Toxic Metal Species and ‘Endogenous’ Metalloproteins at the Blood–Organ Interface: Analytical and Bioinorganic Aspects
Abstract
1. Introduction
2. Metalloproteins Containing a Single Metal as Biomarker
2.1. Blood Plasma/Serum
2.1.1. Decreased Plasma Concentrations of Individual Metalloproteins
2.1.2. Increased Plasma Concentrations of Individual Metalloproteins
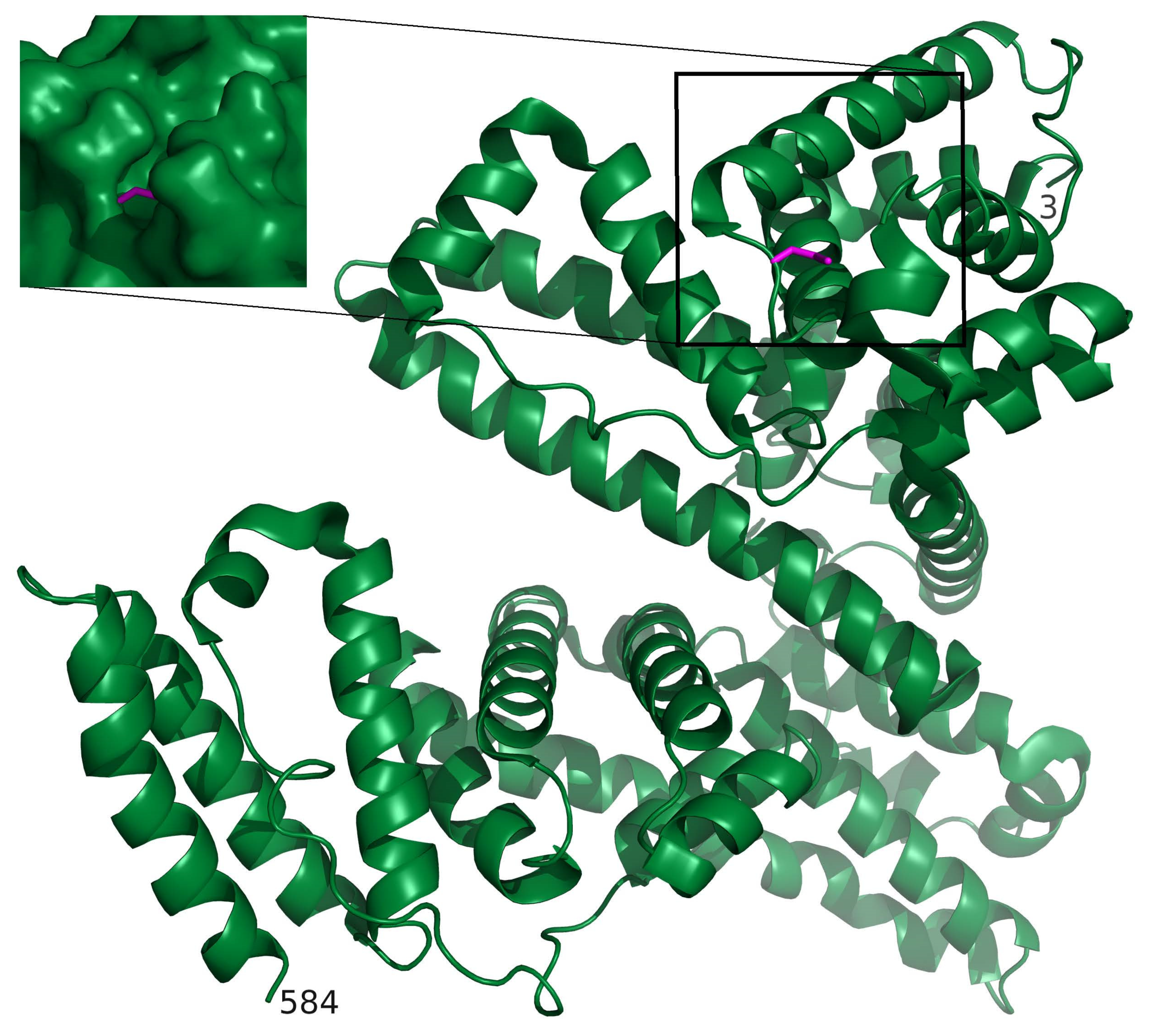
2.2. Red Blood Cells
3. Bi-/Trimetallic Complexes Which Contain Essential and Toxic Metals as Biomarkers
3.1. Blood Plasma/Serum
3.2. Red Blood Cells
4. Dynamic Interactions between Toxic Metal Species, Plasma Proteins and SMW Thiols
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacyna, J.M. Monitoring and assessment of metal contaminants in the air. In Toxicology of Metals; Chang, L.W., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 9–28. [Google Scholar]
- Izatt, R.M.; Izatt, S.R.; Bruening, R.L.; Izatt, N.E.; Moyer, B.A. Challenges to achievement of metal sustainability in our high-tech society. Chem. Soc. Rev. 2014, 43, 2451–2475. [Google Scholar] [CrossRef] [PubMed]
- Kirdyanov, A.V.; Krusic, P.J.; Shishov, V.V.; Vaganov, E.A.; Fertikov, A.I.; Myglan, V.S.; Barinov, V.V.; Brose, J.; Esper, J.; Ilyin, V.A.; et al. Ecological and conceptual consequences of Arctic pollution. Ecol. Lett. 2020, 23, 1827–1837. [Google Scholar] [CrossRef]
- Underwood, E.J. Trace metals in human and animal health. J. Hum. Nutr. 1981, 35, 37–48. [Google Scholar] [CrossRef]
- Bomer, N.; Beverborg, N.G.; Hoes, M.F.; Streng, K.W.; Vermeer, M.; Dokter, M.M.; Ijmker, J.; Anker, S.D.; Cleland, J.G.F.; Hillege, H.L.; et al. Selenium and outcome in heart failure. Eur. J. Heart Fail. 2019, 22, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, N.W.; Beauchemin, D. Simultaneous speciation analysis of arsenic, chromium and selenium in the bioaccessible fraction for realistic assessment of food safety. Anal. Chem. 2018, 257, 230–236. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Parker, D.R.; Clarke, J.M. Metals and micronutrients and food safety issues. Field Crop. Res. 1999, 60, 143–163. [Google Scholar] [CrossRef]
- Centers of Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals; Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2009. [Google Scholar]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Coulthard, T.J.; Macklin, M.G. Modeling long-term contamination in river systems from historical metal mining. Geology 2003, 31, 451–454. [Google Scholar] [CrossRef]
- Rodzik, A.; Pomastowski, P.; Sagandykova, G.M.; Buszewski, B. Interactions of whey proteins with metal ions. Int. J. Mol. Sci. 2020, 21, 2156. [Google Scholar] [CrossRef]
- Buszewski, B.; Žuvela, P.; Król-Górniak, A.; Railean-Plugaru, V.; Rogowska, A.; Wong, M.W.; Yi, M.; Rodzik, A.; Sprynskyy, M.; Pomastowski, P. Interactions of zinc aqua complexes with ovalbumin at the forfront of the Zn2+/ZnO-OVO hybrid complex formation mechanism. Appl. Surf. Sci. 2021, 542, 148641. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metal for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteom. 2002, 1, 845–867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quin, S.; Sun, M.J.; Tang, L.; Yan, X.W.; Kim, T.K.; Caballero, J.; Glusman, G.; Brunkov, M.E.; Soloski, M.J.; et al. Measurement of organ-specific and acute-phase blood protein levels in early lyme disease. J. Proteome Res. 2020, 19, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mu, X.; Zhang, J.; Huang, Q.; Alamdar, A.; Tian, M.; Liu, L.; Shen, H. Serum metabolites reveals that arsenic exposure disrupted lipid and amino acid metabolkism in rats: A step forward in understanding chronic arsenic toxicity. Metallomics 2015, 7, 544–552. [Google Scholar] [CrossRef]
- Dey, K.K.; Wang, H.; Niu, M.; Bai, B.; Wang, X.; Li, Y.; Cho, J.-H.; Tan, H.; Mishra, A.; High, A.A.; et al. Deep undepleted human serum proteome profiling toward biomarker discovery for Alzheimer’s disease. Clin. Proteom. 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Manley, S.A.; Gailer, J. Analysis of the plasma metalloproteome by SEC-ICP-AES: Bridging proteomics and metabolomics. Expert Rev. Proteom. 2009, 6, 251–265. [Google Scholar] [CrossRef]
- Shin, S.-Y.; Fauman, E.B.; Petersen, A.-K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; Yang, T.-P.; et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef]
- Lyon, T.D.B.; Aughey, E.; Scott, R.; Fell, G.S. Cadmium concentration in human kidney in the UK: 1978–1993. J. Environ. Monitor. 1999, 1, 227–231. [Google Scholar] [CrossRef]
- Hood, L. A systems approach to medicine will transform healthcare. In Physical Biology, from Atoms to Medicine; Zewail, A.H., Ed.; Imperial College Press: London, UK, 2008; pp. 337–366. [Google Scholar]
- Dede, E.; Tindall, M.J.; Cherrie, J.W.; Hankin, S.; Collins, C. Physiologically-based pharmacokinetic and toxicokinetic models for estimating human expaosure to five toxic elements through oral ingestion. Environ. Toxicol. Pharmacol. 2018, 57, 104–114. [Google Scholar] [CrossRef]
- Sarpong-Kumankomah, S.; Gailer, J. Application of a novel metallomics tool to probe the fate of metal-based anticancer drugs in blood plasma: Potential, challenges and prospects. Curr. Top. Med. Chem. 2021, 21, 48–58. [Google Scholar] [CrossRef]
- Sturla, S.J.; Boobis, A.R.; Fitzgerald, R.E.; Hoeng, J.; Kavlock, R.J.; Schirmer, K.; Whelan, M.; Wilks, M.F.; Peitsch, M.C. Systems toxicology: From basic research to risk assessment. Chem. Res. Toxicol. 2014, 27, 314–329. [Google Scholar] [CrossRef]
- Gomez-Ariza, J.L.; Jahromi, E.Z.; Gonzalez-Fernandez, M.; Garcia-Barrera, T.; Gailer, J. Liquid chromatography-inductively coupled plasma-based metallomic approaches to probe health-relevant interactions between xenobiotics and mammalian organisms. Metallomics 2011, 3, 566–577. [Google Scholar] [CrossRef]
- Craig, W.Y.; Ledue, T.B.; Ritchie, R.F. Plasma Proteins: Clinical Utility and Interpretation; Dade Behring Inc.: Newark, NJ, USA, 2000. [Google Scholar]
- Da Silva, M.A.O.; Sussulini, A.; Arruda, M.A.Z. Metalloproteomics as an interdisciplinary area involving proteins and metals. Expert Rev. Proteom. 2010, 7, 387–400. [Google Scholar] [CrossRef]
- Barnett, J.P.; Scanlan, D.J.; Blindauer, C.A. Protein fractionation and detection for metalloproteomics: Challenges and approaches. Anal. Bioanal. Chem. 2012, 402, 3311–3322. [Google Scholar] [CrossRef]
- Gailer, J. Arsenic-selenium and mercury-selenium bonds in biology. Coord. Chem. Rev. 2007, 251, 234–254. [Google Scholar] [CrossRef]
- Riuz-Laguna, J.; Garcia-Alfonso, C.; Peinado, J.; Moreno, S.; Leradi, L.A.; Cristaldi, M.; Lopez-Barea, J. Biochemical biomarkers of pollution in Algerian mouse (Mus spretus) to assess the effects of the Aznalcollar disaster on Donana Park (Spain). Biomarkers 2001, 6, 146–160. [Google Scholar]
- Sarpong-Kumankomah, S.; Miller, K.; Gailer, J. Biological chemistry of toxic metals and metalloids, such as arsenic, cadmium and mercury. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2020. [Google Scholar]
- Sussulini, A.; Becker, J.S.; Becker, J.S. Laser ablation ICP-MS: Application in biomedical research. Mass Spectrom. Rev. 2017, 36, 47–57. [Google Scholar] [CrossRef]
- Becker, J.S.; Zoriy, M.; Matusch, A.; Wu, B.; Salber, D.; Palm, C.; Becker, J.S. Bioimaging of metals by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Mass Spectrom. Rev. 2010, 29, 156–175. [Google Scholar] [CrossRef]
- Coufalikova, K.; Benesova, I.; Vaculovic, T.; Kanicky, V.; Preisler, J. LC coupled to ESI, MALDI and ICP MS—A multiple hypehnation for metallproteomic studies. Anal. Chim. Acta 2017, 968, 58–65. [Google Scholar] [CrossRef]
- Hill, A.; Gailer, J. Linking molecular targets of Cd in the bloodstream to organ-based adverse health effects. J. Inorg. Biochem. 2021, 217, 111279. [Google Scholar] [CrossRef]
- Manley, S.A.; Byrns, S.; Lyon, A.W.; Brown, P.; Gailer, J. Simultaneous Cu-, Fe-, and Zn-specific detection of metalloproteins contained in rabbit plasma by size-exclusion chromatography-inductively coupled plasma atomic emission spectroscopy. J. Biol. Inorg. Chem. 2009, 14, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Sarpong-Kumankomah, S.; Gailer, J. Identification of a haptoglobin-hemoglobin complex in human blood plasma. J. Inorg. Biochem. 2019, 201, 110802. [Google Scholar] [CrossRef]
- Broman, L. Separation and characterization of two ceruloplasmins from human serum. Nature 1958, 182, 1655. [Google Scholar] [CrossRef]
- Morgan, E.H. Exchange of iron and transferrin across endothelial surfaces in rat and rabbit. J. Physiol. 1963, 169, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, I.H.; Gitlin, D. Deficiency of ceruloplasmin in patients with hepatolenticular degeneration (Wilson’s Disease). Science 1952, 116, 484–489. [Google Scholar] [CrossRef]
- Rouault, T.A. The role of regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Campbell, C.H.; Brown, R.; Linder, M.C. Circulating ceruloplasmin is an important source of copper for normal and malignant animal cells. Biochim. Biophys. Acta 1981, 678, 27–38. [Google Scholar] [CrossRef]
- George, G.N.; Pickering, I.J.; Harris, H.H.; Gailer, J.; Klein, D.; Lichtmannegger, J.; Summer, K.-H. Tetrathiomolybdate causes formation of hepatic copper-molybdenum clusters in an animal model of Wilson’s Disease. J. Am. Chem. Soc. 2003, 125, 1704–1705. [Google Scholar] [CrossRef]
- Bull, P.C.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef]
- Bonhorst, J.A.; Kipp, A.P.; Haase, H.; Meyer, S.; Schwerdtle, T. The crux of inept biomarkers for risks and benefits of trace elements. Trends Anal. Chem. 2018, 104, 183–190. [Google Scholar] [CrossRef]
- Cannon, W.B. Organization for physiological homeostasis. Physiol. Rev. 1929, 9, 399–431. [Google Scholar] [CrossRef]
- Coverdale, J.P.C.; Barnett, J.P.; Adamu, A.H.; Griffiths, E.J.; Stewart, A.J.; Blindauer, C.A. A metalloproteomic analysis of interactions between plasma proteins and zinc: Elevated fatty acid levels affect zinc distribution. Metallomics 2019, 11, 1805–1819. [Google Scholar] [CrossRef]
- Kimura, F.; Hasegawa, G.; Obayasji, H.; Adachi, T.; Hara, H.; Ohta, T.; Fukui, M.; Kitagawa, Y.; Park, H.; Nakamura, N.; et al. Serum extracellular superoxide dismutase in patients with type 2 diabetes. Diabetes Care 2003, 26, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Inoue, M.; Maehata, E.; Suzuki, S. Relationship of plasma extracellular-superoxide dismutase level with insulin resistance in type 2 diabetic patients. J. Endocrinol. 2004, 181, 413–417. [Google Scholar] [CrossRef]
- Adachi, T.; Wang, J.; Wang, X.L. Age-related change of plasma extracellular-superoxide dismutase. Clin. Chim. Acta 2000, 290, 169–178. [Google Scholar] [CrossRef]
- Yamamoto, M.; Hara, H.; Adachi, T. Effects of homocysteine on the binding of extracellular-superoxide dismutase to the endothelial cell surface. FEBS Lett. 2000, 486, 159–162. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, R.; Garcia-Barrera, T.; Gomez-Ariza, J.L. Characterization of metal profiles in serum during the progression of Alzheimer’s disease. Metallomics 2014, 6, 292–300. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, I.; Zhao, Q.H.; Jiang, R.; Gong, S.G.; Jiang, X.; Xu, X.Q.; He, Y.Y.; Li, Y.; Jing, Z.C. Alteration of extracellular superoxide dismutase in idiopathic pulmonary arterial hypertension. Front. Med. 2020, 7, 509. [Google Scholar] [CrossRef]
- Scassellati, C.; Bonvicini, C.; Benussi, L.; Ghidoni, R.; Squitti, R. Nejurodevelopmental disorders: Metallomics studies for the identification of potential biomarkersassociated to diagnostics and treatment. J. Trace Elem. Med. Biol. 2020, 60, 125499. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Bush, A.I. Metals and Alzheimer’s disease. J. Alzheimer’s Dis. 2006, 10, 145–163. [Google Scholar] [CrossRef]
- Hood, L.; Heath, J.R.; Phelps, M.E.; Lin, B. Systems biology and new technologies enable predictive and preventative medicine. Science 2004, 306, 640–643. [Google Scholar] [CrossRef]
- Price, N.D.; Magis, A.T.; Earls, J.C.; Glusman, G.; Levy, R.; Lausted, C.; McDonald, D.T.; Kuseback, U.; Moss, C.L.; Zhou, Y.; et al. A wellness study of 108 individuals using personal, dense, dynamic data clouds. Nat. Biotechnol. 2017, 35, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Au, F.; Bielecki, A.; Blais, E.; Fisher, M.; Cakmak, S.; Basak, A.; Gomes, J.; Arbuckle, T.E.; Fraser, W.D.; Vincent, R.; et al. Blood metal levels and third trimester maternal plasma matrix metalloproteinases (MMPs). Chemosphere 2016, 159, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Chen, D.C.; Wang, L.; Zhang, X.Y. Sex differences in the association between symptoms and superoxide dismutasein patients with never-treated first-episode schizophrenia. World J. Biol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Garcia-Sevilliano, M.A.; Garcia-Barrera, T.; Navarro, F.; Gomez-Ariza, J.L. Cadmium toxicity in Mus musculus mice based on a metallomic study. Antagonistic interaction between Se and Cd in the bloodstream. Metallomics 2014, 6, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Barea, J.; Gomez-Ariza, J.L. Environmental proteomics and metallomics. Proteomics 2006, 6, S51–S62. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Yamano, Y.; Yamanaka, K.; Hata, A.; Nakadate, T.; Kuroda, Y.; Endo, Y.; Endo, G. Possible production of arsenic hemoglobin adducts via exposure to arsine. J. Occup. Health 2015, 57, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kumarathasan, K.; Gomes, J. Infant and mother related outcomes from exposure to metals with endocrine disrupting properties during pregnancy. Sci. Total Environ. 2016, 569, 1022–1031. [Google Scholar] [CrossRef]
- Gomes, J.; Au, F.; Basak, A.; Cakmak, S.; Vincent, R.; Kumarathasan, P. Maternal blood biomarkers and adverse pregnancy outcomes: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2019, 49, 461–478. [Google Scholar] [CrossRef]
- Harding, A. Panning the proteome for biomarker gold. Science 2012, 337, 1120–1122. [Google Scholar] [CrossRef][Green Version]
- Martelli, A.; Rousselet, E.; Dycke, C.; Bouron, A.; Moulis, J.M. Cadmium toxicity in animal cells by interference with essential elements. Biochimie 2006, 88, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Sarpong-Kumankomah, S.; Gibson, M.A.; Gailer, J. Organ damage by toxic metals is critically determined by the bloodstream. Coord. Chem. Rev. 2018, 374, 376–386. [Google Scholar] [CrossRef]
- Xu, F.F.; Imlay, J.A. Silver(I), mercury(II), cadmium(II) and Zn(II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia Coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. [Google Scholar] [CrossRef] [PubMed]
- Begg, S.L.; Eijkelkamp, B.A.; Luo, Z.; Counago, R.M.; Morey, J.R.; Maher, M.J.; Ong, C.-I.Y.; McEwan, A.G.; Kobe, B.; O’Mara, M.L.; et al. Dysregulation of transition metal ion homeostasis is the molecular basis for cadmium toxicity in Streptococcus pneumoniae. Nat. Commun. 2015, 6, 6418. [Google Scholar] [CrossRef]
- Zalups, R.K. Molecular interactions with mercury in the kidney. Pharmacol. Rev. 2000, 52, 113–143. [Google Scholar]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein. The multipurpose protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef]
- O’Halloran, T.V.; Culotta, V.C. Metallochaperones, an intracellular shuttle service for metal ions. J. Biol. Chem. 2000, 275, 25057–25060. [Google Scholar] [CrossRef]
- Garcia-Sevilliano, M.A.; Garcia-Barrea, T.; Navarro, F.; Gomez-Ariza, J.L. Absolute quantification of superoxide dismutase in cytosol and mitochondria of mice hepatic cells expsoed to mercury by a novel metallomic approach. Anal. Chim. Acta 2014, 842, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sevilliano, M.A.; Rodriguesz-Moro, G.; Garcia-Barrea, T.; Navarro, F.; Gomez-Ariza, J.L. Biological interactions between mercury and seleniumin distribution and detoxifiction processes in mice under controlled exposure. Effects on selenoprotein. Chem. Biol. Interact. 2015, 229, 82–90. [Google Scholar] [CrossRef]
- Usuki, F.; Fujimura, M. Decreased plasma thiol antioxidant barrier amd selenoproteins as potential biomarkers for ongoing methylmercury intoxication and an individual protective capacity. Arch. Toxicol. 2016, 90, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B.; Pretorius, E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics 2014, 6, 748–773. [Google Scholar] [CrossRef]
- Pur, M.R.K.; Hosseini, M.; Faridbod, F.; Ganjali, M.R. Highly sensitive label-free electrochemoluminescence aptasensor for early detection of myoglobin, a biomarker for myocardial infarction. Microchim. Acta 2017, 184, 3529–3537. [Google Scholar] [CrossRef]
- Lang, F.; Gulbins, E.; Lerche, H.; Huber, S.M.; Kempe, D.S.; Foeller, M. Eryptosis, a window to sytemic disease. Cell Physiol. Biochem. 2008, 22, 373–380. [Google Scholar] [CrossRef]
- Miller, K.; Sarpong-Kumankomah, S.; Egorov, A.; Gailer, J. Sample preparation of blood plasma enables baseline separation of iron metalloproteins by SEC-GFAAS. J. Chromatogr. B 2020, 1147, 122147. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; DeZern, A.E.; Kinoshita, T.; Brodsky, R.A. Paroxysmal nocturnal haemoglobinuria. Nat. Rev. Dis. Primers 2017, 3, 1–14. [Google Scholar] [CrossRef]
- Gailer, J. Probing the bioinorganic chemistry of toxic metals in the mammalian bloodstream to advance human health. J. Inorg. Biochem. 2012, 108, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Moitra, S.; Brashier, B.B.; Sahu, S. Occupational cadmium exposure-associated oxidative stress and erythrocyte fragility among jewelry workers in India. Am. J. Ind. Med. 2014, 57, 1064–1072. [Google Scholar] [CrossRef]
- Ordonez, Y.N.; Montes-Bayon, M.; Blanco-Gonzalez, E.; Sanz-Medel, A. Quantitative analysis and simultaneous activity measurements of Cu, Zn-superoxide dismutase in red blood cells by HPLC-ICPMS. Anal. Chem. 2010, 82, 2387–2394. [Google Scholar] [CrossRef]
- Pomazal, K.; Prohaska, C.; Steffan, I.; Reich, G.; Huber, J.F.K. Determination of Cu, Fe, Mn, and Zn in blood fractions by SEC-HPLC-ICP-AES coupling. Analyst 1999, 124, 657–663. [Google Scholar] [CrossRef]
- Gibson, M.A.; Sarpong-Kumankomah, S.; Nehzati, S.; George, G.N.; Gailer, J. Remarkable differences in the biochemical fate of Cd2+, Hg2+, CH3Hg+ and thimerosal in red blood cell lysate. Metallomics 2017, 9, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, E.Z.; Gailer, J. Probing bioinorganic chemistry processes in the bloodstream to gain new insights into the origin of human diseases. Dalton Trans. 2010, 39, 329–336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warren, M.J.; Cooper, J.B.; Wood, S.P.; Shoolingin-Jordan, P.M. Lead poisoning, haem synthesis and 5-aminolaevulinic acid dehydratase. TIBS 1998, 23, 217–221. [Google Scholar] [CrossRef]
- Lamola, A.A.; Yamane, T. Zn protoporphyrin in the erythrocytes of patients with lead intoxication and iron deficiency anemia. Science 1974, 186, 936–938. [Google Scholar] [CrossRef]
- Gonick, H.C. Lead-binding proteins: A review. J. Toxicol. 2011, 2011, 686050. [Google Scholar] [CrossRef]
- Burk, R.F.; Foster, K.A.; Greenfield, P.M.; Kiker, K.W. Binding of simultaneously administered inorganic selenium and mercury to a rat plasma protein. Proc. Soc. Exp. Biol. Med. 1974, 145, 782–785. [Google Scholar] [CrossRef]
- Sasakura, C.; Suzuki, K.T. Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J. Inorg. Biochem. 1998, 71, 159–162. [Google Scholar] [CrossRef]
- Gailer, J.; George, G.N.; Pickering, I.J.; Madden, S.; Prince, R.C.; Yu, E.Y.; Denton, M.B.; Younis, H.S.; Aposhian, H.V. Structural basis of the antagonism between inorganic mercury and selenium in mammals. Chem. Res. Toxicol. 2000, 13, 1135–1142. [Google Scholar] [CrossRef]
- Suzuki, K.T.; Sasakura, C.; Yoneda, S. Binding sites for the (Hg-Se) complex on selenoprotein P. Biochim. Biophys. Acta 1998, 1429, 102–112. [Google Scholar] [CrossRef]
- Khan, M.A.K.; Wang, F. Mercury-selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury-selenium antagonism. Environ. Toxicol. Chem. 2009, 28, 1567–1577. [Google Scholar] [CrossRef]
- Cherdwongcharoensuk, D.; Oliveira, M.J.; Aguas, A.P. In vivo formation and binding of SeHg complexes to the erythrocyte surface. Biol. Trace Elem. Res. 2010, 136, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.K.; Wang, F. Reversible dossolution of glutathione-mediated HgSexS1−x nanoparticles and possible significance in Hg-Se antagonism. Chem. Res. Toxicol. 2009, 22, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.N.; Schindler, D.W.; St. Louis, V.L.; Donald, D.B.; Vladicka, K.E. Forest fire increases mercury accumulation by fishes via food web restructuring and increased mercury inputs. Proc. Natl. Acad. Sci. USA 2006, 103, 19380–19385. [Google Scholar] [CrossRef] [PubMed]
- LeMasters, G.K.; Genaidy, A.M.; Succop, P.; Deddens, J.; Sobeih, T.; Barriera-Viruet, H.; Dunning, K.; Lockey, J. Cancer risk among firefighters: A review and metal-analysis of 32 studies. J. Occup. Environ. Med. 2006, 48, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Manley, S.A.; George, G.N.; Pickering, I.J.; Glass, R.S.; Prenner, E.J.; Yamdagni, R.; Wu, Q.; Gailer, J. The seleno bis (S-glutathionyl) arsinium ion is assembled in erythrocyte lysate. Chem. Res. Toxicol. 2006, 19, 601–607. [Google Scholar] [CrossRef]
- Korbas, M.; Percy, A.J.; Gailer, J.; George, G.N. A possible molecular link between the toxicological effects of arsenic, selenium and methylmercury: Methylmercury(II) seleno bis (S-glutathionyl) arsenic(III). J. Biol. Inorg. Chem. 2008, 13, 461–470. [Google Scholar] [CrossRef]
- De Magalhanes Silva, M.; de Araujo Dantas, M.D.; de Sila Filho, R.C.; dos Santos Sales, M.V.; de Almeida Xavier, J.; Leite, A.C.R.; Goulart, M.O.F.; Grillo, L.A.M.; de Barros, W.A.; de Fatima, A.; et al. Toxicity of thimerosal in biological systems: Conformational changes in human hemoglobin, decrease of oxygen binding, increase of protein glycation and amyloid formation. Intern. J. Biol. Macromol. 2020, 154, 661–671. [Google Scholar] [CrossRef]
- Thomas, D.J.; Smith, J.C. Effects of coadministered low-molecular weight thiol compounds on short-term distribution of methylmercury in the rat. Toxicol. Appl. Pharmacol. 1982, 62, 104–110. [Google Scholar] [CrossRef]
- Simmons-Wills, T.A.; Koh, A.S.; Clarkson, T.W. Transport of a neurotoxicant by molecualr mimicry: The methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT 2. Biochem. J. 2002, 367, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef]
- Huang, J.; Williams, M. Total plasma homocysteine determination using ion-exchange chromatography. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 3143–3153. [Google Scholar] [CrossRef]
- Sagmeister, P.; Gibson, M.A.; McDade, K.H.; Gailer, J. Physiologically relevant plasma D, L-homocysteine concentrations mobilize Cd from human serum albumin. J. Chromatogr. B 2016, 1027, 181–186. [Google Scholar] [CrossRef]
- Araujo, J.E.; Jorge, S.; Santos, H.M.; Chiechi, A.; Galstyan, A.; Lodeiro, C.; Diniz, M.; Kleinman, M.T.; Ljubimova, J.Y.; Capelo, J.L. Proteomic changes driven by urban pollution suggest particulate matter as a deregulator of energy metabolism, mitochondrial activity, and oxidative pathways in the rat brain. Sci. Total Environ. 2019, 687, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, R.E.; Singh, S.P.; Doonan, C.J.; Ross, A.R.S.; Hughes, R.J.; Pickering, I.J.; George, G.N. Molecular mimicry in mercury toxicology. Chem. Res. Toxicol. 2006, 19, 753–759. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skaklny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sylfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef]
- Rabenstein, D.L.; Evans, C.A. The mobility of methylmercury in biological systems. Bioinorg. Chem. 1978, 8, 107–114. [Google Scholar] [CrossRef]
- Nogara, P.A.; Oliveira, C.S.; Schmitz, G.L.; Piquini, P.C.; Farina, M.; Aschner, M.; Rocha, J.B.T. Methylmercury’s chemistry: From the environment to the mammalian brain. Biochim. Biophys. Acta 2019, 1863, 129284. [Google Scholar] [CrossRef]
- Ballatori, N.; Lieberman, M.W.; Wang, W. N-acetylcysteine as an antidote in methylmercury poisoning. Environ. Health Perspect. 1998, 106, 267–271. [Google Scholar] [CrossRef]
- Madejczyk, M.S.; Aremu, D.A.; Simmons-Willis, T.A.; Clarkson, T.W.; Ballatori, N. Accelerated urinary excretion of methylmercury following administration of its antidote N-acetylcysteine requires Mrp2/Abcc2, the apical multidrug resistance-associated protein. J. Pharmacol. Exp. Ther. 2007, 322, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.S.; Simmons-Willis, T.A.; Pritchard, J.B.; Grassl, S.M.; Ballatori, N. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: Transport by the renal organic anion transporter-1. Mol. Pharmacol. 2002, 62, 921–926. [Google Scholar] [CrossRef]
- Campbell, P.G.C.; Gailer, J. Effects of Non-essential Metal Releases on the Environment and Human Health. In Metal Sustainability: Global Challenges, Consequences and Prospects; Izatt, R.M., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2016; pp. 221–252. [Google Scholar]
- Charlet, L.; Chapron, Y.; Faller, P.; Kirsch, R.; Stone, A.T.; Baveye, P.C. Neurodegenerative diseases and exposure to the environmental metals Mn, Pb, and Hg. Coord. Chem. Rev. 2012, 256, 2147–2163. [Google Scholar] [CrossRef]
- Korbas, M.; O’Donoghue, J.L.; Watson, G.E.; Pickering, I.J.; Singh, S.P.; Myers, G.J.; Clarkson, T.W.; George, G.N. The chemical form of mercury in human brain following poisoning or environmental exposure. ACS Chem. Neurosci. 2010, 1, 810–818. [Google Scholar] [CrossRef]
- Im, J.-Y.; Paik, S.-G.; Han, P.-L. Cadmium-induced astroglial death proceeds via glutathione depletion. J. Neurosci. Res. 2006, 83, 301–308. [Google Scholar] [CrossRef]
- Farina, M.; Rocha, J.B.T.; Aschner, M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011, 89, 555–563. [Google Scholar] [CrossRef]
- Costa, L.G.; Aschner, M.; Vitalone, A.; Syversen, T.; Soldin, O.P. Developmental neuropathology of environmental agents. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 87–110. [Google Scholar] [CrossRef]
- Bjorklund, G.; Tinkov, A.A.; Dadar, M.; Rahman, M.M.; Chirumbolo, S.; Skalny, A.V.; Skalnaya, M.G.; Haley, B.E.; Ajsuvakova, O.P.; Aaseth, J. Insights into the potential role of mercury in Alzheimer’s disease. J. Mol. Neurosci. 2019, 67, 511–533. [Google Scholar] [CrossRef]
- Jude, K.M.; Banerjee, A.L.; Haldar, M.K.; Manokaran, S.; Roy, B.; Mallik, S.; Srivastava, D.K.; Christianson, D.W. Ultrahigh resolution crystal structures of human carbonic anhydrase I and II complexed with two-prong inhibitors reveal the molecular basis of high affinity. J. Am. Chem. Soc. 2006, 128, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ning, T.; Xie, T.; Qui, Q.; Zhang, L.; Sun, Y.; Jiang, D.; Fu, K.; Yin, E.; Zhang, W.; et al. Large-scale production of functional human serum albumin from transgenic rice seeds. Proc. Natl. Acad. Sci. USA 2011, 108, 19078–19083. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-Model and the Swiss-PdbViewer: An environment for comparative protein modelling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Balde, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Trasande, L.; Liu, Y. Reducing the staggering costs of environmental disease in children, estimated at $76.6 billion in 2008. Health Aff. 2011, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Prasad, M.N.V. Cadmium minimization in rice: A review. Agron. Sustain. Dev. 2014, 34, 155–173. [Google Scholar] [CrossRef]
- Puschenreiter, M.; Horak, O.; Friesl, W.; Hartl, W. Low-cost agricultural measures to reduce heavy metal transfer to the food chain—A review. Plant Soil Environ. 2005, 51, 1–11. [Google Scholar] [CrossRef]
- Lew, T.T.S.; Park, M.; Cui, J.; Strano, M.S. Plant nanobionic sensors for arsenic detection. Adv. Mater. 2020, 33, 2005683. [Google Scholar] [CrossRef] [PubMed]
- Nachman, K.E.; Punshon, T.; Rardin, L.; Signes-Pastor, A.J.; Murray, C.J.L.; Jackson, B.P.; Guerinot, M.L.; Burke, T.A.; Chen, C.Y.; Ahsan, H.; et al. Opportunities and challenges for dietary arsenic intervention. Environ. Health Perspect. 2018, 126, 084503. [Google Scholar] [CrossRef]
- Orr, S.E.; Bridges, C.C. Chronic kidney disease and exposure to nephrotoxic metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar]
- Weiss, B.; Clarkson, T.W.; Simon, W. Silent latency period in methylmercury poisoning and in neurodegenerative disease. Environ. Health Perspect. 2002, 110, 851–854. [Google Scholar] [CrossRef]
- Marth, J.D. A unified vision of the building blocks of life. Nat. Cell Biol. 2008, 10, 1015–1016. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Genius, S.J.; Frye, R.E. Environmental toxicants and autism spectrum disorders: A systematic review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef]
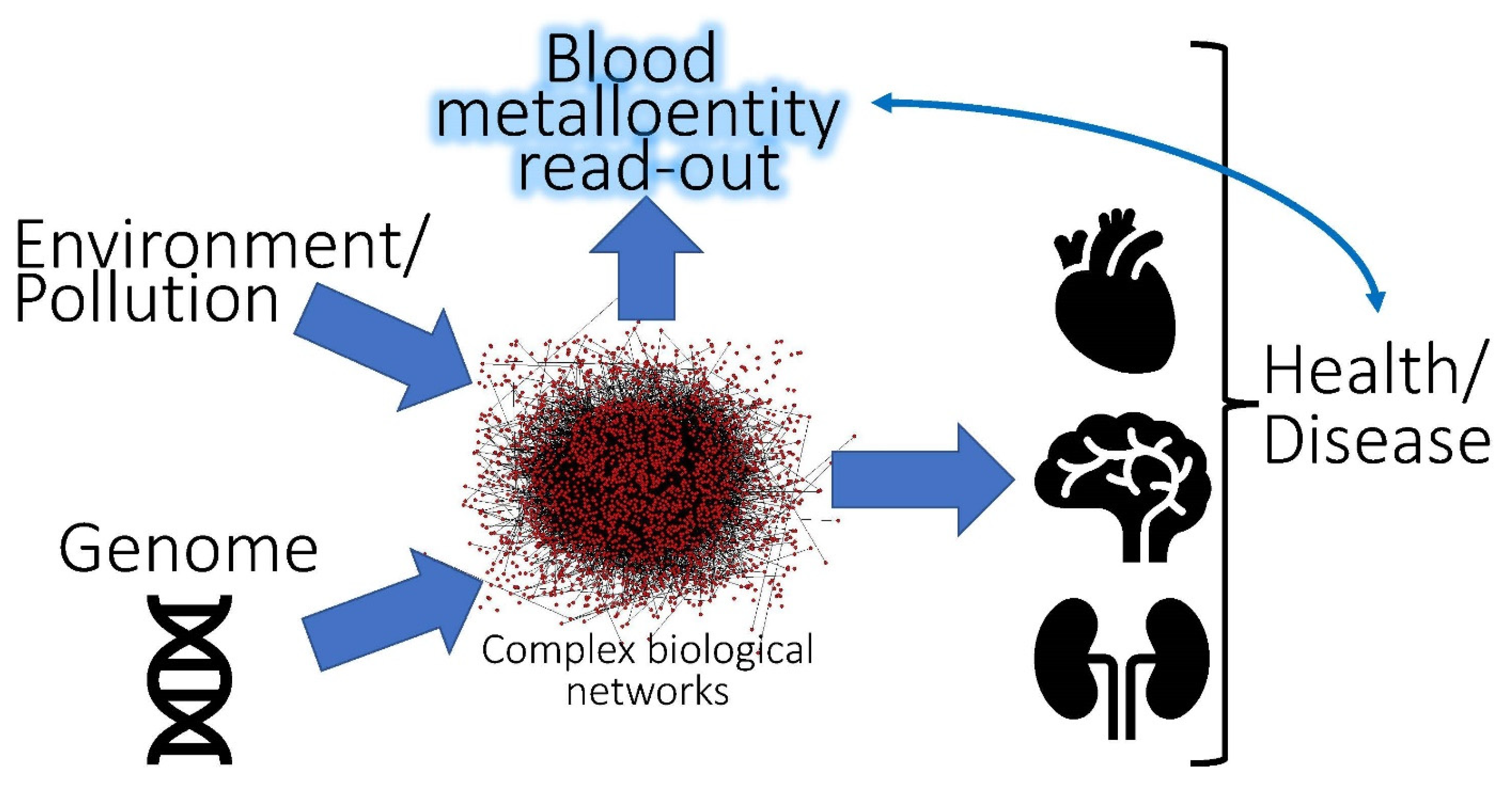
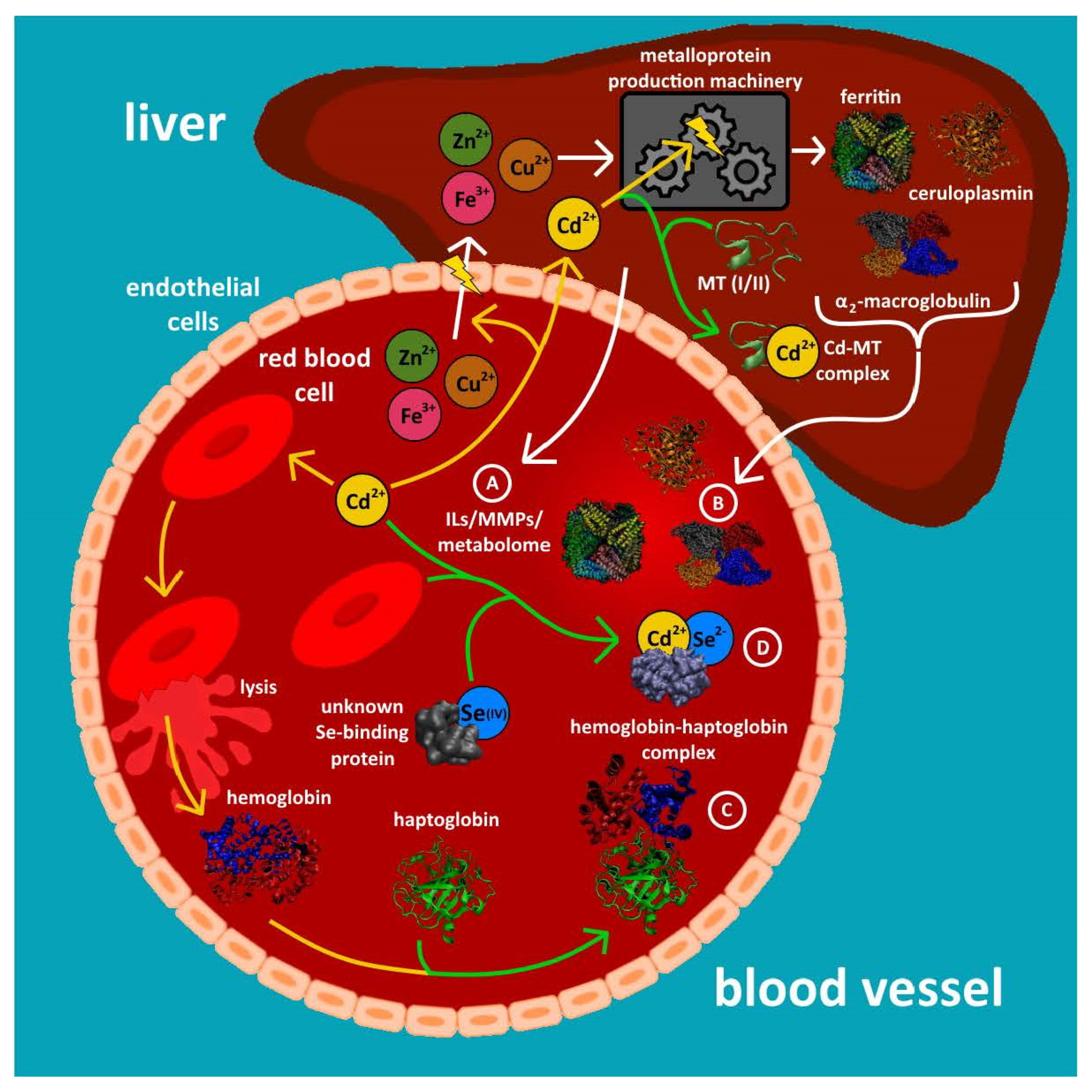
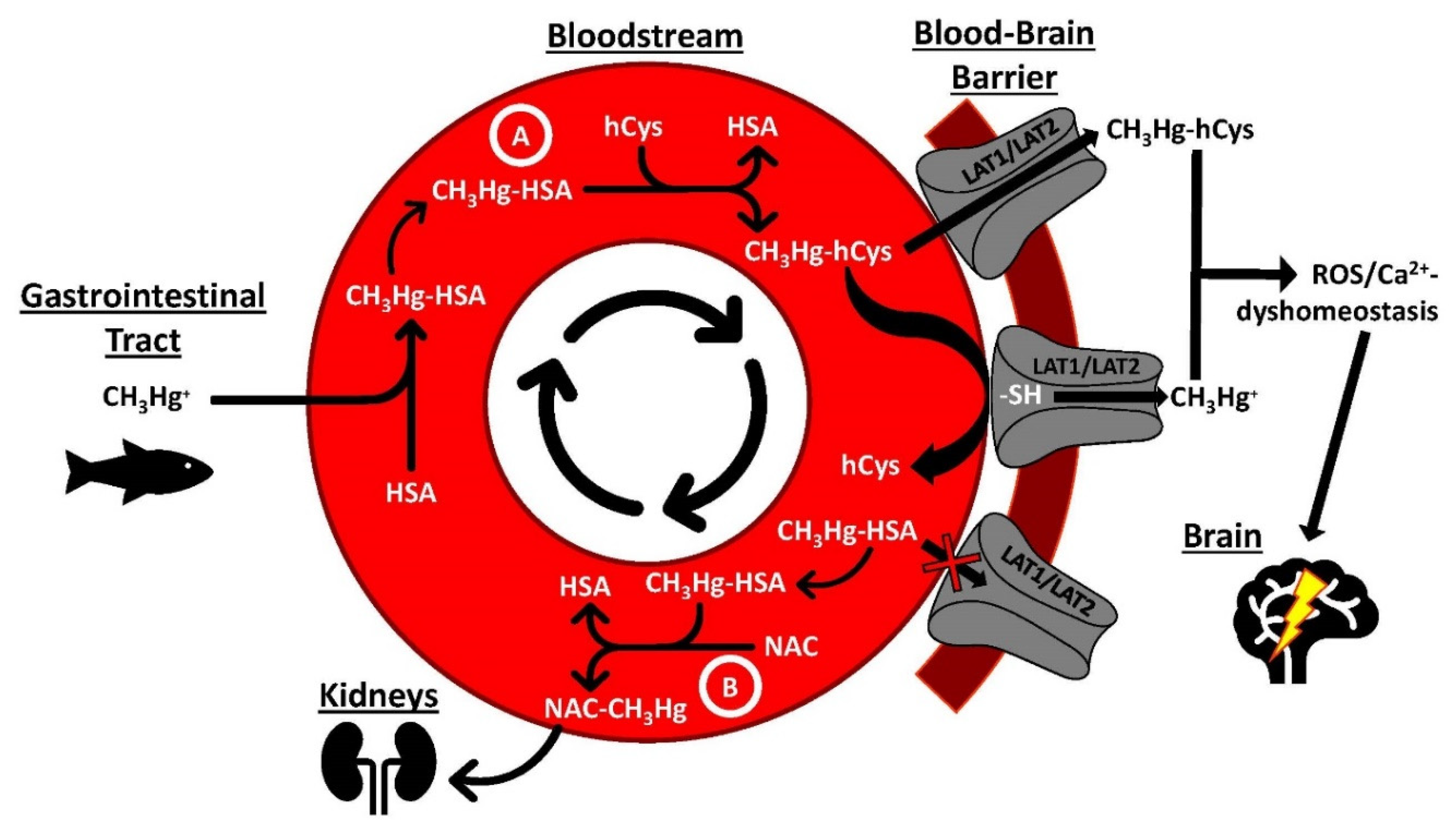
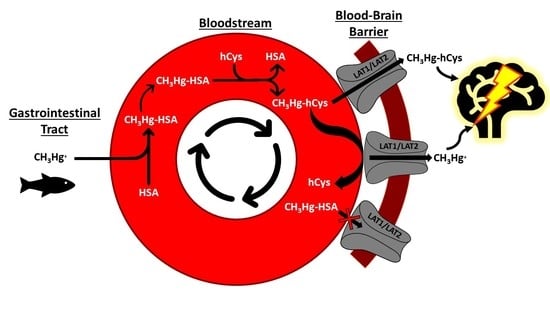
| Metal | Metalloprotein or Entity Which Contains Bound Metal | Molecular Mass (kDa) | Number of Metal Atoms Bound per Protein | Reference |
|---|---|---|---|---|
| Fe | Ferritin | 450 | <4500 | [36] |
| Transferrin | 79.9 | 2 | [36] | |
| Haptoglobin–hemoglobin complex | 86–900 | 2 | [37] | |
| Cu | Blood coagulation factor V | 330 | 1 | [36] |
| Transcuprein | 270 | 0.5 | [36] | |
| Ceruloplasmin | 132 | 6 | [36] | |
| Albumin | 66 | 1 | [36] | |
| Extracellular superoxide dismutase | 165 | 4 | [36] | |
| Peptides and amino acids | <5 | - | [36] | |
| Zn | α2 Macroglobulin | 725 | 5 | [36] |
| Albumin | 66 | 1 | [36] | |
| Extracellular superoxide dismutase | 165 | 4 | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bridle, T.G.; Kumarathasan, P.; Gailer, J. Toxic Metal Species and ‘Endogenous’ Metalloproteins at the Blood–Organ Interface: Analytical and Bioinorganic Aspects. Molecules 2021, 26, 3408. https://doi.org/10.3390/molecules26113408
Bridle TG, Kumarathasan P, Gailer J. Toxic Metal Species and ‘Endogenous’ Metalloproteins at the Blood–Organ Interface: Analytical and Bioinorganic Aspects. Molecules. 2021; 26(11):3408. https://doi.org/10.3390/molecules26113408
Chicago/Turabian StyleBridle, Tristen G., Premkumari Kumarathasan, and Jürgen Gailer. 2021. "Toxic Metal Species and ‘Endogenous’ Metalloproteins at the Blood–Organ Interface: Analytical and Bioinorganic Aspects" Molecules 26, no. 11: 3408. https://doi.org/10.3390/molecules26113408
APA StyleBridle, T. G., Kumarathasan, P., & Gailer, J. (2021). Toxic Metal Species and ‘Endogenous’ Metalloproteins at the Blood–Organ Interface: Analytical and Bioinorganic Aspects. Molecules, 26(11), 3408. https://doi.org/10.3390/molecules26113408