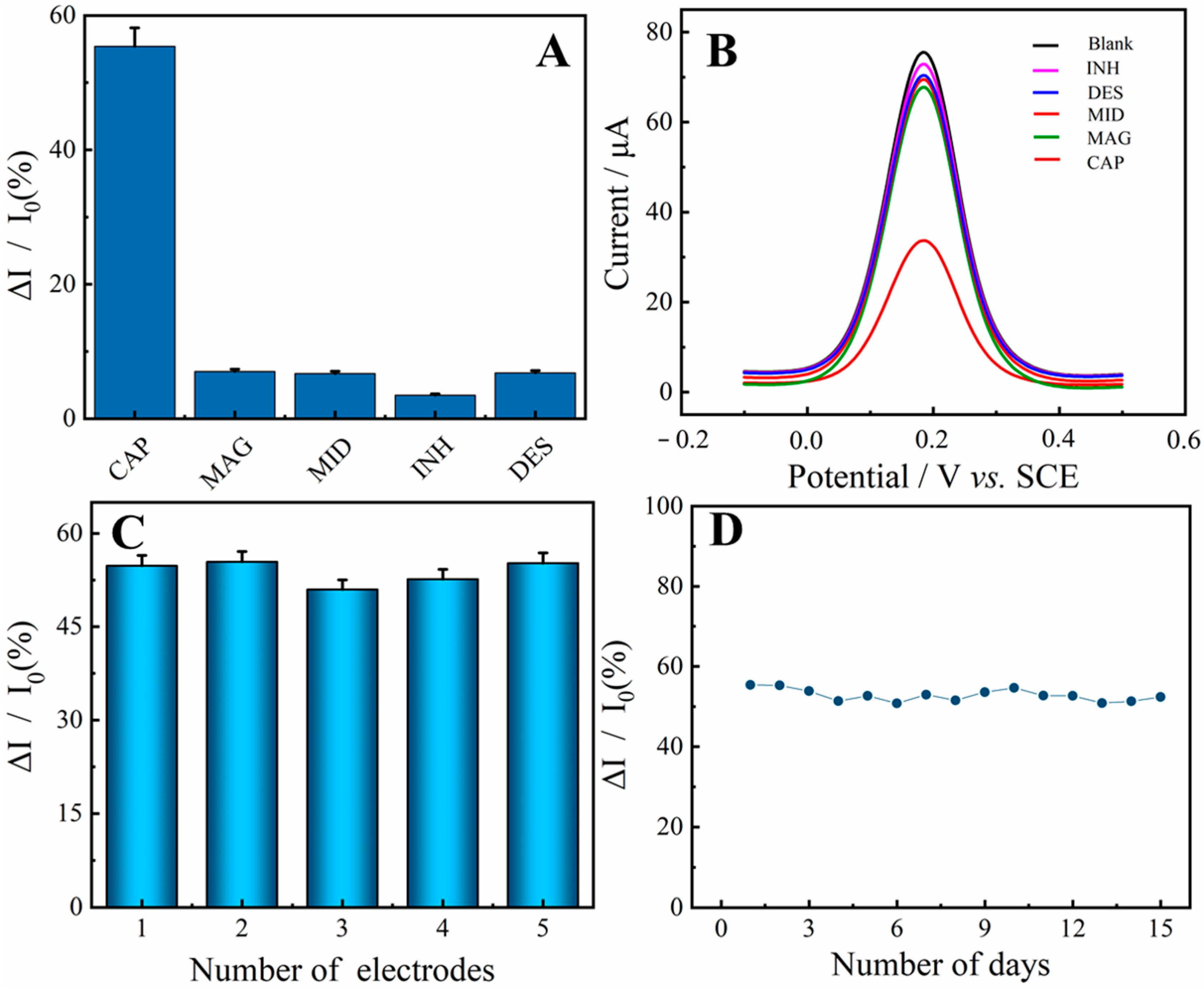
2.6. Selectivity, Reproductivity, and Stability Investigation
2.7. Analysis of Spiked Samples
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instruments
3.3. Preparation of MXene–AuNP Composite
3.4. Preparation of Aptasensor
3.5. Honey Sample Preparation
3.6. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roushani, M.; Rahmati, Z.; Farokhi, S.; Hoseini, S.J.; Fath, R.H. The development of an electrochemical nanoaptasensor to sensing chloramphenicol using a nanocomposite consisting of graphene oxide functionalized with (3-Aminopropyl) triethoxysilane and silver nanoparticles. Mater. Sci. Eng. C 2020, 108, 110388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, X.; Zhang, Y.; Wang, Y.; Wang, B.; Zheng, L.; Zhang, D.; Zhuang, S. Rapid quantitative detection of chloramphenicol in milk by microfluidic immunoassay. Food Chem. 2021, 339, 127857. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yan, X.; Li, M.; Liu, H.; Li, J.; Wang, L.; Wang, K.; Lu, X.; Wang, S.; He, B. Ultrasensitive detection of chloramphenicol using electrochemical aptamer sensor: A mini review. Electrochem. Commun. 2020, 120, 106835. [Google Scholar] [CrossRef]
- Munawar, A.; Tahir, M.A.; Shaheen, A.; Lieberzeit, P.A.; Khan, W.S.; Bajwa, S.Z. Investigating nanohybrid material based on 3D CNTs@Cu nanoparticle composite and imprinted polymer for highly selective detection of chloramphenicol. J. Hazard. Mater. 2018, 342, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Forti, A.F.; Campana, G.; Simonella, A.; Multari, M.; Scortichini, G. Determination of chloramphenicol in honey by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2005, 529, 257–263. [Google Scholar] [CrossRef]
- Bogusz, M.J.; Hassan, H.; Al-Enazi, E.; Ibrahim, Z.; Al-Tufail, M. Rapid determination of chloramphenicol and its glucuronide in food products by liquid chromatography–electrospray negative ionization tandem mass spectrometry. J. Chromatogr. B 2004, 807, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, P.; Bedi, J.S.; Aulakh, R.S.; Gill, J.P.S.; Kumar, A. Validation of HPLC Multi-residue Method for Determination of Fluoroquinolones, Tetracycline, Sulphonamides and Chloramphenicol Residues in Bovine Milk. Food Anal. Method 2019, 12, 338–346. [Google Scholar] [CrossRef]
- Sample, R.B.; Glick, M.R.; Kleiman, M.B.; Smith, J.W.; Oei, T.O. High-pressure liquid chromatographic assay of chloramphenicol in biological fluids. Antimicrob. Agents Chemother. 1979, 15, 491–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akter Mou, S.; Islam, R.; Shoeb, M.; Nahar, N. Determination of chloramphenicol in meat samples using liquid chromatography–tandem mass spectrometry. Food Sci. Nutr. 2021, 9, 5670–5675. [Google Scholar] [CrossRef]
- Sánchez-Brunete, C.; Albero, B.; Miguel, E.; Tadeo, J.L. Rapid Method for Determination of Chloramphenicol Residues in Honey Using Gas Chromatography-Mass Spectrometry. B Environ. Contam. Tox. 2005, 75, 459–465. [Google Scholar] [CrossRef]
- Tu, X.; Gao, F.; Ma, X.; Zou, J.; Yu, Y.; Li, M.; Qu, F.; Huang, X.; Lu, L. Mxene/carbon nanohorn/β-cyclodextrin-Metal-organic frameworks as high-performance electrochemical sensing platform for sensitive detection of carbendazim pesticide. J. Hazard. Mater. 2020, 396, 122776. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Lu, L.; Leng, J.; Yu, Y.; Wang, W.; Jiang, M.; Bai, L. An enhanced electrochemical platform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of Sunset Yellow and Tartrazine. Food Chem. 2016, 190, 889–895. [Google Scholar] [CrossRef] [PubMed]
- de la Rica, R.; Pejoux, C.; Matsui, H. Assemblies of Functional Peptides and Their Applications in Building Blocks for Biosensors. Adv. Funct. Mater. 2011, 21, 1018–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangiri Dehaghani, F.; Zare, H.R.; Shekari, Z. Measurement of aflatoxin M1 in powder and pasteurized milk samples by using a label–free electrochemical aptasensor based on platinum nanoparticles loaded on Fe–based metal–organic frameworks. Food Chem. 2020, 310, 125820. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhang, H.; Li, H.; Zhu, Q.; He, H. Ingenious construction of an electrochemical aptasensor based on a Au@COF/GO-NH2 composite with excellent detection performance. J. Mater. Chem. C. 2021, 9, 4576–4582. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, L.; Ma, K.; Xu, B.; Liu, L.; Tian, W. Label-Free Aptamer-Based Biosensor for Specific Detection of Chloramphenicol Using AIE Probe and Graphene Oxide. ACS Omega 2018, 3, 12886–12892. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Ma, X.; Ma, X.; Yue, T.; Sheng, Q. A label-free aptasensor for ochratoxin a detection with signal amplification strategies on ultrathin micron-sized 2D MOF sheets. Sens. Actuators B Chem. 2021, 334, 129682. [Google Scholar] [CrossRef]
- Lu, M.; Cao, C.; Wang, F.; Liu, G. A polyethyleneimine reduced graphene oxide/gold nanocubes based electrochemical aptasensor for chloramphenicol detection using single-stranded DNA-binding protein. Mater. Des. 2021, 199, 109409–109417. [Google Scholar] [CrossRef]
- Dong, J.; Chen, F.; Xu, L.; Yan, P.; Qian, J.; Chen, Y.; Yang, M.; Li, H. Fabrication of sensitive photoelectrochemical aptasensor using Ag nanoparticles sensitized bismuth oxyiodide for determination of chloramphenicol. Microchem. J. 2022, 107317–107328. [Google Scholar] [CrossRef]
- Zhao, C.; Jing, T.; Dong, M.; Pan, D.; Guo, J.; Tian, J.; Wu, M.; Naik, N.; Huang, M.; Guo, Z. A Visible Light Driven Photoelectrochemical Chloramphenicol Aptasensor Based on a Gold Nanoparticle-Functionalized 3D Flower-like MoS2/TiO2 Heterostructure. Langmuir 2022, 38, 2276–2286. [Google Scholar] [CrossRef]
- Xiong, D.; Shi, Y.; Yang, H.Y. Rational design of MXene-based films for energy storage: Progress, prospects. Mater. Today 2021, 46, 183–211. [Google Scholar] [CrossRef]
- Ma, X.; Tu, X.; Gao, F.; Xie, Y.; Huang, X.; Fernandez, C.; Qu, F.; Liu, G.; Lu, L.; Yu, Y. Hierarchical porous MXene/amino carbon nanotubes-based molecular imprinting sensor for highly sensitive and selective sensing of fisetin. Sens. Actuators B Chem. 2020, 309, 127815. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, M.; Anasori, B.; Maleski, K.; Ren, C.E.; Li, J.; Byles, B.W.; Pomerantseva, E.; Wang, G.; Gogotsi, Y. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy 2016, 26, 513–523. [Google Scholar] [CrossRef]
- Zou, G.; Zhang, Z.; Guo, J.; Liu, B.; Zhang, Q.; Fernandez, C.; Peng, Q. Synthesis of MXene/Ag Composites for Extraordinary Long Cycle Lifetime Lithium Storage at High Rates. ACS Appl. Mater. Inter. 2016, 8, 22280–22286. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gao, F.; Zou, J.; Liu, S.; Li, M.; Gao, Y.; Yu, Y.; Wang, X.; Lu, L. MXene@Ag-based ratiometric electrochemical sensing strategy for effective detection of carbendazim in vegetable samples. Food Chem. 2021, 360, 130006. [Google Scholar] [CrossRef] [PubMed]
- Feng, E.; Zheng, T.; Tian, Y. Dual-Mode Au Nanoprobe Based on Surface Enhancement Raman Scattering and Colorimetry for Sensitive Determination of Telomerase Activity Both in Cell Extracts and in the Urine of Patients. ACS Sens. 2019, 4, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Jiang, X.; Li, Y.; Lu, X.; Luan, S.; Wang, Y.; Li, Y.; Gao, F. Metal-organic frameworks-derived MnO2/Mn3O4 microcuboids with hierarchically ordered nanosheets and Ti3C2 MXene/Au NPs composites for electrochemical pesticide detection. J. Hazard. Mater. 2019, 373, 367–376. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, C.; Yan, W.; Guo, Y.; Shuang, S.; Dong, C.; Bi, Y. Design of a facile and label-free electrochemical aptasensor for detection of atrazine. Talanta 2019, 201, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Yalikun, N.; Mamat, X.; Li, Y.; Hu, X.; Wågberg, T.; Dong, Y.; Hu, G. Synthesis of an iron-nitrogen co-doped ordered mesoporous carbon-silicon nanocomposite as an enhanced electrochemical sensor for sensitive and selective determination of chloramphenicol. Colloids Surf. B Biointerfaces 2018, 172, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Roushani, M.; Rahmati, Z.; Hoseini, S.J.; Hashemi Fath, R. Impedimetric ultrasensitive detection of chloramphenicol based on aptamer MIP using a glassy carbon electrode modified by 3-ampy-RGO and silver nanoparticle. Colloids Surf. B Biointerfaces 2019, 183, 110451. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Huang, Y.; Tang, D.; Cui, H.; Cao, H. A competitive colorimetric chloramphenicol assay based on the non-cross-linking deaggregation of gold nanoparticles coated with a polyadenine-modified aptamer. Microchim. Acta 2018, 185, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, Q.; Geng, L.; Shu, X.; Wang, Y. A “signal-on” photoelectrochemical aptasensor based on graphene quantum dots-sensitized TiO2 nanotube arrays for sensitive detection of chloramphenicol. Talanta 2019, 197, 28–35. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wang, S. An electrochemical aptasensor based on PEI-C3N4/AuNWs for determination of chloramphenicol via exonuclease-assisted signal amplification. Mikrochim. Acta 2021, 188, 22. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Luo, C.; Cheng, W.; Mao, W.; Zhang, D.; Ding, S. A simple and sensitive electrochemical aptasensor for determination of Chloramphenicol in honey based on target-induced strand release. J. Electroanal. Chem. 2012, 687, 89–94. [Google Scholar] [CrossRef]


| Materials | Methods | Linear Range (μM) | LOD (μM) | Reference |
|---|---|---|---|---|
| Si-Fe/NOMC a | Electrochemical sensors | 1–500 | 0.3 | [29] |
| AgNP/3-ampy b-RGO | Molecular imprinting polymer | 1 × 10−6–1 × 10−3 | 0.3 × 10−6 | [30] |
| AuNPs | Colorimetric | 3 × 10−6–0.309 | 0.4 × 10−6 | [31] |
| GODs/TiO2 NTs | Photoelectrochemical | 0.5 × 10−3–0.1 | 0.579 × 10−6 | [32] |
| PEI-C3N4 c/AuNCs | Electrochemical aptasensor | 5 × 10−6–1 | 2.08 × 10−6 | [33] |
| MXene–AuNPs | Electrochemical aptasensor | 0.1 × 10−6–10 × 10−3 | 0.03 × 10−6 | This work |
| Samples | Added (pM) | Founded (pM) | Recovery (%) | by HPLC Method |
|---|---|---|---|---|
| 0 | 0 | 0.02 | 102.0 | 0.018 |
| 1 | 50 | 53 | 106.0 | 54.85 |
| 2 | 500 | 483 | 96.6 | 512.70 |
| 3 | 1000 | 983 | 98.3 | 1024.01 |
| 4 | 5000 | 4788 | 95.7 | 4906.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhong, W.; Yu, Q.; Zou, J.; Gao, Y.; Liu, S.; Zhang, S.; Wang, X.; Lu, L. MXene–AuNP-Based Electrochemical Aptasensor for Ultra-Sensitive Detection of Chloramphenicol in Honey. Molecules 2022, 27, 1871. https://doi.org/10.3390/molecules27061871
Yang J, Zhong W, Yu Q, Zou J, Gao Y, Liu S, Zhang S, Wang X, Lu L. MXene–AuNP-Based Electrochemical Aptasensor for Ultra-Sensitive Detection of Chloramphenicol in Honey. Molecules. 2022; 27(6):1871. https://doi.org/10.3390/molecules27061871
Chicago/Turabian StyleYang, Jing, Wei Zhong, Qi Yu, Jin Zou, Yansha Gao, Shuwu Liu, Songbai Zhang, Xiaoqiang Wang, and Limin Lu. 2022. "MXene–AuNP-Based Electrochemical Aptasensor for Ultra-Sensitive Detection of Chloramphenicol in Honey" Molecules 27, no. 6: 1871. https://doi.org/10.3390/molecules27061871
APA StyleYang, J., Zhong, W., Yu, Q., Zou, J., Gao, Y., Liu, S., Zhang, S., Wang, X., & Lu, L. (2022). MXene–AuNP-Based Electrochemical Aptasensor for Ultra-Sensitive Detection of Chloramphenicol in Honey. Molecules, 27(6), 1871. https://doi.org/10.3390/molecules27061871








