The (Bio)Chemistry of Non-Transferrin-Bound Iron
Abstract
:1. Introduction
2. NTBI: Non-Transferrin-Bound Iron
3. The Chemical Characterization of NTBI
4. Ferric Citrate Chemistry
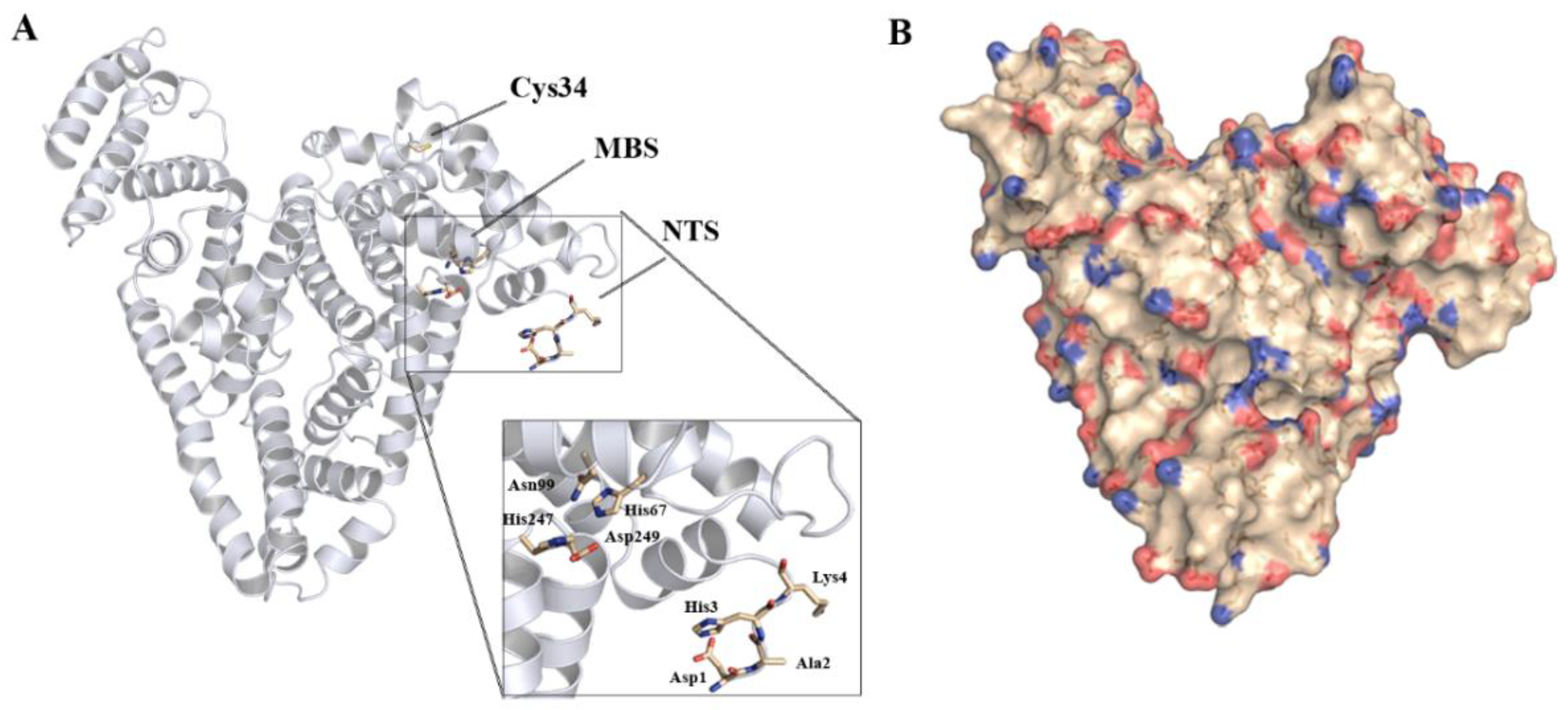
5. Iron Binding by Serum Albumin
6. NTBI Detection and Quantification
7. NTBI in Iron Related Disorders
8. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crichton, R.R. The Importance of Iron for Biological Systems. In Inorganic Biochemistry of Iron Metabolism, 2nd ed.; Crichton, R.R., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001; pp. 17–48. [Google Scholar]
- Sánchez, M.; Sabio, L.; Gálvez, N.; Capdevila, M.; Dominguez-Vera, J.M. Iron chemistry at the service of life. IUBMB Life 2017, 69, 382–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koppenol, W.H.; Hider, R.H. Iron and redox cycling. Do’s and don’ts. Free Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef]
- Silva, A.M.N.; Moniz, T.; de Castro, B.; Rangel, M. Human transferrin: An inorganic biochemistry perspective. Coord. Chem. Rev. 2021, 449, 214186. [Google Scholar] [CrossRef]
- Cazzola, M.; Huebers, H.A.; Sayers, M.H.; MacPhail, A.P.; Eng, M.; Finch, C.A. Transferrin saturation, plasma iron turnover, and transferrin uptake in normal humans. Blood 1985, 66, 935–939. [Google Scholar] [CrossRef] [Green Version]
- Brissot, P.; Loréal, O. Role of non-transferrin-bound iron in the pathogenesis of iron overload and toxicity. Adv. Exp. Med. Biol. 2002, 509, 45–53. [Google Scholar] [CrossRef]
- Sugiura, T.; Dohi, Y.; Takase, H.; Fujii, S.; Seo, Y.; Ohte, N. Analytical evaluation of serum non-transferrin-bound iron and its relationships with oxidative stress and cardiac load in the general population. Medicine 2021, 100, e24722. [Google Scholar] [CrossRef]
- Vinchi, F.; Porto, G.; Simmelbauer, A.; Altamura, S.; Passos, S.T.; Garbowski, M.; Silva, A.M.N.; Spaich, S.; Seide, S.E.; Sparla, R.; et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur. Heart J. 2020, 41, 2681–2695. [Google Scholar] [CrossRef]
- Roghi, A.; Poggiali, E.; Duca, L.; Mafrici, A.; Pedrotti, P.; Paccagnini, S.; Brenna, S.; Galli, A.; Consonni, D.; Cappellini, M.D. Role of Non-Transferrin-Bound Iron in the pathogenesis of cardiotoxicity in patients with ST-elevation myocardial infarction assessed by Cardiac Magnetic Resonance Imaging. Int. J. Cardiol. 2015, 199, 326–332. [Google Scholar] [CrossRef]
- Piga, A.; Longo, F.; Duca, L.; Roggero, S.; Vinciguerra, T.; Calabrese, R.; Hershko, C.; Cappellini, M.D. High nontransferrin bound iron levels and heart disease in thalassemia major. Am. J. Hematol. 2009, 84, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, S.A.; Brown, K.E. Iron-Induced Liver Injury: A Critical Reappraisal. Int. J. Mol. Sci. 2019, 20, 2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [Green Version]
- Cabantchik, I.Z.; Hershko, C. Plasma non transferrin bound iron—NTBI revisited. Implications for systemic iron overload and in iv iron supplementation. Am. J. Hematol. 2022, 97, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Silva, A.M.; Podinovskaia, M.; Ma, Y. Monitoring the efficiency of iron chelation therapy: The potential of nontransferrin-bound iron. Ann. N. Y. Acad. Sci. 2010, 1202, 94–99. [Google Scholar] [CrossRef] [PubMed]
- de Swart, L.; Hendriks, J.C.; van der Vorm, L.N.; Cabantchik, Z.I.; Evans, P.J.; Hod, E.A.; Brittenham, G.M.; Furman, Y.; Wojczyk, B.; Janssen, M.C.; et al. Second international round robin for the quantification of serum non-transferrin-bound iron and labile plasma iron in patients with iron-overload disorders. Haematologica 2016, 101, 38–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hershko, C.; Graham, G.; Bates, G.W.; Rachmilewitz, E.A. Non-specific serum iron in thalassaemia: An abnormal serum iron fraction of potential toxicity. Br. J. Haematol. 1978, 40, 255–263. [Google Scholar] [CrossRef]
- Anuwatanakulchai, M.; Pootrakul, P.; Thuvasethakul, P.; Wasi, P. Non-transferrin plasma iron in beta-thalassaemia/Hb E and haemoglobin H diseases. Scand. J. Haematol. 1984, 32, 153–158. [Google Scholar] [CrossRef]
- Wang, W.C.; Ahmed, N.; Hanna, M. Non-transferrin-bound iron in long-term transfusion in children with congenital anemias. J. Pediatr. 1986, 108, 552–557. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Bomford, A.; Polson, R.J.; Halliwell, B. Nontransferrin-bound iron in plasma from hemochromatosis patients: Effect of phlebotomy therapy. Blood 1988, 72, 1416–1419. [Google Scholar] [CrossRef] [Green Version]
- Gosriwatana, I.; Loreal, O.; Lu, S.; Brissot, P.; Porter, J.; Hider, R.C. Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Anal. Biochem. 1999, 273, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Loréal, O.; Gosriwatana, I.; Guyader, D.; Porter, J.; Brissot, P.; Hider, R.C. Determination of non-transferrin-bound iron in genetic hemochromatosis using a new HPLC-based method. J. Hepatol. 2000, 32, 727–733. [Google Scholar] [CrossRef]
- Breuer, W.; Hershko, C.; Cabantchik, Z.I. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus. Sci. 2000, 23, 185–192. [Google Scholar] [CrossRef]
- Lee, D.H.; Liu, D.Y.; Jacobs, D.R., Jr.; Shin, H.R.; Song, K.; Lee, I.K.; Kim, B.; Hider, R.C. Common presence of non-transferrin-bound iron among patients with type 2 diabetes. Diabetes Care 2006, 29, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Dresow, B.; Petersen, D.; Fischer, R.; Nielsen, P. Non-transferrin-bound iron in plasma following administration of oral iron drugs. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2008, 21, 273–276. [Google Scholar] [CrossRef]
- Halliwell, B.; Aruoma, O.I.; Mufti, G.; Bomford, A. Bleomycin-detectable iron in serum from leukaemic patients before and after chemotherapy. Therapeutic implications for treatment with oxidant-generating drugs. FEBS Lett. 1988, 241, 202–204. [Google Scholar] [CrossRef] [Green Version]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loréal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta 2012, 1820, 403–410. [Google Scholar] [CrossRef]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef] [Green Version]
- Breuer, W.; Ermers, M.J.; Pootrakul, P.; Abramov, A.; Hershko, C.; Cabantchik, Z.I. Desferrioxamine-chelatable iron, a component of serum non-transferrin-bound iron, used for assessing chelation therapy. Blood 2001, 97, 792–798. [Google Scholar] [CrossRef] [Green Version]
- Esposito, B.P.; Breuer, W.; Sirankapracha, P.; Pootrakul, P.; Hershko, C.; Cabantchik, Z.I. Labile plasma iron in iron overload: Redox activity and susceptibility to chelation. Blood 2003, 102, 2670–2677. [Google Scholar] [CrossRef] [Green Version]
- Pootrakul, P.; Breuer, W.; Sametband, M.; Sirankapracha, P.; Hershko, C.; Cabantchik, Z.I. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded β-thalassemia/HbE patients treated with an oral chelator. Blood 2004, 104, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Kakhlon, O.; Cabantchik, Z.I. The labile iron pool: Characterization, measurement, and participation in cellular processes(1). Free Radic. Biol. Med. 2002, 33, 1037–1046. [Google Scholar] [CrossRef]
- de Valk, B.; Addicks, M.A.; Gosriwatana, I.; Lu, S.; Hider, R.C.; Marx, J.J. Non-transferrin-bound iron is present in serum of hereditary haemochromatosis heterozygotes. Eur. J. Clin. Investig. 2000, 30, 248–251. [Google Scholar] [CrossRef]
- Van Campenhout, A.; Van Campenhout, C.; Lagrou, A.R.; Moorkens, G.; De Block, C.; Manuel-y-Keenoy, B. Iron-binding antioxidant capacity is impaired in diabetes mellitus. Free Radic. Biol. Med. 2006, 40, 1749–1755. [Google Scholar] [CrossRef]
- Porter, J.B.; Walter, P.B.; Neumayr, L.D.; Evans, P.; Bansal, S.; Garbowski, M.; Weyhmiller, M.G.; Harmatz, P.R.; Wood, J.C.; Miller, J.L.; et al. Mechanisms of plasma non-transferrin bound iron generation: Insights from comparing transfused diamond blackfan anaemia with sickle cell and thalassaemia patients. Br. J. Haematol. 2014, 167, 692–696. [Google Scholar] [CrossRef]
- Knutson, M.D. Non-transferrin-bound iron transporters. Free Radic. Biol. Med. 2019, 133, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.; Baker, S.M.; Morgan, E.H. Characterisation of non-transferrin-bound iron (ferric citrate) uptake by rat hepatocytes in culture. Biochim. Biophys. Acta 1998, 1380, 21–30. [Google Scholar] [CrossRef]
- Arezes, J.; Costa, M.; Vieira, I.; Dias, V.; Kong, X.L.; Fernandes, R.; Vos, M.; Carlsson, A.; Rikers, Y.; Porto, G.; et al. Non-transferrin-bound iron (NTBI) uptake by T lymphocytes: Evidence for the selective acquisition of oligomeric ferric citrate species. PLoS ONE 2013, 8, e79870. [Google Scholar] [CrossRef] [Green Version]
- Brissot, P.; Wright, T.L.; Ma, W.L.; Weisiger, R.A. Efficient clearance of non-transferrin-bound iron by rat liver. Implications for hepatic iron loading in iron overload states. J. Clin. Investig. 1985, 76, 1463–1470. [Google Scholar] [CrossRef] [Green Version]
- Graham, R.M.; Morgan, E.H.; Baker, E. Characterisation of citrate and iron citrate uptake by cultured rat hepatocytes. J. Hepatol. 1998, 29, 603–613. [Google Scholar] [CrossRef]
- Wright, T.L.; Brissot, P.; Ma, W.L.; Weisiger, R.A. Characterization of non-transferrin-bound iron clearance by rat liver. J. Biol. Chem. 1986, 261, 10909–10914. [Google Scholar] [CrossRef]
- Craven, C.M.; Alexander, J.; Eldridge, M.; Kushner, J.P.; Bernstein, S.; Kaplan, J. Tissue distribution and clearance kinetics of non-transferrin-bound iron in the hypotransferrinemic mouse: A rodent model for hemochromatosis. Proc. Natl. Acad. Sci. USA 1987, 84, 3457–3461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkitkasemwong, S.; Wang, C.-Y.; Coffey, R.; Zhang, W.; Chan, A.; Biel, T.; Kim, J.-S.; Hojyo, S.; Fukada, T.; Knutson, M.D. SLC39A14 Is Required for the Development of Hepatocellular Iron Overload in Murine Models of Hereditary Hemochromatosis. Cell Metab. 2015, 22, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-Y.; Knutson, M.D. Hepatocyte divalent metal-ion transporter-1 is dispensable for hepatic iron accumulation and non-transferrin-bound iron uptake in mice. Hepatology 2013, 58, 788–798. [Google Scholar] [CrossRef] [Green Version]
- Coffey, R.; Knutson, M.D. The plasma membrane metal-ion transporter ZIP14 contributes to nontransferrin-bound iron uptake by human β-cells. Am. J. Physiol. Cell Physiol. 2017, 312, C169–C175. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Aydemir, F.; Nam, H.; Knutson, M.D.; Cousins, R.J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13612–13617. [Google Scholar] [CrossRef] [Green Version]
- Ji, C.; Kosman, D.J. Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J. Neurochem. 2015, 133, 668–683. [Google Scholar] [CrossRef] [Green Version]
- Oudit, G.Y.; Sun, H.; Trivieri, M.G.; Koch, S.E.; Dawood, F.; Ackerley, C.; Yazdanpanah, M.; Wilson, G.J.; Schwartz, A.; Liu, P.P.; et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat. Med. 2003, 9, 1187–1194. [Google Scholar] [CrossRef]
- Tsushima, R.G.; Wickenden, A.D.; Bouchard, R.A.; Oudit, G.Y.; Liu, P.P.; Backx, P.H. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: Possible implications in iron overload. Circ. Res. 1999, 84, 1302–1309. [Google Scholar] [CrossRef] [Green Version]
- Parkes, J.G.; Olivieri, N.F.; Templeton, D.M. Characterization of Fe2+ and Fe3+ transport by iron-loaded cardiac myocytes. Toxicology 1997, 117, 141–151. [Google Scholar] [CrossRef]
- Tulpule, K.; Robinson, S.R.; Bishop, G.M.; Dringen, R. Uptake of ferrous iron by cultured rat astrocytes. J. Neurosci. Res. 2010, 88, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Haldar, S.; Tripathi, A.; Qian, J.; Beserra, A.; Suda, S.; McElwee, M.; Turner, J.; Hopfer, U.; Singh, N. Prion protein promotes kidney iron uptake via its ferrireductase activity. J. Biol. Chem. 2015, 290, 5512–5522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, A.K.; Haldar, S.; Qian, J.; Beserra, A.; Suda, S.; Singh, A.; Hopfer, U.; Chen, S.G.; Garrick, M.D.; Turner, J.R.; et al. Prion protein functions as a ferrireductase partner for ZIP14 and DMT1. Free. Radic. Biol. Med. 2015, 84, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, Z.L.; Durley, A.P.; Man, T.K.; Gitlin, J.D. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc. Natl. Acad. Sci. USA 1999, 96, 10812–10817. [Google Scholar] [CrossRef] [Green Version]
- Vulpe, C.D.; Kuo, Y.M.; Murphy, T.L.; Cowley, L.; Askwith, C.; Libina, N.; Gitschier, J.; Anderson, G.J. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat. Genet. 1999, 21, 195–199. [Google Scholar] [CrossRef]
- Healy, J.; Tipton, K. Ceruloplasmin and what it might do. J. Neural Transm. 2007, 114, 777–781. [Google Scholar] [CrossRef]
- Crichton, R.R.; Pierre, J.L. Old iron, young copper: From Mars to Venus. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2001, 14, 99–112. [Google Scholar] [CrossRef] [Green Version]
- May, P.M.; Linder, P.W.; Williams, D.R. Computer simulation of metal-ion equilibria in biofluids: Models for the low-molecular-weight complex distribution of calcium(II), magnesium(II), manganese(II), iron(III), copper(II), zinc(II), and lead(II) ions in human blood plasma. J. Chem. Soc. Dalton Trans. 1977, 6, 588–595. [Google Scholar] [CrossRef]
- Grootveld, M.; Bell, J.D.; Halliwell, B.; Aruoma, O.I.; Bomford, A.; Sadler, P.J. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J. Biol. Chem. 1989, 264, 4417–4422. [Google Scholar] [CrossRef]
- Simpson, R.J.; Cooper, C.E.; Raja, K.B.; Halliwell, B.; Evans, P.J.; Aruoma, O.I.; Singh, S.; Konijn, A.M. Non-transferrin-bound iron species in the serum of hypotransferrinaemic mice. Biochim. Biophys. Acta 1992, 1156, 19–26. [Google Scholar] [CrossRef]
- Evans, R.W.; Rafique, R.; Zarea, A.; Rapisarda, C.; Cammack, R.; Evans, P.J.; Porter, J.B.; Hider, R.C. Nature of non-transferrin-bound iron: Studies on iron citrate complexes and thalassemic sera. J. Biol. Inorg. Chem. 2008, 13, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, S.; Lachowicz, J.I.; Nowakowski, M.E.; Jaremko, M.; Jaremko, Ł. Towards the functional high-resolution coordination chemistry of blood plasma human serum albumin. J. Inorg. Biochem. 2019, 198, 110716. [Google Scholar] [CrossRef] [PubMed]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C. Nature of nontransferrin-bound iron. Eur. J. Clin. Investig. 2002, 32, 50–54. [Google Scholar] [CrossRef]
- Silva, A.M.N.; Hider, R.C. Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation. Biochim. Biophys. Acta 2009, 1794, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef]
- Ariga, T.; Hazama, K.; Yanagisawa, S.; Yoneyama, T. Chemical forms of iron in xylem sap from graminaceous and non-graminaceous plants. Soil Sci. Plant Nutr. 2014, 60, 460–469. [Google Scholar] [CrossRef] [Green Version]
- Rellán-Alvarez, R.; Giner-Martínez-Sierra, J.; Orduna, J.; Orera, I.; Rodríguez-Castrillón, J.A.; García-Alonso, J.I.; Abadía, J.; Alvarez-Fernández, A. Identification of a tri-iron(III), tri-citrate complex in the xylem sap of iron-deficient tomato resupplied with iron: New insights into plant iron long-distance transport. Plant Cell Physiol. 2010, 51, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.M.N.; Kong, X.; Hider, R.C. Determination of the pKa value of the hydroxyl group in the alpha-hydroxycarboxylates citrate, malate and lactate by 13C NMR: Implications for metal coordination in biological systems. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2009, 22, 771–778. [Google Scholar] [CrossRef]
- Gautier-Luneau, I.; Merle, C.; Phanon, D.; Lebrun, C.; Biaso, F.; Serratrice, G.; Pierre, J.-L. New Trends in the Chemistry of Iron(III) Citrate Complexes: Correlations between X-ray Structures and Solution Species Probed by Electrospray Mass Spectrometry and Kinetics of Iron Uptake from Citrate by Iron Chelators. Chem.—Eur. J. 2005, 11, 2207–2219. [Google Scholar] [CrossRef]
- Bino, A.; Shweky, I.; Cohen, S.; Bauminger, E.R.; Lippard, S.J. A Novel Nonairon(III) Citrate Complex: A “Ferric Triple-Decker”. Inorg. Chem. 1998, 37, 5168–5172. [Google Scholar] [CrossRef]
- Matzapetakis, M.; Raptopoulou, C.P.; Tsohos, A.; Papaefthymiou, V.; Moon, N.; Salifoglou, A. Synthesis, Spectroscopic and Structural Characterization of the First Mononuclear, Water Soluble Iron−Citrate Complex, (NH4)5Fe(C6H4O7)2·2H2O. J. Am. Chem. Soc. 1998, 120, 13266–13267. [Google Scholar] [CrossRef]
- Shweky, I.; Bino, A.; Goldberg, D.P.; Lippard, S.J. Syntheses, Structures, and Magnetic Properties of Two Dinuclear Iron(III) Citrate Complexes. Inorg. Chem. 1994, 33, 5161–5162. [Google Scholar] [CrossRef]
- Fukushima, T.; Sia, A.K.; Allred, B.E.; Nichiporuk, R.; Zhou, Z.; Andersen, U.N.; Raymond, K.N. Bacillus cereus iron uptake protein fishes out an unstable ferric citrate trimer. Proc. Natl. Acad. Sci. USA 2012, 109, 16829–16834. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.M.N.; Kong, X.; Parkin, M.C.; Cammack, R.; Hider, R.C. Iron(III) citrate speciation in aqueous solution. Dalton Trans. 2009, 40, 8616–8625. [Google Scholar] [CrossRef]
- Yue, W.W.; Grizot, S.; Buchanan, S.K. Structural Evidence for Iron-free Citrate and Ferric Citrate Binding to the TonB-dependent Outer Membrane Transporter FecA. J. Mol. Biol. 2003, 332, 353–368. [Google Scholar] [CrossRef]
- Ferguson, A.D.; Chakraborty, R.; Smith, B.S.; Esser, L.; van der Helm, D.; Deisenhofer, J. Structural Basis of Gating by the Outer Membrane Transporter FecA. Science 2002, 295, 1715–1719. [Google Scholar] [CrossRef]
- Thorstensen, K.; Romslo, I. Uptake of iron from transferrin by isolated hepatocytes. Biochim. Biophys. Acta 1984, 804, 200–208. [Google Scholar] [CrossRef]
- Trinder, D.; Morgan, E. Mechanisms of ferric citrate uptake by human hepatoma cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 1998, 275, G279–G286. [Google Scholar] [CrossRef]
- Kosman, D.J. A holistic view of mammalian (vertebrate) cellular iron uptake. Metallomics 2020, 12, 1323–1334. [Google Scholar] [CrossRef]
- Sherman, H.G.; Jovanovic, C.; Stolnik, S.; Baronian, K.; Downard, A.J.; Rawson, F.J. New Perspectives on Iron Uptake in Eukaryotes. Front. Mol. Biosci. 2018, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Vukosav, P.; Mlakar, M.; Tomišić, V. Revision of iron(III)–citrate speciation in aqueous solution. Voltammetric and spectrophotometric studies. Anal. Chim. Acta 2012, 745, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Adam, F.I.; Bounds, P.L.; Kissner, R.; Koppenol, W.H. Redox properties and activity of iron-citrate complexes: Evidence for redox cycling. Chem. Res. Toxicol. 2015, 28, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Bal, W.; Sokołowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Sadler, P.J.; Tucker, A.; Viles, J.H. Involvement of a lysine residue in the N-terminal Ni2+ and Cu2+ binding site of serum albumins. Eur. J. Biochem. 1994, 220, 193–200. [Google Scholar] [CrossRef]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.T.; Blindauer, C.A. Albumin as a zinc carrier: Properties of its high-affinity zinc-binding site. Biochem. Soc. Trans. 2008, 36, 1317–1321. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.J.; Blindauer, C.A.; Berezenko, S.; Sleep, D.; Sadler, P.J. Interdomain zinc site on human albumin. Proc. Natl. Acad. Sci. USA 2003, 100, 3701. [Google Scholar] [CrossRef] [Green Version]
- Sadler, P.J.; Viles, J.H. 1H and (113)Cd NMR Investigations of Cd(2+) and Zn(2+) Binding Sites on Serum Albumin: Competition with Ca(2+), Ni(2+), Cu(2+), and Zn(2+). Inorg. Chem. 1996, 35, 4490–4496. [Google Scholar] [CrossRef]
- Goumakos, W.; Laussac, J.P.; Sarkar, B. Binding of cadmium(II) and zinc(II) to human and dog serum albumins. An equilibrium dialysis and 113Cd-NMR study. Biochem. Cell Biol. 1991, 69, 809–820. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Christodoulou, J.; Parkinson, J.A.; Barnham, K.J.; Tucker, A.; Woodrow, J.; Sadler, P.J. Cisplatin binding sites on human albumin. J. Biol. Chem. 1998, 273, 14721–14730. [Google Scholar] [CrossRef] [Green Version]
- Talib, J.; Beck, J.L.; Ralph, S.F. A mass spectrometric investigation of the binding of gold antiarthritic agents and the metabolite [Au(CN)2]− to human serum albumin. JBIC J. Biol. Inorg. Chem. 2006, 11, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, X.-P.; Chen; Xia, Y.-L.; Jiang, Y. Human Serum Albumin−Mercurial Species Interactions. J. Proteome Res. 2007, 6, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 Å resolution. Protein Eng. Des. Sel. 1999, 12, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Kragh-Hansen, U.; Vorum, H. Quantitative analyses of the interaction between calcium ions and human serum albumin. Clin. Chem. 1993, 39, 202–208. [Google Scholar] [CrossRef]
- Vorum, H.; Fisker, K.; Otagiri, M.; Pedersen, A.O.; Kragh-Hansen, U. Calcium ion binding to clinically relevant chemical modifications of human serum albumin. Clin. Chem. 1995, 41, 1654–1661. [Google Scholar] [CrossRef]
- Anghileri, L.J. Fate of intravenously injected iron compounds: Ferric-fructose complex, iron-EDTA, ferric hydroxide and iron-albumin labeled with 59Fe. Biochem. Pharmacol. 1967, 16, 2033–2036. [Google Scholar] [CrossRef]
- Loban, A.; Kime, R.; Powers, H. Iron-Binding Antioxidant Potential of Plasma Albumin. Clin. Sci. 1997, 93, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, L.; Shen, D.; Wu, H.; Liu, Q. Oxygen-dependent Oxidation of Fe(II) to Fe(III) and Interaction of Fe(III) with Bovine Serum Albumin, Leading to a Hysteretic Effect on the Fluorescence of Bovine Serum Albumin. J. Fluoresc. 2008, 18, 193–201. [Google Scholar] [CrossRef]
- Coddington, A.; Perkins, D.J. The binding of Fe+++ to native and chemically modified human serum albumin in the presence of sodium citrate. Biochim. Biophys. Acta 1960, 44, 361–363. [Google Scholar] [CrossRef]
- Løvstad, R.A. Interaction of serum albumin with the Fe(III)-citrate complex. Int. J. Biochem. 1993, 25, 1015–1017. [Google Scholar] [CrossRef]
- Spiro, T.G.; Pape, L.; Saltman, P. Hydrolytic polymerization of ferric citrate. I. Chemistry of the polymer. J. Am. Chem. Soc. 1967, 89, 5555–5559. [Google Scholar] [CrossRef]
- Singh, S.; Hider, R.C.; Porter, J.B. A direct method for quantification of non-transferrin-bound iron. Anal. Biochem. 1990, 186, 320–323. [Google Scholar] [CrossRef]
- Evans, P.J.; Halliwell, B. Measurement of iron and copper in biological systems: Bleomycin and copper-phenanthroline assays. Methods Enzymol. 1994, 233, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Podinovskaia, M.; Evans, P.J.; Emma, G.; Schaible, U.E.; Porter, J.; Hider, R.C. A novel method for non-transferrin-bound iron quantification by chelatable fluorescent beads based on flow cytometry. Biochem. J. 2014, 463, 351–362. [Google Scholar] [CrossRef]
- Garbowski, M.W.; Ma, Y.; Fucharoen, S.; Srichairatanakool, S.; Hider, R.; Porter, J.B. Clinical and methodological factors affecting non-transferrin-bound iron values using a novel fluorescent bead assay. Transl. Res. 2016, 177, 19–30.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, E.M.; Hendriks, J.C.; van Tits, B.L.; Evans, P.J.; Breuer, W.; Liu, D.Y.; Jansen, E.H.; Jauhiainen, K.; Sturm, B.; Porter, J.B.; et al. Results of an international round robin for the quantification of serum non-transferrin-bound iron: Need for defining standardization and a clinically relevant isoform. Anal. Biochem. 2005, 341, 241–250. [Google Scholar] [CrossRef]
- Fawwaz, R.A.; Winchell, H.S.; Pollycove, M.; Sargent, T. Hepatic Iron Deposition in Humans: I. First-Pass Hepatic Deposition of Intestinally Absorbed Iron in Patients with Low Plasma Latent Iron-Binding Capacity. Blood 1967, 30, 417–424. [Google Scholar] [CrossRef]
- Noetzli, L.J.; Papudesi, J.; Coates, T.D.; Wood, J.C. Pancreatic iron loading predicts cardiac iron loading in thalassemia major. Blood 2009, 114, 4021–4026. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, V.N.; McDonald, C.J.; Ostini, L.; Lusby, P.E.; Wockner, L.F.; Ramm, G.A.; Wallace, D.F. Hepatic iron deposition does not predict extrahepatic iron loading in mouse models of hereditary hemochromatosis. Am. J. Pathol. 2012, 181, 1173–1179. [Google Scholar] [CrossRef]
- Kishimoto, M.; Endo, H.; Hagiwara, S.; Miwa, A.; Noda, M. Immunohistochemical findings in the pancreatic islets of a patient with transfusional iron overload and diabetes: Case report. J. Med. Investig. 2010, 57, 345–349. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.-P.; Hayashi, K. Selective iron deposition in pancreatic islet B cells of transfusional iron-overloaded autopsy cases. Pathol. Int. 1994, 44, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Marku, A.; Galli, A.; Marciani, P.; Dule, N.; Perego, C.; Castagna, M. Iron Metabolism in Pancreatic Beta-Cell Function and Dysfunction. Cells 2021, 10, 2841. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Parkes, J.G.; Templeton, D.M. Differential accumulation of non-transferrin-bound iron by cardiac myocytes and fibroblasts. J. Mol. Cell. Cardiol. 2003, 35, 505–514. [Google Scholar] [CrossRef]
- Link, G.; Athias, P.; Grynberg, A.; Pinson, A.; Hershko, C. Effect of iron loading on transmembrane potential, contraction, and automaticity of rat ventricular muscle cells in culture. J. Lab. Clin. Med. 1989, 113, 103–111. [Google Scholar]
- Leoncini, S.; Rossi, V.; Signorini, C.; Tanganelli, I.; Comporti, M.; Ciccoli, L. Oxidative stress, erythrocyte ageing and plasma non-protein-bound iron in diabetic patients. Free Radic. Res. 2008, 42, 716–724. [Google Scholar] [CrossRef]
- Sulieman, M.; Asleh, R.; Cabantchik, Z.I.; Breuer, W.; Aronson, D.; Suleiman, A.; Miller-Lotan, R.; Hammerman, H.; Levy, A.P. Serum chelatable redox-active iron is an independent predictor of mortality after myocardial infarction in individuals with diabetes. Diabetes Care 2004, 27, 2730–2732. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Real, J.M.; Lopez-Bermejo, A.; Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef] [Green Version]
- Wlazlo, N.; van Greevenbroek, M.M.; Ferreira, I.; Jansen, E.H.; Feskens, E.J.; van der Kallen, C.J.; Schalkwijk, C.G.; Bravenboer, B.; Stehouwer, C.D. Iron metabolism is associated with adipocyte insulin resistance and plasma adiponectin: The Cohort on Diabetes and Atherosclerosis Maastricht (CODAM) study. Diabetes Care 2013, 36, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Wlazlo, N.; van Greevenbroek, M.M.; Ferreira, I.; Jansen, E.H.; Feskens, E.J.; van der Kallen, C.J.; Schalkwijk, C.G.; Bravenboer, B.; Stehouwer, C.D. Iron metabolism is prospectively associated with insulin resistance and glucose intolerance over a 7-year follow-up period: The CODAM study. Acta Diabetol. 2015, 52, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Ayton, S.; Wang, Y.; Diouf, I.; Schneider, J.A.; Brockman, J.; Morris, M.C.; Bush, A.I. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry 2020, 25, 2932–2941. [Google Scholar] [CrossRef]
- van Bergen, J.M.; Li, X.; Hua, J.; Schreiner, S.J.; Steininger, S.C.; Quevenco, F.C.; Wyss, M.; Gietl, A.F.; Treyer, V.; Leh, S.E.; et al. Colocalization of cerebral iron with Amyloid beta in Mild Cognitive Impairment. Sci. Rep. 2016, 6, 35514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everett, J.; Collingwood, J.F.; Tjendana-Tjhin, V.; Brooks, J.; Lermyte, F.; Plascencia-Villa, G.; Hands-Portman, I.; Dobson, J.; Perry, G.; Telling, N.D. Nanoscale synchrotron X-ray speciation of iron and calcium compounds in amyloid plaque cores from Alzheimer’s disease subjects. Nanoscale 2018, 10, 11782–11796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plascencia-Villa, G.; Ponce, A.; Collingwood, J.F.; Arellano-Jiménez, M.J.; Zhu, X.; Rogers, J.T.; Betancourt, I.; José-Yacamán, M.; Perry, G. High-resolution analytical imaging and electron holography of magnetite particles in amyloid cores of Alzheimer’s disease. Sci. Rep. 2016, 6, 24873. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Jeandriens, J.; Parkes, H.G.; So, P.-W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: Evidence of ferroptosis. Redox Biol. 2020, 32, 101494. [Google Scholar] [CrossRef]
- Jakaria, M.; Belaidi, A.A.; Bush, A.I.; Ayton, S. Ferroptosis as a mechanism of neurodegeneration in Alzheimer’s disease. J. Neurochem. 2021, 159, 804–825. [Google Scholar] [CrossRef]
- Dexter, D.T.; Wells, F.R.; Agid, F.; Agid, Y.; Lees, A.J.; Jenner, P.; Marsden, C.D. Increased nigral iron content in postmortem parkinsonian brain. Lancet 1987, 330, 1219–1220. [Google Scholar] [CrossRef]
- Dexter, D.T.; Wells, F.R.; Lees, A.J.; Agid, F.; Agid, Y.; Jenner, P.; Marsden, C.D. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J. Neurochem. 1989, 52, 1830–1836. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Brandel, J.P.; Galle, P.; Javoy-Agid, F.; Agid, Y. Iron and aluminum increase in the substantia nigra of patients with Parkinson’s disease: An X-ray microanalysis. J. Neurochem. 1991, 56, 446–451. [Google Scholar] [CrossRef]
- Kalpouzos, G.; Mangialasche, F.; Falahati, F.; Laukka, E.J.; Papenberg, G. Contributions of HFE polymorphisms to brain and blood iron load, and their links to cognitive and motor function in healthy adults. Neuropsychopharmacol. Rep. 2021, 41, 393–404. [Google Scholar] [CrossRef]
- Nandar, W.; Connor, J.R. HFE gene variants affect iron in the brain. J. Nutr. 2011, 141, 729s–739s. [Google Scholar] [CrossRef]
- Kaplan, J.; Craven, C.; Alexander, J.; Kushner, J.; Lamb, J.; Bernstein, S. Regulation of the Distribution of Tissue Iron. Ann. N. Y. Acad. Sci. 1988, 526, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Metafratzi, Z.; Argyropoulou, M.I.; Kiortsis, D.N.; Tsampoulas, C.; Chaliassos, N.; Efremidis, S.C. T2 relaxation rate of basal ganglia and cortex in patients with β-thalassaemia major. Br. J. Radiol. 2001, 74, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Chan, G.C.F.; Chu, J.; Chan, Q.; Ha, S.Y.; Moseley, M.E.; Khong, P.L. MR Quantitative susceptibility imaging for the evaluation of iron loading in the brains of patients with β-thalassemia major. Am. J. Neuroradiol. 2014, 35, 1085–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, A.K.; Karmakar, S.; Asthana, A.; Ashok, A.; Desai, V.; Baksi, S.; Singh, N. Transport of Non-Transferrin Bound Iron to the Brain: Implications for Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Bradbury, M.W.B. Transport of iron in the blood-brain-cerebrospinal fluid system. J. Neurochem. 1997, 69, 443–454. [Google Scholar] [CrossRef]
- Bishop, G.M.; Dang, T.N.; Dringen, R.; Robinson, S.R. Accumulation of Non-Transferrin-Bound Iron by Neurons, Astrocytes, and Microglia. Neurotox. Res. 2011, 19, 443–451. [Google Scholar] [CrossRef]
- Dziuba, N.; Hardy, J.; Lindahl, P.A. Low-molecular-mass iron in healthy blood plasma is not predominately ferric citrate. Metallomics 2018, 10, 802–817. [Google Scholar] [CrossRef]
- Løvstad, R.A. A kinetic study of the coupled iron-ceruloplasmin catalyzed oxidation of ascorbate in the presence of albumin. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 1995, 8, 328–331. [Google Scholar] [CrossRef]
- Matias, C.; Belnap, D.W.; Smith, M.T.; Stewart, M.G.; Torres, I.F.; Gross, A.J.; Watt, R.K. Citrate and albumin facilitate transferrin iron loading in the presence of phosphate. J. Inorg. Biochem. 2017, 168, 107–113. [Google Scholar] [CrossRef]
- Sohn, Y.S.; Ghoti, H.; Breuer, W.; Rachmilewitz, E.; Attar, S.; Weiss, G.; Cabantchik, Z.I. The role of endocytic pathways in cellular uptake of plasma non-transferrin iron. Haematologica 2012, 97, 670–678. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.M.N.; Rangel, M. The (Bio)Chemistry of Non-Transferrin-Bound Iron. Molecules 2022, 27, 1784. https://doi.org/10.3390/molecules27061784
Silva AMN, Rangel M. The (Bio)Chemistry of Non-Transferrin-Bound Iron. Molecules. 2022; 27(6):1784. https://doi.org/10.3390/molecules27061784
Chicago/Turabian StyleSilva, André M. N., and Maria Rangel. 2022. "The (Bio)Chemistry of Non-Transferrin-Bound Iron" Molecules 27, no. 6: 1784. https://doi.org/10.3390/molecules27061784
APA StyleSilva, A. M. N., & Rangel, M. (2022). The (Bio)Chemistry of Non-Transferrin-Bound Iron. Molecules, 27(6), 1784. https://doi.org/10.3390/molecules27061784






