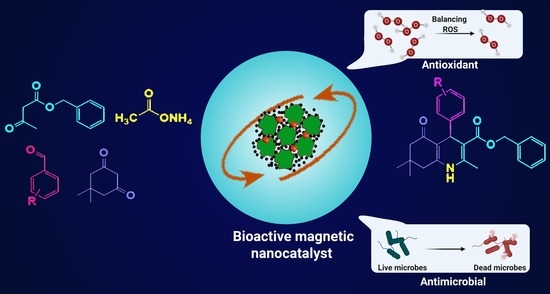
Ionic Liquid-Assisted Fabrication of Bioactive Heterogeneous Magnetic Nanocatalyst with Antioxidant and Antibacterial Activities for the Synthesis of Polyhydroquinoline Derivatives
Abstract
:1. Introduction
2. Results
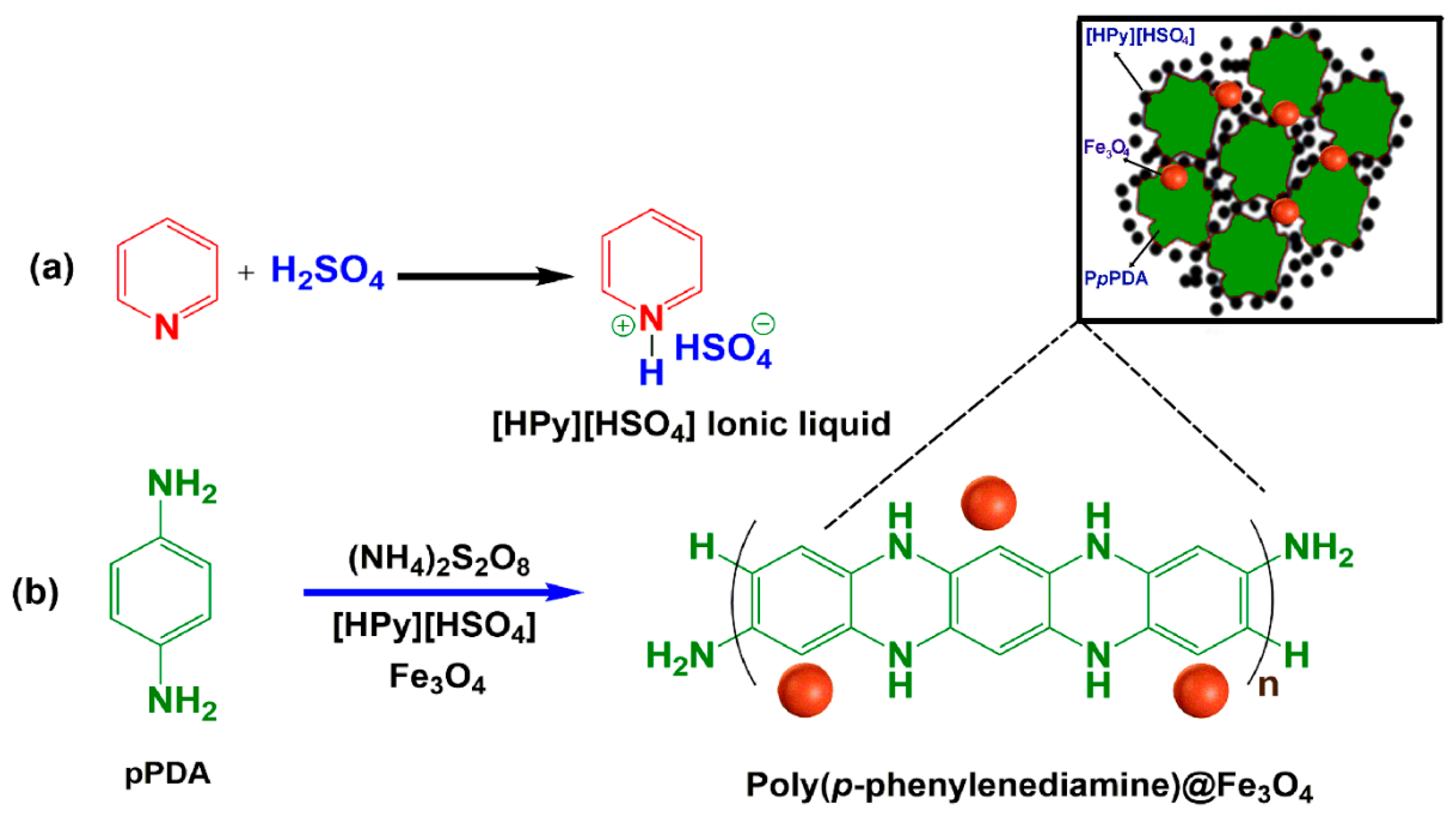
2.1. Characterization of Polymer-Based Catalyst
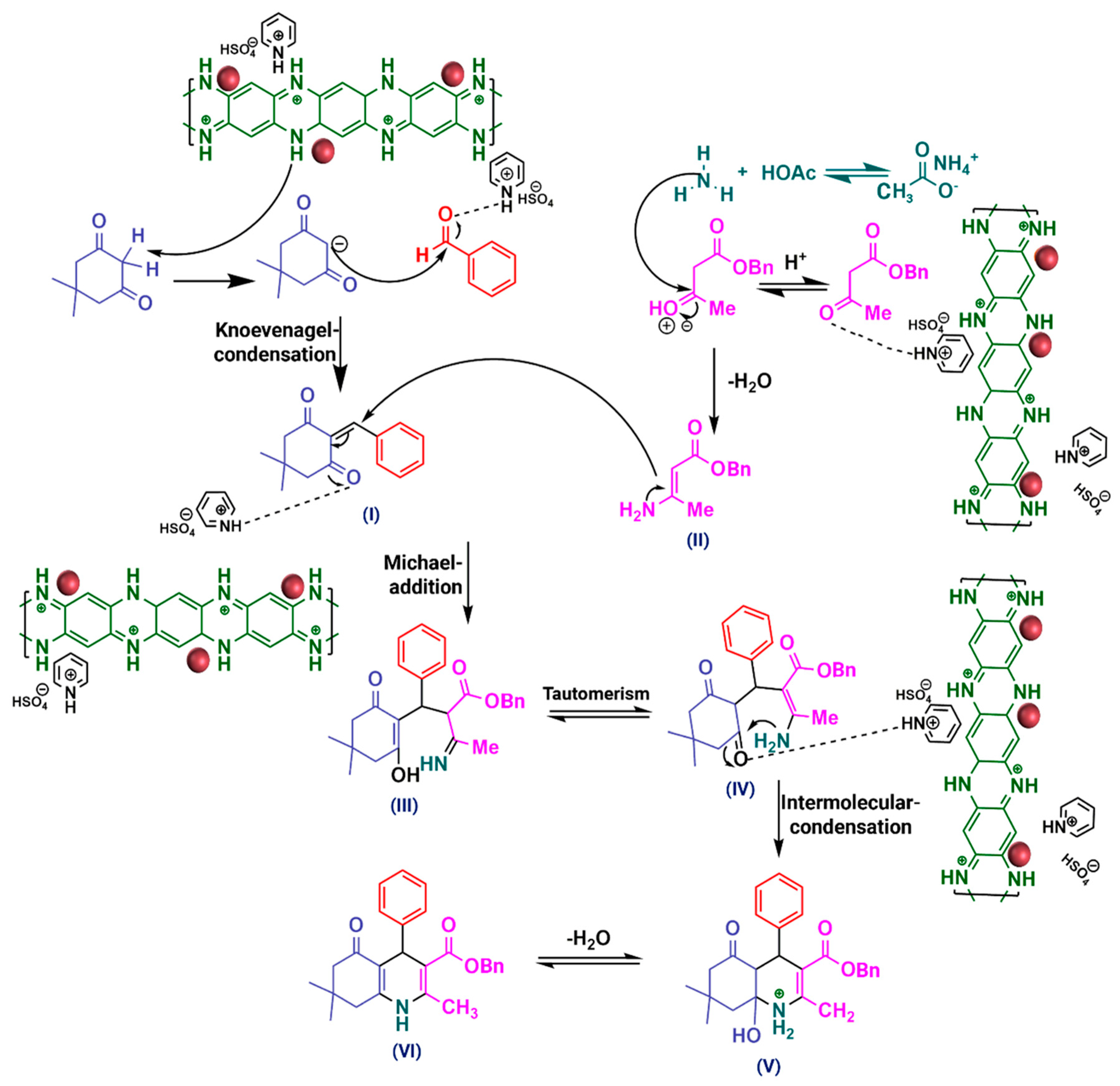
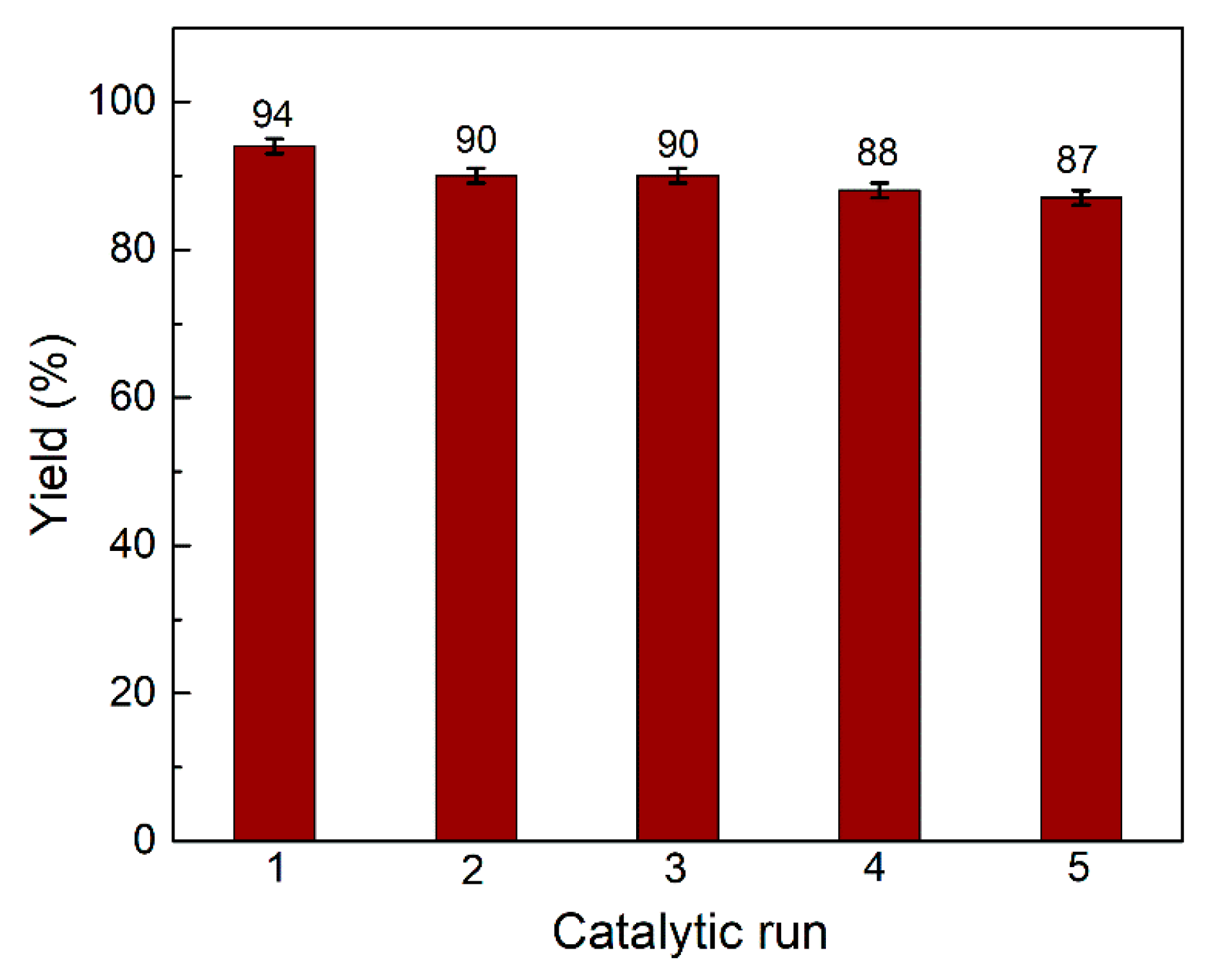
2.2. Evaluation of the Catalytic Activity of PpPDA@Fe3O4 Nanocomposite
3. Experimental Section
3.1. Materials
3.2. Preparation of Iron Oxide Nanoparticles
3.3. Pyridinium Hydrogen Sulfate [HPy][HSO4] Preparation
3.4. Fabrication of Poly(p-Phenylenediamine)@Fe3O4 in [HPy][HSO4] Ionic Liquid
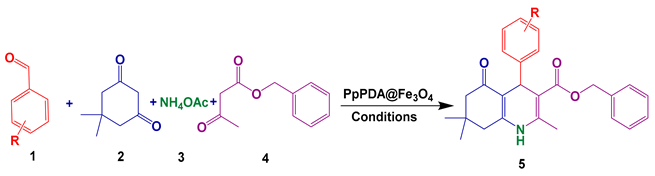
3.5. Overall Route for the Synthesis of Polyhydroquinoline Derivatives
3.6. Antioxidant Activity
3.7. Antibacterial Activity
3.8. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, P.; Liang, S.; Zong, M.-H.; Lou, W.-Y. Ionic liquids for regulating biocatalytic process: Achievements and perspectives. Biotechnol. Adv. 2021, 51, 107702. [Google Scholar] [CrossRef] [PubMed]
- Cull, S.G.; Holbrey, J.D.; Vargas-Mora, V.; Seddon, K.R.; Lye, G.J. Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations. Biotechnol. Bioeng. 2000, 69, 227–233. [Google Scholar] [CrossRef]
- Erbeldinger, M.; Mesiano, A.J.; Russell, A.J. Enzymatic catalysis of formation of Z-aspartame in ionic liquid−an alternative to enzymatic catalysis in organic solvents. Biotechnol. Prog. 2000, 16, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Posvyatenko, A.V.; Larin, S.S.; Ananikov, V.P. Ionic liquids: Prospects for nucleic acid handling and delivery. Nucleic Acids Res. 2021, 49, 1201–1234. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Abbott, A.P.; McKenzie, K.J. Application of ionic liquids to the electrodeposition of metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Pahovnik, D.; Žagar, E.; Kogej, K.; Vohlídal, J.; Žigon, M. Polyaniline nanostructures prepared in acidic aqueous solutions of ionic liquids acting as soft templates. Eur. Polym. J. 2013, 49, 1381–1390. [Google Scholar] [CrossRef]
- Harun, M.H.; Saion, E.; Kassim, A.; Yahya, N.; Mahmud, E. Conjugated Conducting Polymers: A Brief Overview. Sens. Peterbrgh. NH 2007, 2, 63–68. [Google Scholar]
- Zare, E.N.; Agarwal, T.; Zarepour, A.; Pinelli, F.; Zarrabi, A.; Rossi, F.; Ashrafizadeh, M.; Maleki, A.; Shahbazi, M.-A.; Maiti, T.K. Electroconductive multi-functional polypyrrole composites for biomedical applications. Appl. Mater. Today 2021, 24, 101117. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M.; Ashna, A. Synthesis of conductive poly (3-aminobenzoic acid) nanostructures with different shapes in acidic ionic liquids medium. J. Mol. Liq. 2018, 271, 514–521. [Google Scholar] [CrossRef]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Donescu, D.; Fierascu, R.C.; Ghiurea, M.; Manaila-Maximean, D.; Nicolae, C.A.; Somoghi, R.; Spataru, C.I.; Stanica, N.; Raditoiu, V.; Vasile, E. Synthesis and magnetic properties of inverted core-shell polyaniline-ferrite composite. Appl. Surf. Sci. 2017, 414, 8–17. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef]
- Livi, S.; Gérard, J.F.; Duchet-Rumeau, J. Ionic liquids as polymer additives. In Applications of Ionic Liquids in Polymer Science and Technology; Mecerreyes, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–21. ISBN 9783662449035. [Google Scholar]
- Krishna, A.; Laslau, C.; Waterhouse, G.I.N.; Zujovic, Z.D.; Travas-Sejdic, J. Effect of ionic liquid on polyaniline chemically synthesised under falling-pH conditions. Chem. Pap. 2013, 67, 995–1001. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Y.; Liu, Z.; Huang, J.; Han, B.; Sun, Z.; Du, J. Synthesis of polyaniline nanofibrous networks with the aid of an amphiphilic ionic liquid. J. Nanosci. Nanotechnol. 2006, 6, 227–230. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; De Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Bahadorikhalili, S.; Ma’mani, L.; Mahdavi, H.; Shafiee, A. Palladium catalyst supported on PEGylated imidazolium based phosphinite ionic liquid-modified magnetic silica core–shell nanoparticles: A worthy and highly water-dispersible catalyst for organic reactions in water. RSC Adv. 2015, 5, 71297–71305. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Maleki, A. Design and synthesis of a new magnetic aromatic organo-silane star polymer with unique nanoplate morphology and hyperthermia application. J. Nanostructure Chem. 2021, 11, 751–767. [Google Scholar] [CrossRef]
- Marzouk, A.A.; Abu-Dief, A.M.; Abdelhamid, A.A. Hydrothermal preparation and characterization of ZnFe2O4 magnetic nanoparticles as an efficient heterogeneous catalyst for the synthesis of multi-substituted imidazoles and study of their anti-inflammatory activity. Appl. Organomet. Chem. 2018, 32, e3794. [Google Scholar] [CrossRef]
- Babudurai, M.; Nwakanma, O.; Romero-Nuñez, A.; Manisekaran, R.; Subramaniam, V.; Castaneda, H.; Jantrania, A. Mechanical activation of TiO2/Fe2O3 nanocomposite for arsenic adsorption: Effect of ball-to-powder ratio and milling time. J. Nanostructure Chem. 2021, 11, 619–632. [Google Scholar] [CrossRef]
- Halim, E.M.; Perrot, H.; Sel, O.; Debiemme-Chouvy, C.; Lafdi, K.; El Rhazi, M. Electrosynthesis of hierarchical Cu2O–Cu(OH)2 nanodendrites supported on carbon nanofibers/poly(para-phenylenediamine) nanocomposite as high-efficiency catalysts for methanol electrooxidation. Int. J. Hydrog. Energy 2021, 46, 19926–19938. [Google Scholar] [CrossRef]
- Su, X.; Yao, Y.; Tian, J.; Liu, J.; Wang, Z.; You, Y.; Huang, L.; Wu, C. Investigation of the durability of a poly-p-phenylenediamine/carbon black composite for the oxygen reduction reaction. Chin. J. Catal. 2016, 37, 1096–1102. [Google Scholar] [CrossRef]
- Mirzaei-Mosbat, M.; Ghorbani-Vaghei, R.; Karamshahi, Z. Layered double hydroxides@poly(p-phenylenediamine)@Cu as a novel catalyst for the synthesis of pyrrole derivatives: Preparation and characterization. Res. Chem. Intermed. 2020, 46, 1613–1628. [Google Scholar] [CrossRef]
- Karamshahi, Z.; Ghorbani-Vaghei, R. Facile synthesis of indolizines using layered double hydroxides@poly(p-phenylenediamine) as a catalyst with a green tool (neat technology). Appl. Organomet. Chem. 2020, 34, e5347. [Google Scholar] [CrossRef]
- Ortiz, M.E.; Núñez-Vergara, L.J.; Camargo, C.; Squella, J.A. Oxidation of Hantzsch 1, 4-dihydropyridines of pharmacological significance by electrogenerated superoxide. Pharm. Res. 2004, 21, 428–435. [Google Scholar] [CrossRef]
- Bossert, F.; Meyer, H.; Wehinger, E. 4-Aryldihydropyridines, a New Class of Highly Active Calcium Antagonists. Angew. Chem. Int. Ed. 1981, 20, 762–769. [Google Scholar] [CrossRef]
- Kazemi, M.; Mohammadi, M. Magnetically Recoverable Catalysts: Catalysis in Synthesis of Polyhydroquinolines. Appl. Organomet. Chem. 2020, 34, 42–48. [Google Scholar] [CrossRef]
- Ahankar, H.; Ramazani, A.; Joo, S.W. Magnetic nickel ferrite nanoparticles as an efficient catalyst for the preparation of polyhydroquinoline derivatives under microwave irradiation in solvent-free conditions. Res. Chem. Intermed. 2016, 42, 2487–2500. [Google Scholar] [CrossRef]
- Balaji, G.L.; Rajesh, K.; Venkatesh, M.; Sarveswari, S.; Vijayakumar, V. Ultrasound-promoted synthesis of bi-, tri- and tetrapodal polyhydroquinolines, 1,4-dihydropyridines and the corresponding pyridines. RSC Adv. 2014, 4, 39–46. [Google Scholar] [CrossRef]
- Amer, I.; Brandt, S. Synthesis and characterization of Poly(p-phenylenediamine) and its derivatives using aluminium triflate as a co-catalyst. Cogent Eng. 2018, 5, 1499701. [Google Scholar] [CrossRef]
- Arya, V.; Philip, L. Adsorption of pharmaceuticals in water using Fe3O4 coated polymer clay composite. Microporous Mesoporous Mater. 2016, 232, 273–280. [Google Scholar] [CrossRef]
- Muhammad, A.; Bilal, S. Comparative study of the adsorption of Acid Blue 40 on polyaniline, magnetic oxide and their composites: Synthesis, characterization and application. Materials 2019, 12, 2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, K.-S.; Lee, Y.H.; Musa, A.; Salmah, A.A.; Zamri, I. Use of Fe3O4 nanoparticles for enhancement of biosensor response to the herbicide 2, 4-dichlorophenoxyacetic acid. Sensors 2008, 8, 5775–5791. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, A.; Zare, E.N.; Zamani, M. Acidic ionic liquid-mediated preparation of shaped electrically conductive poly (p-phenylenediamine). J. Polym. Res. 2021, 28, 224. [Google Scholar] [CrossRef]
- Mathew, J.; Sathishkumar, M.; Kothurkar, N.K.; Senthilkumar, R.; Narayanan, B.S. Polyaniline/Fe3O4-RGO nanocomposites for microwave absorption. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 310, p. 12138. [Google Scholar]
- Kechrakos, D.; Trohidou, K.N. Magnetic properties of dipolar interacting single-domain particles. Phys. Rev. B 1998, 58, 12169. [Google Scholar] [CrossRef] [Green Version]
- Behbahani, F.K.; Homafar, M. Synthesis of polyhydroquinoline derivatives through the hantzsch four component using iron (III) phosphate as a catalyst. Synth. React. Inorg. Met. Nano-Metal Chem. 2012, 42, 291–295. [Google Scholar] [CrossRef]
- Wu, S.; Sun, A.; Zhai, F.; Wang, J.; Xu, W.; Zhang, Q.; Volinsky, A.A. Fe3O4 magnetic nanoparticles synthesis from tailings by ultrasonic chemical co-precipitation. Mater. Lett. 2011, 65, 1882–1884. [Google Scholar] [CrossRef]
- Tao, D.J.; Wu, Y.T.; Zhou, Z.; Geng, J.; Hu, X.B.; Zhang, Z.B. Kinetics for the esterification reaction of n -butanol with acetic acid catalyzed by noncorrosive brønsted acidic ionic liquids. Ind. Eng. Chem. Res. 2011, 50, 1989–1996. [Google Scholar] [CrossRef]


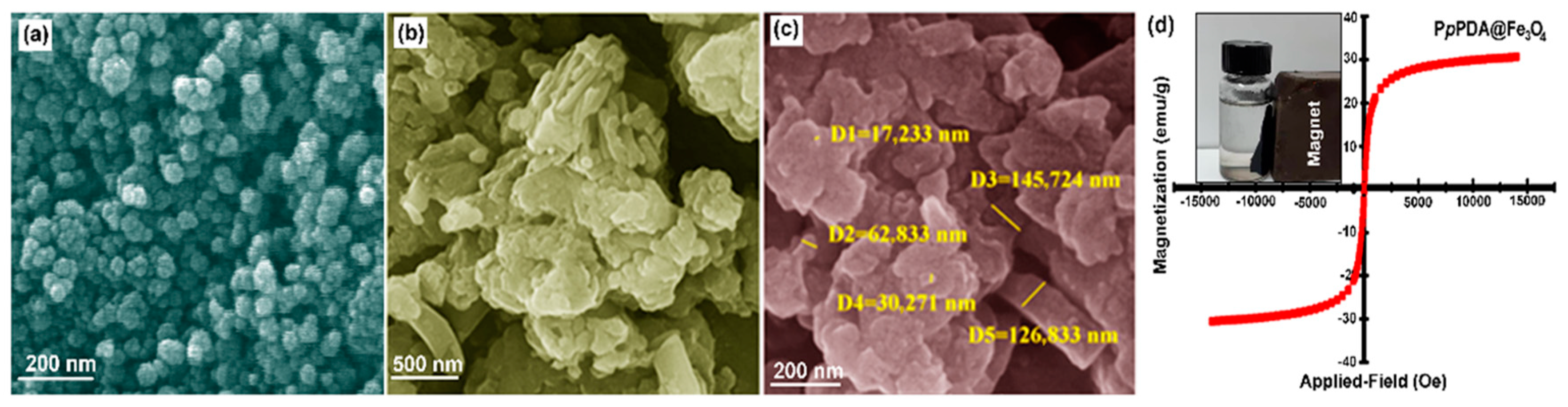
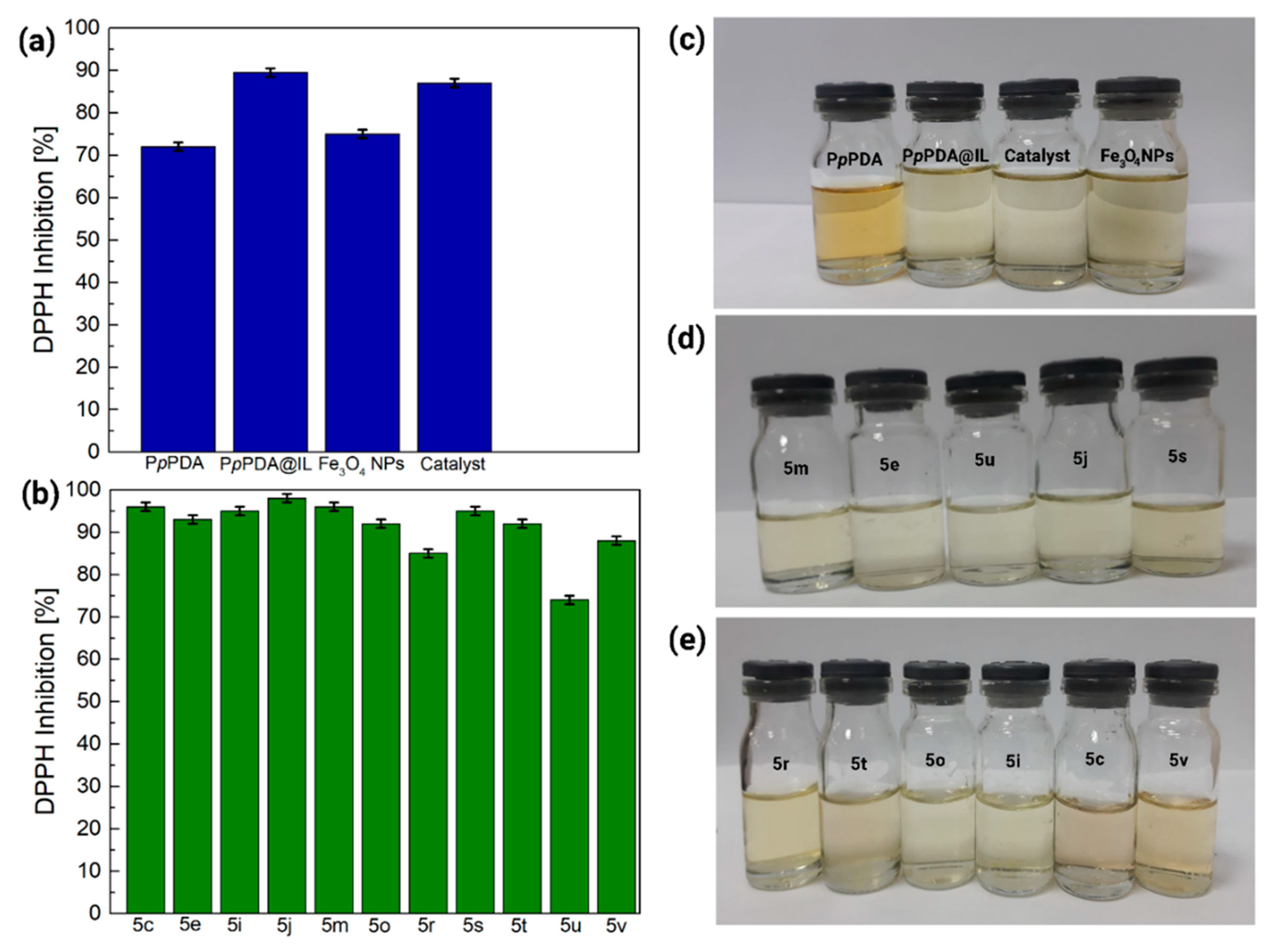
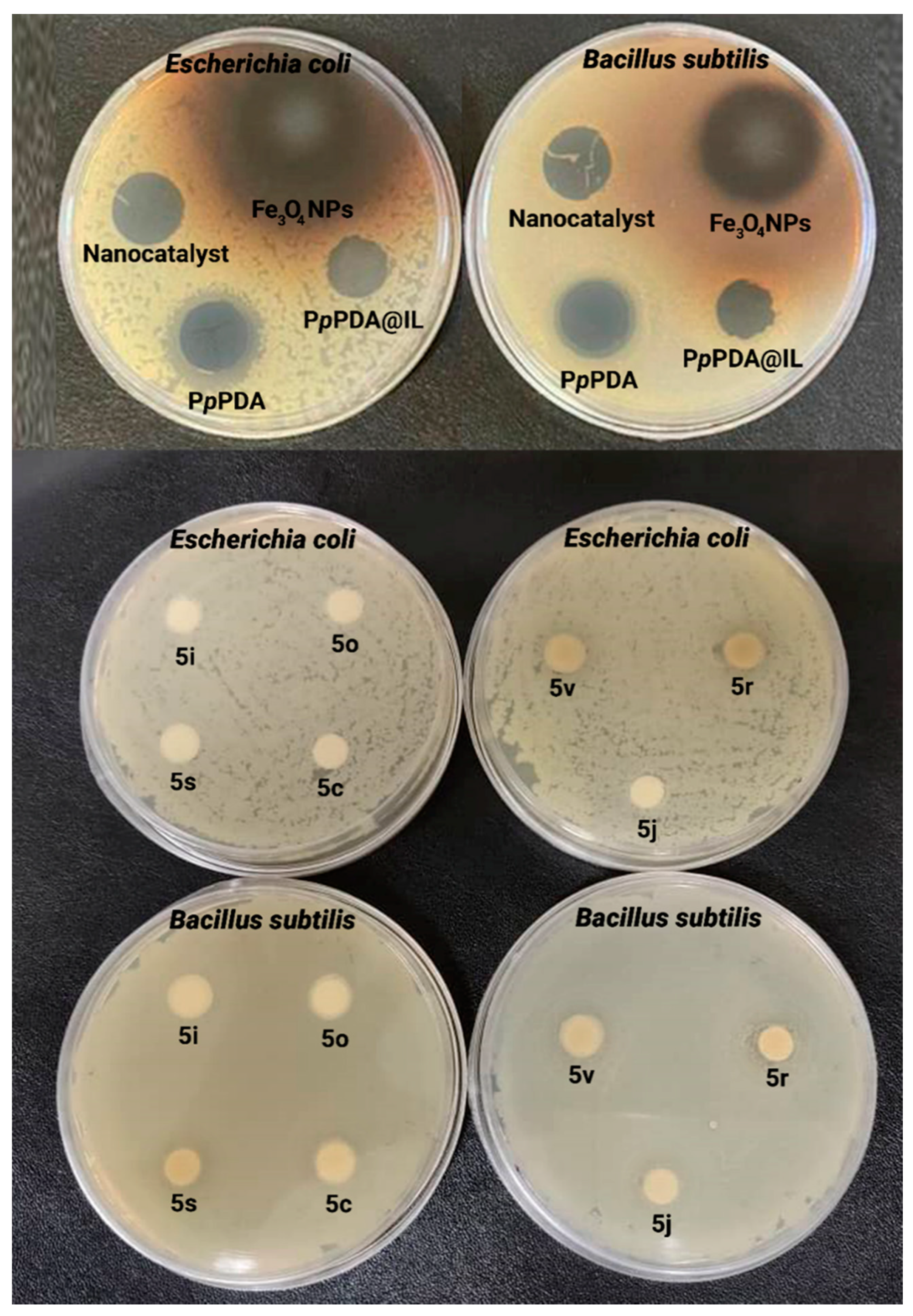
| Samples | C% | H% | N% | S% | O% |
|---|---|---|---|---|---|
| PpPDA | 54.42 (46.31) | 2.86 (5.30) | 27.4 (14.73) | 1.32 (11.24) | 14.00 (22.43) |
| PpPDA@Fe3O4 | 52.00 (46.28) | 2.90 (5.27) | 26.45 (14.70) | 1.25 (11.21) | 17.40 (22.55) |
| Samples | Solvent | |||||
|---|---|---|---|---|---|---|
| H2O | MeOH | EtOH | THF | DMF | NMP | |
| PpPDA@Fe3O4 a | -- | -- | -- | -- | -- | -- |
| PpPDA a | +- | +- | ++ | +- | ++ | ++ |
| PpPDA b | -- | +- | +- | -- | +- | +- |

| Entry | Solvent | Catalyst (g) | Temp. (°C) | Yield (% b) | Time (min) |
|---|---|---|---|---|---|
| 1 | EtOH | 0.03 | Reflux | 85 | 60 |
| 2 | H2O | 0.03 | Reflux | 50 | 240 |
| 3 | THF | 0.03 | Reflux | 45 | 240 |
| 4 | Hexane | 0.03 | Reflux | 20 | 240 |
| 5 | CHCl3 | 0.03 | Reflux | 40 | 240 |
| 6 | - | 0.03 | 80 | 50 | 240 |
| 7 | EtOH | 0.03 | 40 | 75 | 240 |
| 8 | EtOH | 0.03 | 60 | 80 | 240 |
| 9 | EtOH | 0.03 | R.T. | 55 | 240 |
| 10 | EtOH | 0.04 | Reflux | 40 | 60 |
| 11 | EtOH | 0.02 | Reflux | 60 | 60 |
| 12 | EtOH | 0.04 | Reflux | 94 | 30 |
| 13 | EtOH | 0.06 | Reflux | 96 | 30 |
| Entry | Catalyst | Catalyst (g) | Temp. (°C) | Time (min) | Yield (% b) |
|---|---|---|---|---|---|
| 1 | Without catalyst | - | Reflux | 60 | 40 |
| 2 | PpPDA in the absence [HPy][HSO4] | 0.04 | Reflux | 60 | 90 |
| 3 | PpPDA in the presence of [HPy][HSO4] | 0.04 | Reflux | 25 | 95 |
| 4 | PpPDA@Fe3O4 | 0.04 | Reflux | 30 | 94 |
| 5 | PpPDA@Fe3O4 | 0.02 | Reflux | 60 | 60 |
| 6 | PpPDA@Fe3O4 | 0.03 | Reflux | 60 | 85 |
| 7 | PpPDA@Fe3O4 | 0.06 | Reflux | 30 | 96 |
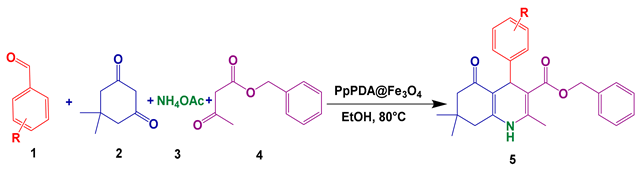

| Entry | Product | Time (min) | Yield % b | Observed M.P (°C) |
|---|---|---|---|---|
| 1 |  5a | 30 | 94 | 172–174 |
| 2 |  5b | 25 | 94 | 148–150 |
| 3 | 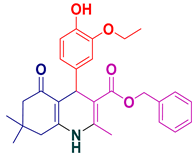 5c | 25 | 97 | 210–212 |
| 4 | 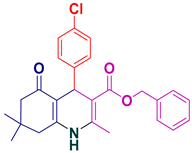 5d | 20 | 93 | 135–137 |
| 5 | 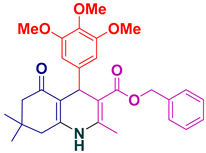 5e | 20 | 92 | 140–142 |
| 6 | 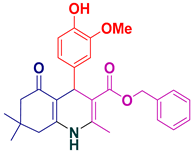 5f | 25 | 94 | 160–162 |
| 7 |  5g | 30 | 93 | 130–132 |
| 8 | 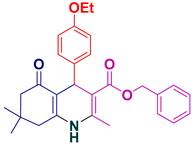 5h | 30 | 95 | 154–156 |
| 9 |  5i | 35 | 90 | 169–171 |
| 10 |  5j | 30 | 92 | 126–128 |
| 11 | 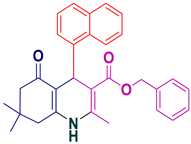 5k | 20 | 96 | 200–202 |
| 12 | 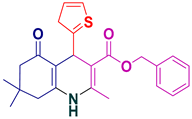 5l | 35 | 92 | 216–218 |
| 13 | 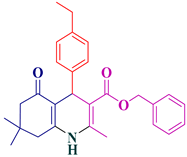 5m | 40 | 90 | 165–167 |
| 14 | 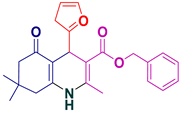 5n | 35 | 90 | 188–190 |
| 15 | 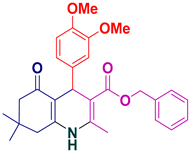 5o | 30 | 91 | 151–153 (187–189) [39] |
| 16 | 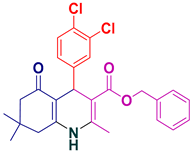 5p | 20 | 92 | 147–149 |
| 17 | 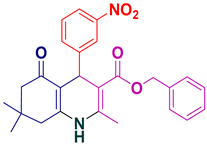 5q | 20 | 94 | 219–221 |
| 18 | 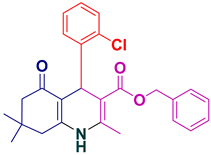 5r | 20 | 96 | 178–180 |
| 19 | 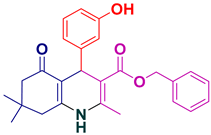 5s | 30 | 93 | 212–214 |
| 20 | 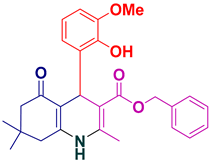 5t | 30 | 90 | 248–250 |
| 21 | 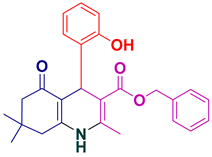 5u | 35 | 92 | 212–215 |
| 22 | 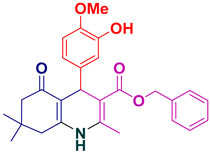 5v | 25 | 94 | 141–143 |
| 23 | 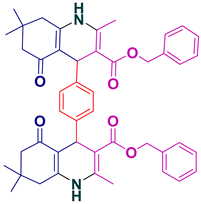 5w | 25 | 95 | 327–330 |
| 24 |  5x | 40 | 92 | 161–163 |
| Entry | Compound | Inhibition Zone (mm) | |
|---|---|---|---|
| Bacillus subtilis Gram-Positive (+) | Escherichia coli Gram-Negative (−) | ||
| 1 | Fe3O4 NPs | 24 ± 1.2 | 25 ± 1.2 |
| 2 | PpPDA@IL | 9 ± 0.6 | NE a |
| 3 | PpPDA | 20 ± 1.2 | 22 ± 1.7 |
| 4 | Nanocatalyst | 9 ± 0.6 | 8 ± 0.6 |
| 5 | 5c | 8 ± 0.6 | NE |
| 6 | 5i | NE | NE |
| 7 | 5j | 10 ± 1.0 | NE |
| 8 | 5o | NE | NE |
| 9 | 5r | 12 ± 1.0 | 12 ± 1.0 |
| 10 | 5s | 9 ± 0.7 | NE |
| 11 | 5v | 11 ± 0.7 | 10 ± 1.0 |
| 12 | Gentamicin | 26 ± 1.2 | 19.6 ± 0.7 |
| 13 | Chloramphenicol | 22.3 ± 1.7 | 20.7 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirani Nezhad, S.; Nazarzadeh Zare, E.; Davarpanah, A.; Pourmousavi, S.A.; Ashrafizadeh, M.; Kumar, A.P. Ionic Liquid-Assisted Fabrication of Bioactive Heterogeneous Magnetic Nanocatalyst with Antioxidant and Antibacterial Activities for the Synthesis of Polyhydroquinoline Derivatives. Molecules 2022, 27, 1748. https://doi.org/10.3390/molecules27051748
Mirani Nezhad S, Nazarzadeh Zare E, Davarpanah A, Pourmousavi SA, Ashrafizadeh M, Kumar AP. Ionic Liquid-Assisted Fabrication of Bioactive Heterogeneous Magnetic Nanocatalyst with Antioxidant and Antibacterial Activities for the Synthesis of Polyhydroquinoline Derivatives. Molecules. 2022; 27(5):1748. https://doi.org/10.3390/molecules27051748
Chicago/Turabian StyleMirani Nezhad, Shefa, Ehsan Nazarzadeh Zare, Azimeh Davarpanah, Seied Ali Pourmousavi, Milad Ashrafizadeh, and Alan Prem Kumar. 2022. "Ionic Liquid-Assisted Fabrication of Bioactive Heterogeneous Magnetic Nanocatalyst with Antioxidant and Antibacterial Activities for the Synthesis of Polyhydroquinoline Derivatives" Molecules 27, no. 5: 1748. https://doi.org/10.3390/molecules27051748
APA StyleMirani Nezhad, S., Nazarzadeh Zare, E., Davarpanah, A., Pourmousavi, S. A., Ashrafizadeh, M., & Kumar, A. P. (2022). Ionic Liquid-Assisted Fabrication of Bioactive Heterogeneous Magnetic Nanocatalyst with Antioxidant and Antibacterial Activities for the Synthesis of Polyhydroquinoline Derivatives. Molecules, 27(5), 1748. https://doi.org/10.3390/molecules27051748