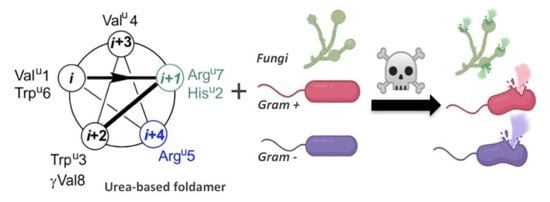
Design of Oligourea-Based Foldamers with Antibacterial and Antifungal Activities
Abstract
:1. Introduction
2. Results and Discussion
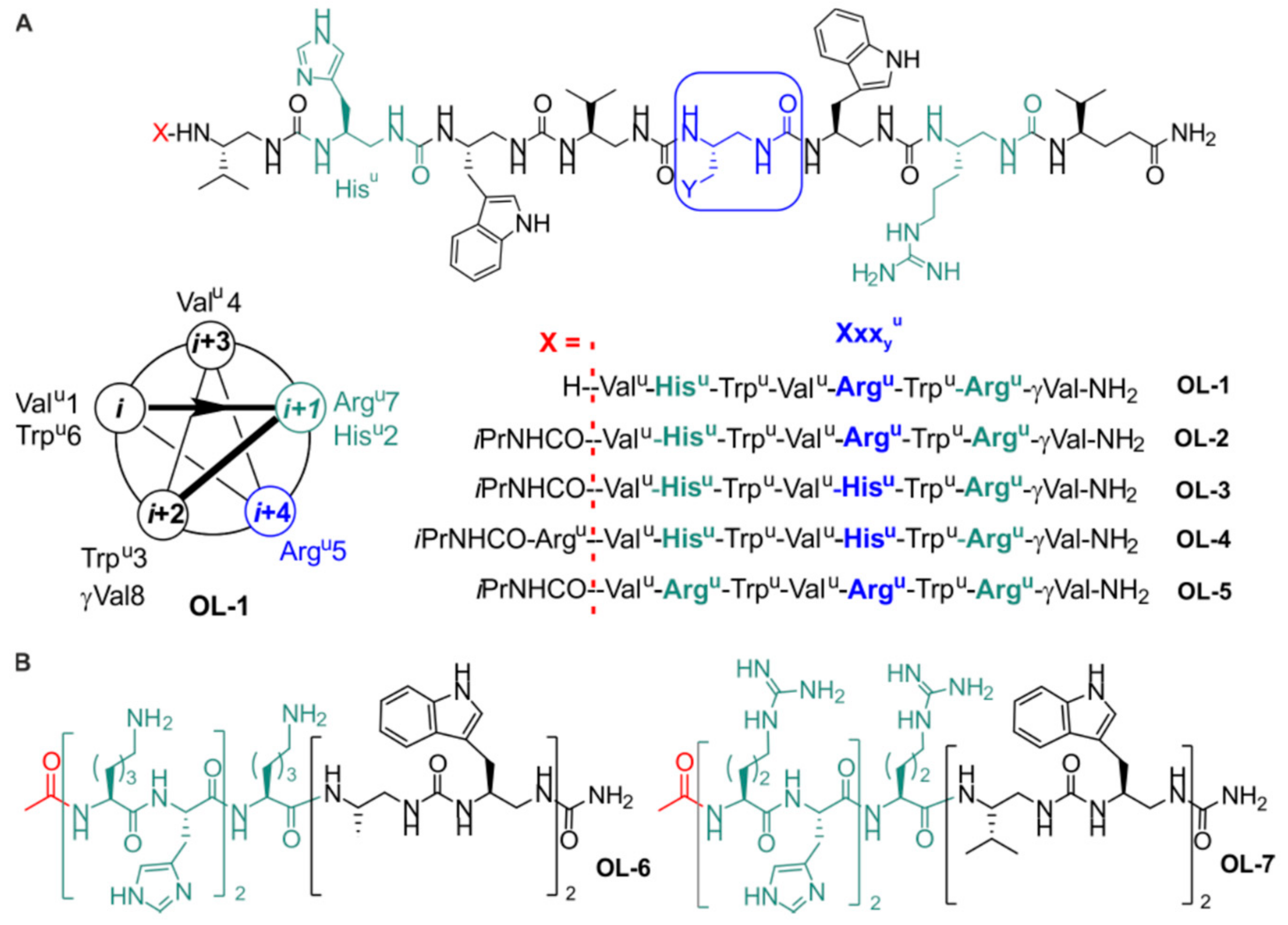
2.1. Design and Synthesis of the Foldamers
2.2. Evaluation of the Antibacterial Activity
2.3. Antibacterial Activity in the Presence of Serum
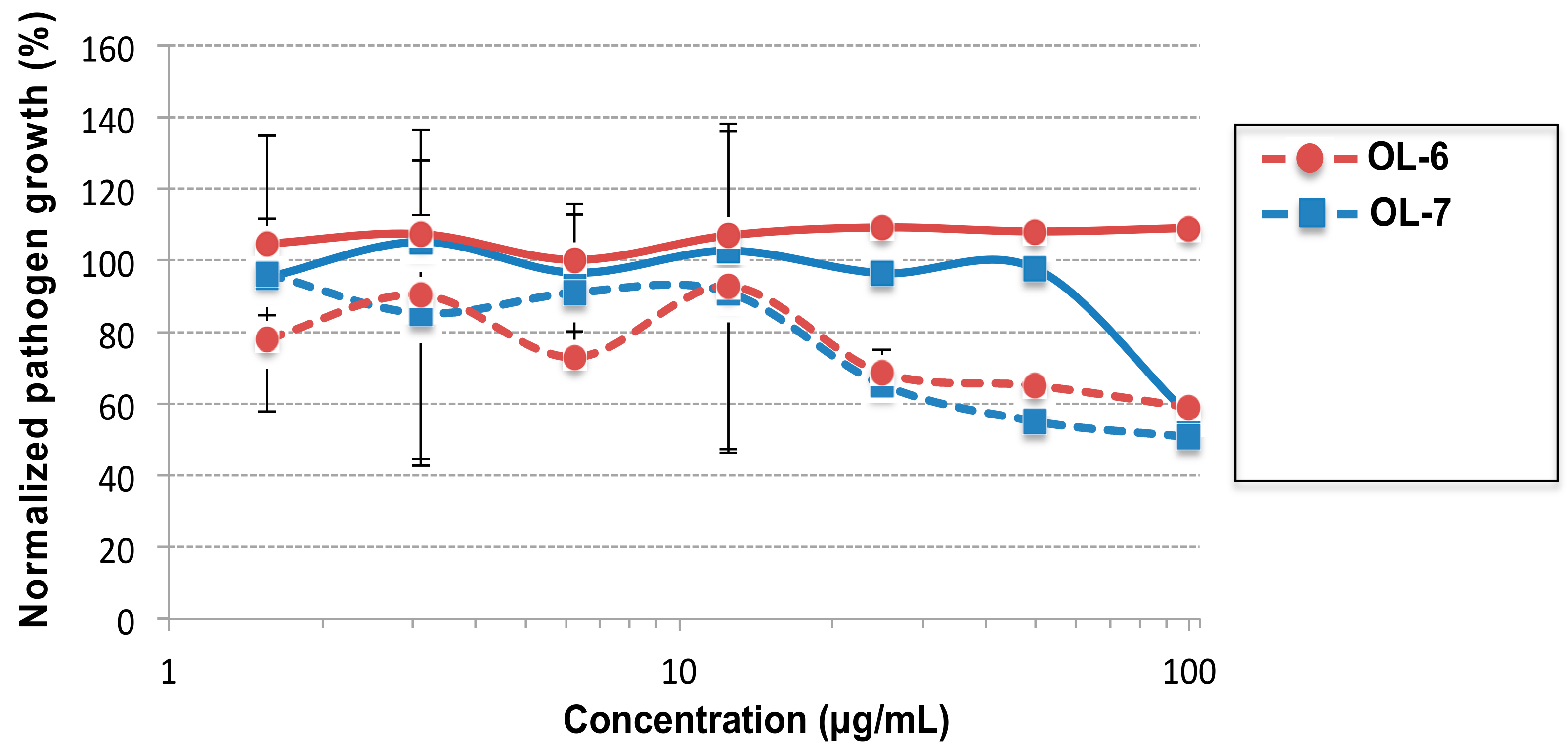
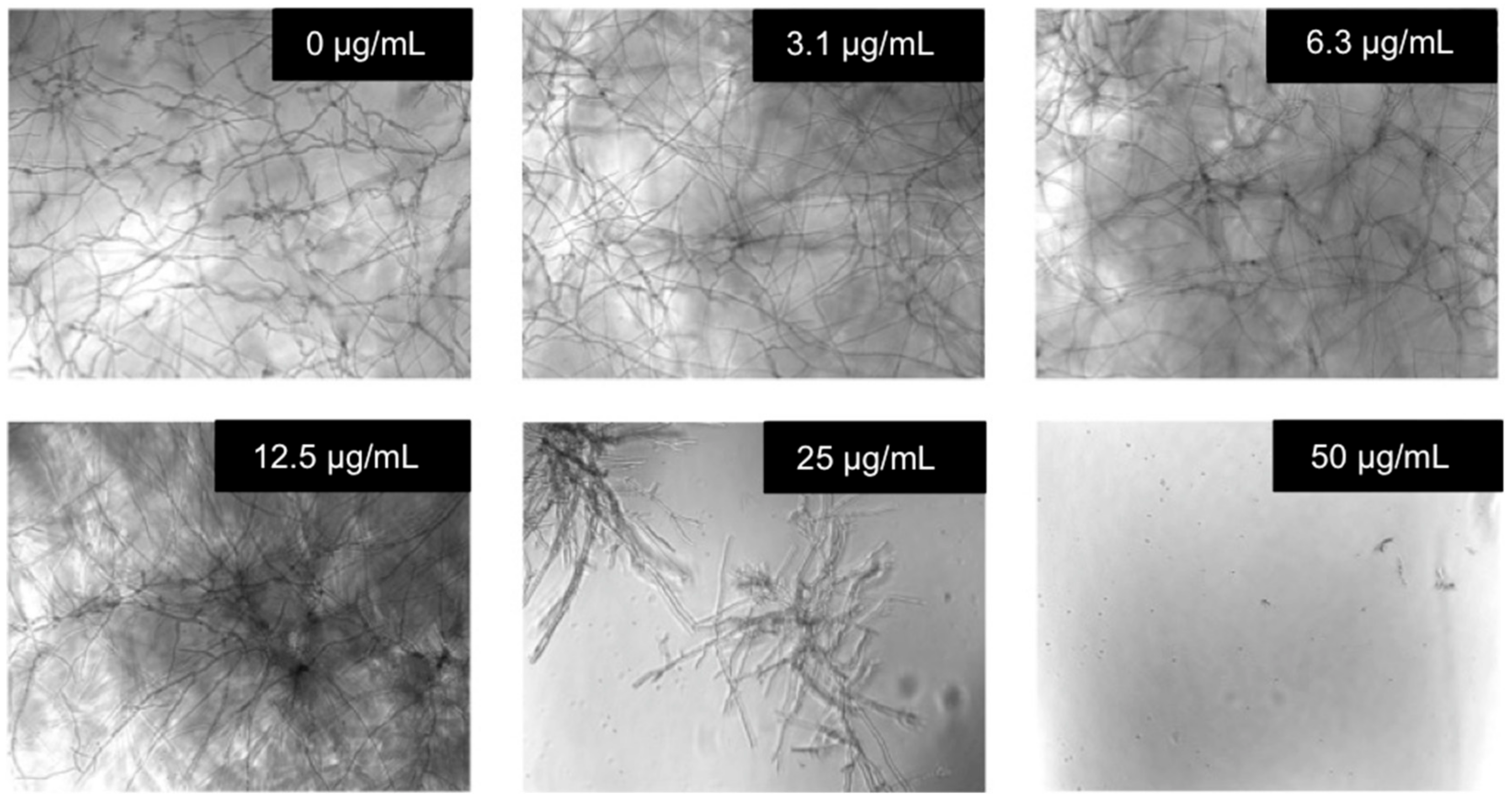
2.4. Evaluation of the Antifungal Activity
3. Experimental
3.1. Synthesis of Amphipathic Foldamer and Related Peptide Hybrids
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Lactate Dehydrogenase Assay
3.5. Antibacterial Assays
3.6. Antifungal Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- de Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; van der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourtada, R.; Herce, H.D.; Yin, D.J.; Moroco, J.A.; Wales, T.E.; Engen, J.R.; Walensky, L.D. Design of stapled antimicrobial peptides that are stable, nontoxic and kill antibiotic-resistant bacteria in mice. Nat. Biotechnol. 2019, 37, 1186–1197. [Google Scholar] [CrossRef]
- Guichard, G.; Huc, I. Synthetic foldamers. Chem. Commun. (Camb.) 2011, 47, 5933–5941. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Frolov, A.I.; Knerr, L.; Drury, W.J., 3rd; Valeur, E. Therapeutic Potential of Foldamers: From Chemical Biology Tools To Drug Candidates? J. Med. Chem. 2016, 59, 9599–9621. [Google Scholar] [CrossRef]
- Pasco, M.; Dolain, C.; Guichard, G. Foldamers in Medicinal Chemistry. In Comprehensive Supramolecular Chemistry II; Atwood, J.L., Ed.; Elsevier: Oxford, UK, 2017; Volume 5, pp. 89–125. [Google Scholar]
- Hamuro, Y.; Schneider, J.P.; DeGrado, W.F. De Novo Design of Antibacterial β-Peptides. J. Am. Chem. Soc. 1999, 121, 12200–12201. [Google Scholar] [CrossRef]
- Schmitt, M.A.; Weisblum, B.; Gellman, S.H. Unexpected Relationships between Structure and Function in α,β-Peptides: Antimicrobial Foldamers with Heterogeneous Backbones. J. Am. Chem. Soc. 2004, 126, 6848–6849. [Google Scholar] [CrossRef]
- Chongsiriwatana, N.P.; Patch, J.A.; Czyzewski, A.M.; Dohm, M.T.; Ivankin, A.; Gidalevitz, D.; Zuckermann, R.N.; Barron, A.E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 2794–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shyam, R.; Charbonnel, N.; Job, A.; Blavignac, C.; Forestier, C.; Taillefumier, C.; Faure, S. 1,2,3-Triazolium-Based Cationic Amphipathic Peptoid Oligomers Mimicking Antimicrobial Helical Peptides. ChemMedChem 2018, 13, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Molchanova, N.; Wang, H.; Hansen, P.R.; Høiby, N.; Nielsen, H.M.; Franzyk, H. Antimicrobial Activity of α-Peptide/β-Peptoid Lysine-Based Peptidomimetics Against Colistin-Resistant Pseudomonas aeruginosa Isolated From Cystic Fibrosis Patients. Front. Microbiol. 2019, 10, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnel, C.; Legrand, B.; Simon, M.; Clavié, M.; Masnou, A.; Jumas-Bilak, E.; Kang, Y.K.; Licznar-Fajardo, P.; Maillard, L.T.; Masurier, N. Tailoring the Physicochemical Properties of Antimicrobial Peptides onto a Thiazole-Based γ-Peptide Foldamer. J. Med. Chem. 2020, 63, 9168–9180. [Google Scholar] [CrossRef]
- Wei, L.; Wang, M.; Gao, R.; Fatirkhorani, R.; Cai, J. Antibacterial activity of lipo-α/sulfono-γ-AA hybrid peptides. Eur. J. Med. Chem. 2020, 186, 111901. [Google Scholar] [CrossRef]
- Mensa, B.; Kim, Y.H.; Choi, S.; Scott, R.; Caputo, G.A.; DeGrado, W.F. Antibacterial Mechanism of Action of Arylamide Foldamers. Antimicrob. Agents Chemother. 2011, 55, 5043–5053. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Doerksen, R.J.; Tew, G.N. Synthesis of urea oligomers and their antibacterial activity. Chem. Commun. (Camb.) 2005, 12, 1537–1539. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.H.; Li, B.; Dolain, C.; Pasco, M.; Guichard, G. Urea based foldamers. Methods Enzymol. 2021, 656, 59–92. [Google Scholar]
- Fischer, L.; Claudon, P.; Pendem, N.; Miclet, E.; Didierjean, C.; Ennifar, E.; Guichard, G. The canonical helix of urea oligomers at atomic resolution: Insights into folding-induced axial organization. Angew. Chem. Int. Ed. Engl. 2010, 49, 1067–1070. [Google Scholar] [CrossRef]
- Teyssières, E.; Corre, J.P.; Antunes, S.; Rougeot, C.; Dugave, C.; Jouvion, G.; Claudon, P.; Mikaty, G.; Douat, C.; Goossens, P.L.; et al. Proteolytically Stable Foldamer Mimics of Host-Defense Peptides with Protective Activities in a Murine Model of Bacterial Infection. J. Med. Chem. 2016, 59, 8221–8232. [Google Scholar] [CrossRef]
- Antunes, S.; Corre, J.-P.; Mikaty, G.; Douat, C.; Goossens, P.L.; Guichard, G. Effect of replacing main-chain ureas with thiourea and guanidinium surrogates on the bactericidal activity of membrane active oligourea foldamers. Bioorg. Med. Chem. 2017, 25, 4245–4252. [Google Scholar] [CrossRef] [PubMed]
- Claudon, P.; Violette, A.; Lamour, K.; Decossas, M.; Fournel, S.; Heurtault, B.; Godet, J.; Mély, Y.; Jamart-Grégoire, B.; Averlant-Petit, M.-C.; et al. Consequences of isostructural main-chain modifications for the design of antimicrobial foldamers: Helical mimics of host-defense peptides based on a heterogeneous amide/urea backbone. Angew. Chem. Int. Ed. Engl. 2010, 49, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Violette, A.; Fournel, S.; Lamour, K.; Chaloin, O.; Frisch, B.; Briand, J.-P.; Monteil, H.; Guichard, G. Mimicking helical antibacterial peptides with nonpeptidic folding oligomers. Chem. Biol. 2006, 13, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.J.; Kim, D.T.; Steinman, L.; Fathman, C.G.; Rothbard, J.B. Polyarginine enters cells more efficiently than other polycationic homopolymers. J. Pept. Res. 2000, 56, 318–325. [Google Scholar] [CrossRef]
- Cutrona, K.J.; Kaufman, B.A.; Figueroa, D.M.; Elmore, D.E. Role of arginine and lysine in the antimicrobial mechanism of histone-derived antimicrobial peptides. FEBS Lett. 2015, 589 Pt B, 3915–3920. [Google Scholar] [CrossRef] [Green Version]
- Deslouches, B.; Hasek, M.L.; Craigo, J.K.; Steckbeck, J.D.; Montelaro, R.C. Comparative functional properties of engineered cationic antimicrobial peptides consisting exclusively of tryptophan and either lysine or arginine. J. Med. Microbiol. 2016, 65, 554–565. [Google Scholar] [CrossRef]
- Aisenbrey, C.; Douat, C.; Kichler, A.; Guichard, G.; Bechinger, B. Characterization of the DNA and Membrane Interactions of a Bioreducible Cell-Penetrating Foldamer in its Monomeric and Dimeric Form. J. Phys. Chem. B 2020, 124, 4476–4486. [Google Scholar] [CrossRef]
- Bornerie, M.; Brion, A.; Guichard, G.; Kichler, A.; Douat, C. Delivery of siRNA by tailored cell-penetrating urea-based foldamers. Chem. Commun. (Camb.) 2021, 57, 1458–1461. [Google Scholar] [CrossRef]
- Douat, C.; Aisenbrey, C.; Antunes, S.; Decossas, M.; Lambert, O.; Bechinger, B.; Kichler, A.; Guichard, G. A cell-penetrating foldamer with a bioreducible linkage for intracellular delivery of DNA. Angew. Chem. Int. Ed. Engl. 2015, 54, 11133–11137. [Google Scholar] [CrossRef]
- Douat, C.; Bornerie, M.; Antunes, S.; Guichard, G.; Kichler, A. Hybrid Cell-Penetrating Foldamer with Superior Intracellular Delivery Properties and Serum Stability. Bioconjug. Chem. 2019, 30, 1133–1139. [Google Scholar] [CrossRef] [Green Version]
- Bahnsen, J.S.; Franzyk, H.; Sandberg-Schaal, A.; Nielsen, H.M. Antimicrobial and cell-penetrating properties of penetratin analogs: Effect of sequence and secondary structure. Biochim. Biophys. Acta 2013, 1828, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fremaux, J.; Mauran, L.; Pulka-Ziach, K.; Kauffmann, B.; Odaert, B.; Guichard, G. α-Peptide-Oligourea Chimeras: Stabilization of Short α-Helices by Non-Peptide Helical Foldamers. Angew. Chem. Int. Ed. Engl. 2015, 54, 9816–9820. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Kanaoka, S.; Sato, T.; Aoshima, S.; Kuroda, K. Block versus Random Amphiphilic Copolymers as Antibacterial Agents. Biomacromolecules 2011, 12, 3581–3591. [Google Scholar] [CrossRef] [PubMed]
- Cussol, L.; Mauran-Ambrosino, L.; Buratto, J.; Belorusova, A.; Neuville, M.; Osz, J.; Fribourg, S.; Fremaux, J.; Dolain, C.; Goudreau, S.R.; et al. Structural Basis for α-Helix Mimicry and Inhibition of Protein-Protein Interactions with Oligourea Foldamers. Angew. Chem. Int. Ed. Engl. 2021, 60, 2296–2303. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, C.E.; Ueno, S.; Avison, M.B. Enhanced membrane permeabilization and antibacterial activity of a disulfide-dimerized magainin analogue. Biochemistry 2003, 42, 402–409. [Google Scholar] [CrossRef]
- Laurent, Q.; Berthet, M.; Cheng, Y.; Sakai, N.; Barluenga, S.; Winssinger, N.; Matile, S. Probing for Thiol-Mediated Uptake into Bacteria. Chembiochem 2020, 21, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Ge, Y.; MacDonald, D.L.; Holroyd, K.J.; Thornsberry, C.; Wexler, H.; Zasloff, M. In Vitro Antibacterial Properties of Pexiganan, an Analog of Magainin. Antimicrob. Agents Chemother. 1999, 43, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, A.J.; Pomerantz, W.C.; Neilsen, K.J.; Gellman, S.H.; Palecek, S.P. Effect of sequence and structural properties on 14-helical beta-peptide activity against Candida albicans planktonic cells and biofilms. ACS Chem. Biol. 2009, 4, 567–579. [Google Scholar] [CrossRef]
- Karlsson, A.J.; Pomerantz, W.C.; Weisblum, B.; Gellman, S.H.; Palecek, S.P. Antifungal activity from 14-helical beta-peptides. J. Am. Chem. Soc. 2006, 128, 12630–12631. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.O.; Atici, S.; Akkoç, G.; Yakut, N.; İkizoğlu, N.B.; Eralp, E.E.; Soysal, A.; Bakir, M. Neurologic Adverse Events Associated with Voriconazole Therapy: Report of Two Pediatric Cases. Case Rep. Infect. Dis. 2016, 2016, 3989070. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Oligourea | S. aureus ATCC 25923 | MRSA | P. aeruginosa ATCC 27853 | E. coli ATCC 25922 |
|---|---|---|---|---|
| OL-1 | 12.5 * | 50 | 12.5 | 12.5 |
| OL-2 | 6.25 | 12.5 | 25 | 6.25 |
| OL-3 | 12.5 | 12.5 | 50 | 12.5 |
| OL-4 | 6.25 | 6.25 | 50 | 6.25 |
| OL-5 | 12.5 | 6.25 | 12.5 | 6.25 |
| Oligourea | S. aureus ATCC 25923 | MRSA | P. aeruginosa ATCC 27853 | E. coli ATCC 25922 |
|---|---|---|---|---|
| OL-5 | 12.5 | 6.25 | 12.5 | 6.25 |
| [Lysu 2, 5, 7]OL-5 | 12.5 | 12.5 | 25 | 12.5 |
| Oligourea | C. albicans 18804 |
|---|---|
| OL-1 | 12.5 |
| OL-2 | 12.5 |
| OL-3 | 12.5 |
| OL-4 | 12.5 |
| OL-5 | 6.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tallet, L.; Frisch, E.; Bornerie, M.; Medemblik, C.; Frisch, B.; Lavalle, P.; Guichard, G.; Douat, C.; Kichler, A. Design of Oligourea-Based Foldamers with Antibacterial and Antifungal Activities. Molecules 2022, 27, 1749. https://doi.org/10.3390/molecules27051749
Tallet L, Frisch E, Bornerie M, Medemblik C, Frisch B, Lavalle P, Guichard G, Douat C, Kichler A. Design of Oligourea-Based Foldamers with Antibacterial and Antifungal Activities. Molecules. 2022; 27(5):1749. https://doi.org/10.3390/molecules27051749
Chicago/Turabian StyleTallet, Lorène, Emilie Frisch, Mégane Bornerie, Claire Medemblik, Benoît Frisch, Philippe Lavalle, Gilles Guichard, Céline Douat, and Antoine Kichler. 2022. "Design of Oligourea-Based Foldamers with Antibacterial and Antifungal Activities" Molecules 27, no. 5: 1749. https://doi.org/10.3390/molecules27051749
APA StyleTallet, L., Frisch, E., Bornerie, M., Medemblik, C., Frisch, B., Lavalle, P., Guichard, G., Douat, C., & Kichler, A. (2022). Design of Oligourea-Based Foldamers with Antibacterial and Antifungal Activities. Molecules, 27(5), 1749. https://doi.org/10.3390/molecules27051749