Investigation of Different Winemaking Protocols to Mitigate Smoke Taint Character in Wine
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis
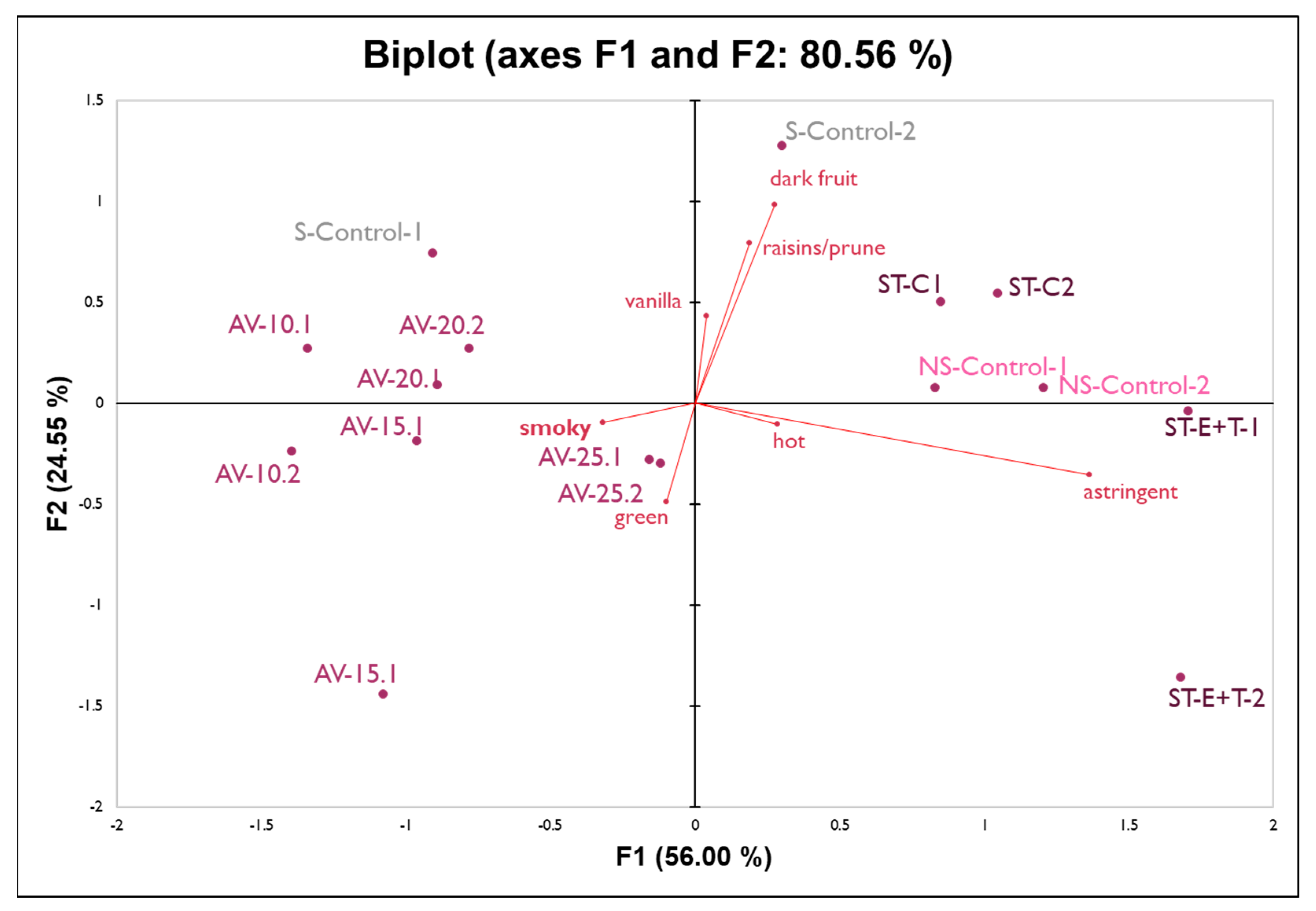
2.2. Sensory Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Grapes and General Winemaking
4.3. Wine Treatments
4.3.1. Yeast Strains
4.3.2. Oak and Tannin Additions
4.3.3. Skin Contact Time and Maceration Enzyme
4.3.4. Fermentation Temperature
4.4. Wine Analysis
4.5. Gas Chromatography-Mass Spectrometry (GC-MS)
4.6. Descriptive Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchinson, J.A.; Vargo, J.; Milet, M.; French, N.H.; Billmire, M.; Johnson, J.; Hoshiko, S. The San Diego 2007 wildfires and Medi-Cal emergency department presentations, inpatient hospitalizations, and outpatient visits: An observational study of smoke exposure periods and a bidirectional case-crossover analysis. J. PLoS Med. 2018, 15, e1002601. [Google Scholar] [CrossRef] [PubMed]
- Fried, J.S.; Torn, M.S.; Mills, E. The impact of climate change on wildfire severity: A regional forecast for northern California. Clim. Change 2004, 64, 169–191. [Google Scholar] [CrossRef]
- Linuma, Y.; Keywood, M.; Herrmann, H. Characterization of primary and secondary organic aerosols in Melbourne airshed: The influence of biogenic emissions, wood smoke and bushfires. Atmos. Environ. 2016, 130, 54–63. [Google Scholar]
- Wentworth, G.R.; Aklilu, Y.-A.; Landis, M.S.; Hsu, Y.-M. Impacts of a large boreal wildfire on ground level atmospheric concentrations of PAHs, VOCs and ozone. Atmos. Environ. 2018, 178, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Hamilton, R.F.; Smith, G.C. The Missoula Valley Semivolatile and Volatile Organic Compound Study: Seasonal Average Concentrations. J. Air Waste Manag. 2005, 55, 1007–1013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kennison, K.R.; Gibberd, M.R.; Pollnitz, A.P.; Wilkinson, K.L. Smoke-Derived Taint in Wine: The Release of Smoke-Derived Volatile Phenols during Fermentation of Merlot Juice following Grapevine Exposure to Smoke. J. Agric. Food Chem. 2008, 56, 7379–7383. [Google Scholar] [CrossRef]
- Krstic, M.P.; Johnson, D.L.; Herderich, M.J. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust. J. Grape Wine Res. 2015, 21, 537–553. [Google Scholar] [CrossRef]
- Kennison, K. Bushfire Generated Smoke Taint in Grapes and Wine; Final Report to Grape and Wine Research and Development Corporation, Project Number: RD 05/02-3; Department of Agriculture and Food: Perth, WA, Australia, 2009.
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of Several Volatile Phenols and Their Glycoconjugates to Smoke-Related Sensory Properties of Red Wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Ristic, R.; Fudge, A.L.; Pinchbeck, K.A.; Bei, R.; Fuentes, S.; Hayasaka, Y.; Tyerman, S.; Wilkinson, K. Impact of grapevine exposure to smoke on vine physiology and the composition and sensory properties of wine. Theor. Exp. Plant Phys. 2016, 28, 67–83. [Google Scholar] [CrossRef]
- Kennison, K.R.; Wilkinson, K.L.; Pollnitz, A.P.; Williams, H.G.; Gibberd, M.R. Effect of timing and duration of grapevine exposure to smoke on the composition and sensory properties of wine. Aust. J. Grape Wine Res. 2009, 15, 228–237. [Google Scholar] [CrossRef]
- Mirabelli-Montan, Y.A.; Marangon, M.; Graça, A.; Mayr Marangon, C.M.; Wilkinson, K.L. Techniques for Mitigating the Effects of Smoke Taint While Maintaining Quality in Wine Production: A Review. Molecules 2021, 26, 1672. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.I.; Dhesi, M.K.; Eggers, N.J. Effect of Pre- and Postveraison Smoke Exposure on Guaiacol and 4-Methylguaiacol Concentration in Mature Grapes. Am. J. Enol. Vitic. 2009, 60, 98–103. [Google Scholar]
- Singh, D.P.; Chong, H.H.; Pitt, K.M.; Cleary, M.; Dokoozlian, N.K.; Downey, M.O. Guaiacol and 4-methylguaiacol accumulate in wines made from smoke-affected fruit because of hydrolysis of their conjugates. Aust. J. Grape Wine Res. 2011, 17, S13–S21. [Google Scholar] [CrossRef]
- Parker, M.; Capone, D.L.; Francis, I.L.; Herderich, M.J. Aroma Precursors in Grapes and Wine: Flavor Release during Wine Production and Consumption. J. Agric. Food Chem. 2018, 66, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.; Ristic, R.; Capone, D.; Puglisi, C.; Pagay, V.; Culbert, J.; Jiang, W.; Herderich, M.; Tuke, J.; Wilkinson, K. Uptake and Glycosylation of Smoke-Derived Volatile Phenols by Cabernet Sauvignon Grapes and Their Subsequent Fate during Winemaking. Molecules 2020, 25, 3720. [Google Scholar] [CrossRef]
- Caffrey, A.; Lerno, L.; Rumbaugh, A.; Girardello, R.; Zweigenbaum, J.; Oberholster, A.; Ebeler, S.E. Changes in Smoke-Taint Volatile-Phenol Glycosides in Wildfire Smoke-Exposed Cabernet Sauvignon Grapes throughout Winemaking. Am. J. Enol. Vitic. 2019, 70, 373. [Google Scholar] [CrossRef]
- De-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V. Levels of higher alcohols inducing aroma changes and modulating experts’ preferences in wine model solutions. Aust. J. Grape Wine Res. 2017, 23, 162–169. [Google Scholar] [CrossRef]
- Mayr, C.M.; Parker, M.; Baldock, G.A.; Black, C.A.; Pardon, K.H.; Williamson, P.O.; Herderich, M.J.; Francis, I.L. Determination of the Importance of In-Mouth Release of Volatile Phenol Glycoconjugates to the Flavor of Smoke-Tainted Wines. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; Osidacz, P.; Pinchbeck, K.A.; Hayasaka, Y.; Fudge, A.L.; Wilkinson, K.L. The effect of winemaking techniques on the intensity of smoke taint in wine. Aust. J. Grape Wine Res. 2011, 17, S29–S40. [Google Scholar] [CrossRef]
- King, E.S.; Heymann, H. The Effect of Reduced Alcohol on the Sensory Profiles and Consumer Preferences of White Wine. J. Sens. Stud. 2014, 29, 33–42. [Google Scholar] [CrossRef]
- Fryer, J.A.; Collins, T.S.; Tomasino, E. Evaluation of Different Interstimulus Rinse Protocols on Smoke Attribute Perception in Wildfire-Affected Wines. Molecules 2021, 26, 5444. [Google Scholar] [CrossRef] [PubMed]
- CALFire. Incidents and Statistics. 16 January 2019; CA.gov: Glendale, CA, USA, 2019.
- McKay, M.; Bauer, F.F.; Panzeri, V.; Buica, A. Investigation of olfactory interactions of low levels of five off-flavour causing compounds in a red wine matrix. Food Res. Int. 2020, 128, 108878. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; van der Hulst, L.; Capone, D.L.; Wilkinson, K.L. Impact of Bottle Aging on Smoke-Tainted Wines from Different Grape Cultivars. J. Agric. Food Chem. 2017, 65, 4146–4152. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.M.; Geue, J.P.; Holt, H.E.; Pearson, W.P.; Jeffery, D.W.; Francis, I.L. Characterization of the Key Aroma Compounds in Shiraz Wine by Quantitation, Aroma Reconstitution, and Omission Studies. J. Agric. Food Chem. 2014, 62, 4528–4536. [Google Scholar] [CrossRef]
- King, E.S.; Dunn, R.L.; Heymann, H. The influence of alcohol on the sensory perception of red wines. Food Qual. Prefer. 2013, 28, 235–243. [Google Scholar] [CrossRef]
- Pollnitz, A.P.; Pardon, K.H.; Sykes, M.; Sefton, M.A. The Effects of Sample Preparation and Gas Chromatograph Injection Techniques on the Accuracy of Measuring Guaiacol, 4-Methylguaiacol and Other Volatile Oak Compounds in Oak Extracts by Stable Isotope Dilution Analyses. J. Agric. Food Chem. 2004, 52, 3244–3252. [Google Scholar] [CrossRef]
- Lerno, L.; Reichwage, M.; Ponangi, R.; Hearne, L.; Block, D.E.; Oberholster, A. Effects of Cap and Overall Fermentation Temperature on Phenolic Extraction in Cabernet Sauvignon Fermentations. Am. J. Enol. Vitic. 2015, 66, 444–453. [Google Scholar] [CrossRef]
- Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. The effect of a commercial pectolytic enzyme on grape skin cell wall degradation and colour evolution during the maceration process. Food Chem. 2012, 130, 626–631. [Google Scholar] [CrossRef]
- Noestheden, M.; Thiessen, K.; Dennis, E.G.; Tiet, B.; Zandberg, W.F. Quantitating Organoleptic Volatile Phenols in Smoke-Exposed Vitis vinifera Berries. J. Agric. Food Chem. 2017, 65, 8418–8425. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Baldock, G.A.; Parker, M.; Pardon, K.H.; Black, C.A.; Herderich, M.J.; Jeffery, D.W. Glycosylation of Smoke-Derived Volatile Phenols in Grapes as a Consequence of Grapevine Exposure to Bushfire Smoke. J. Agric. Food Chem. 2010, 58, 10989–10998. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: Ithaca, NY, USA, 2010. [Google Scholar]



| Wine | Alcohol (%) | pH | TA (g/L) | Residual Sugar (g/L) |
|---|---|---|---|---|
| Panel 1 (Impact of yeast/oak) | ||||
| OFV6 | 16.68 ± 0.18 a | 3.78 ± 0.02 e | 6.18 ± 0.25 a | 1.21 ± 0.45 b |
| EC1118 | 16.35 ± 0.11 a | 3.75 ± 0.01 b | 5.60 ± 0.24 c | 1.01 ± 0.24 b |
| BDX | 15.44 ± 0.40 c | 3.78 ± 0.07 d | 5.63 ± 0.11 c | 6.88 ± 0.60 a |
| D80 | 15.89 ± 0.14 b | 3.72 ± 0.01 bc | 5.99 ± 0.02 ab | 0.76 ± 0.29 bc |
| D254 | 16.63 ± 0.05 a | 3.73 ± 0.02 bc | 5.90 ± 0.03 abc | 0.96 ± 0.23 b |
| NB | 15.90 ± 0.01 b | 3.80 ± 0.04 cd | 5.62 ± 0.11 c | 0.75 ± 0.04 bc |
| NF | 15.74 ± 0.08 bc | 3.77 ± 0.00 b | 5.73 ± 0.18 bc | 0.74 ± 0.08 bc |
| QT | 15.89 ± 0.11 b | 3.82 ± 0.00 a | 5.63 ± 0.11 c | 0.75 ± 0.03 bc |
| Unsmoked | 14.72 ± 0.06 d | 3.88 ± 0.03 a | 5.94 ± 0.10 abc | 0.42 ± 0.10 c |
| Panel 2 (Impact of fermentation temperature/maceration enzymes and tannin) | ||||
| AV_25 | 14.83 ± 0.47 cd | 3.81 ± 0.01 d | 5.40 ± 0.09 cd | 0.44 ± 0.06 b |
| AV_20 | 14.92 ± 0.06 cd | 3.82 ± 0.01 d | 5.22 ± 0.05 d | 0.49 ± 0.29 b |
| AV_15 | 15.14 ± 0.02 bcd | 3.83 ± 0.01 d | 5.47 ± 0.14 c | 0.52 ± 0.06 b |
| AV_10 | 15.18 ± 0.29 bcd | 3.64 ± 0.05 e | 5.48 ± 0.08 c | 0.41 ± 0.35 b |
| ST_C | 15.49 ± 0.14 b | 3.96 ± 0.01 a | 4.91 ± 0.02 e | 0.38 ± 0.01 b |
| ST_E+T | 15.30 ± 0.17 bc | 3.85 ± 0.01 b | 5.42 ± 0.08 cd | 0.53 ± 0.01 b |
| S_Control | 15.51 ± 0.16 d | 3.71 ± 0.02 d | 6.42 ± 0.16 a | 0.61 ± 0.18 b |
| NS_Control | 14.30 ± 0.02 a | 3.70 ± 0.03 c | 6.21 ± 0.21 b | 0.24 ± 0.02 a |
| Wine | Guaiacol | Creosol | o-Cresol | 4-Ethylguaiacol | p-Cresol | m-Cresol | 4-Ethylphenol | Syringol | 4-Methylsyringol |
|---|---|---|---|---|---|---|---|---|---|
| Panel 1 (Impact of yeast/oak) | |||||||||
| OFV6 | 5.7 a | 0.8 cd | 2.5 ab | 0.3 ab | 1.7 cdef | 2.4 ab | 2.8 a | 54.6 a | 8.0 d |
| EC1118 | 4.8 cd | 0.8 cd | 2.5 ab | 0.3 b | 1.8 bcde | 2.4 ab | 1.2 a | 51.5 ab | 8.3 cd |
| BDX | 5.5 a | 0.8 cd | 2.6 ab | 0.4 a | 1.5 ef | 2.6 ab | 1.4 a | 53.1 ab | 8.3 cd |
| D80 | 4.7 d | 0.9 bc | 2.5 ab | 0.3 ab | 1.4 f | 2.3 b | 1.1 a | 42.9 e | 8.1 cd |
| D254 | 5.4 ab | 1.0 bc | 2.6 a | 0.4 ab | 2.0 abc | 2.6 a | 2.8 a | 50.7 bc | 8.4 cd |
| NB | 5.0 bcd | 0.7 d | 2.4 b | 0.4 ab | 2.2 ab | 2.4 ab | 1.4 a | 46.8 d | 9.8 b |
| NF | 5.2 abc | 1.9 a | 2.6 a | 0.3 ab | 2.4 a | 2.6 ab | 1.6 a | 48.3 cd | 8.5 c |
| QT | 4.9 cd | 1.0 b | 2.6 a | 0.3 b | 1.9 bcd | 2.5 ab | 1.3 a | 50.6 bc | 11.6 a |
| Unsmoked | 2.7 e | 0.2 e | 1.2 c | 0.1 c | 1.6 def | 0.8 c | 1.1 a | 37.9 f | 6.6 e |
| Pr > F(Model) | 0.000 | <0.0001 | <0.0001 | 0.000 | 0.245 | 0.036 | 0.502 | <0.0001 | <0.0001 |
| Panel 2 (Impact of fermentation temperature/maceration enzymes and tannin) | |||||||||
| AV_25 | 7.7 a | 0.9 b | 3.6 a | 0.2 c | 3.5 bc | 4.2 c | 2.1 bc | 95.3 a | 15.6 a |
| AV_20 | 7.5 a | 0.9 b | 3.8 a | 0.2 c | 4.0 ab | 4.8 b | 2.2 bc | 91.9 b | 15.9 a |
| AV_15 | 7.7 a | 1.0 ab | 3.2 ab | 0.3 bc | 4.4 a | 5.5 a | 3.3 a | 87.1 c | 16.0 a |
| AV_10 | 6.7 b | 0.9 ab | 3.5 a | 0.4 b | 3.5 bc | 4.7 b | 2.9 ab | 65.2 d | 14.6 b |
| ST_C | 5.9 c | 0.5 cd | 1.7 cd | 0.3 bc | 2.6 d | 1.9 e | 1.8 c | 50.2 e | 8.4 c |
| ST_E+T | 7.4 a | 0.6 c | 2.3 bcd | 0.3 bc | 3.3 c | 1.8 e | 1.3 c | 49.4 e | 8.4 c |
| S_Control | 4.3 d | 1.1 a | 2.8 abc | 0.6 a | 2.1 d | 2.6 d | 2.2 bc | 37.9 f | 8.1 c |
| NS_Control | 2.6 e | 0.3 d | 1.611 d | 0.247 c | 2.1 d | 1.0 f | 1.4 c | 35.4 f | 6.4 d |
| Pr > F(Model) | <0.0001 | <0.0001 | 0.004 | 0.002 | <0.0001 | <0.0001 | 0.004 | <0.0001 | <0.0001 |
| Sample | Guaiacol | Creosol | o-Cresol | 4-Ethylguaiacol | p-Cresol | m-Cresol | 4-Ethylphenol | Syringol |
|---|---|---|---|---|---|---|---|---|
| AV_25 | ||||||||
| After one day of cold soak | 7.1 d | 2.5 b | 3.7 bc | 14.1 cd | 0.3 c | 2.9 d | 4.9 cd | 3.9 b |
| After 2 days of fermentation | 7.9 cd | 2.6 b | 4.0 b | 15.3 c | 0.4 b | 3.5 d | 5.0 cd | 4.3 b |
| After 4 days of fermentation | 8.6 bc | 2.9 a | 4.4 a | 17.4 bc | 0.6 a | 5.2 ab | 5.5 bc | 4.4 ab |
| After 7 days of fermentation | 9.6 ab | 3.1 a | 4.7 a | 19.9 ab | 0.6 a | 5.8 a | 6.1 ab | 4.6 ab |
| After pressing | 10.2 a | 3.1 a | 4.6 a | 22.2 a | 0.6 a | 5.0 b | 6.2 a | 5.5 a |
| AV_20 | ||||||||
| After 2 days of fermentation | 7.8 b | 2.5 b | 4.2 b | 14.6 b | 0.3 b | 3.3 c | 5.1 b | 4.5 a |
| After 7 days of fermentation | 8.7 a | 2.7 a | 5.1 a | 17.4 a | 0.4 a | 5.6 a | 5.4 a | 4.6 a |
| After pressing | 8.6 a | 2.6 a | 5.0 a | 17.0 a | 0.4 a | 4.9 b | 5.3 ab | 4.6 a |
| AV_15 | ||||||||
| After 2 days of fermentation | 7.3 c | 2.4 a | 3.8 b | 13.4 b | 0.4 a | 2.8 b | 4.8 a | 4.4 b |
| After 7 days of fermentation | 7.8 b | 2.46 a | 4.5 a | 15.2 ab | 0.4 a | 3.7 ab | 4.8 a | 4.3 b |
| After pressing | 8.2 a | 2.6 a | 4.7 a | 16.6 a | 0.4 a | 4.5 a | 5.1 a | 4.5 a |
| AV_10 | ||||||||
| After 7 days of cold soak | 6.7 a | 2.4 a | 3.5 b | 11.9 b | 0.3 b | 2.9 a | 4.6 a | 4.1 a |
| After pressing | 6.9 a | 2.2 b | 4.0 a | 13.8 a | 0.6 a | 3.5 a | 4.4 a | 4.1 a |
| After 7 days of fermentation | 6.1 b | 1.9 c | 3.1 c | 8.6 c | 0.2 c | 2.2 b | 3.8 b | 3.7 b |
| Wines | Fig/Dried Fruit | Mineral | Honey | Petrol | Ashy Aftertaste | Sweet | Hot | Astringent | Viscous |
|---|---|---|---|---|---|---|---|---|---|
| BDX_1 | 2.0 | 1.1 | 0.8 | 1.0 | 2.3 | 2.9 | 3.7 | 3.7 | 3.8 |
| BDX_2 | 3.3 | 0.8 | 1.4 | 0.3 | 1.3 | 3.0 | 2.7 | 3.2 | 4.2 |
| D254_1 | 1.8 | 0.9 | 0.8 | 0.5 | 2.5 | 2.4 | 3.1 | 4.3 | 3.5 |
| D254_2 | 1.9 | 1.6 | 1.0 | 0.7 | 2.3 | 2.9 | 3.2 | 4.3 | 3.5 |
| D80_1 | 2.1 | 0.8 | 0.5 | 0.3 | 1.6 | 2.7 | 3.0 | 3.6 | 3.5 |
| D80_2 | 1.5 | 1.1 | 0.8 | 0.5 | 2.4 | 2.3 | 3.0 | 3.4 | 3.1 |
| EC1118_1 | 2.2 | 1.2 | 0.9 | 0.3 | 2.6 | 2.6 | 3.7 | 4.0 | 3.0 |
| EC1118_2 | 2.5 | 1.5 | 0.6 | 0.5 | 2.3 | 2.3 | 2.7 | 4.0 | 3.2 |
| NB_1 | 2.3 | 1.0 | 0.7 | 0.3 | 2.4 | 2.0 | 3.8 | 3.9 | 2.9 |
| NB_2 | 1.9 | 1.1 | 0.8 | 0.4 | 2.4 | 2.2 | 3.4 | 3.5 | 3.5 |
| NF_1 | 2.0 | 1.5 | 0.6 | 0.3 | 2.3 | 1.9 | 2.4 | 3.4 | 2.8 |
| NF_2 | 2.2 | 0.9 | 0.6 | 0.6 | 1.7 | 2.5 | 3.2 | 3.9 | 3.2 |
| OFV6_1 | 2.1 | 1.1 | 0.6 | 0.4 | 2.0 | 2.4 | 3.5 | 3.3 | 3.5 |
| OFV6_2 | 2.7 | 0.8 | 0.7 | 0.5 | 2.0 | 2.3 | 4.0 | 3.8 | 3.6 |
| QT_1 | 1.9 | 1.3 | 0.7 | 0.3 | 2.9 | 1.8 | 4.2 | 3.7 | 3.2 |
| QT_2 | 2.0 | 1.5 | 0.4 | 0.3 | 2.0 | 1.7 | 3.1 | 4.0 | 2.9 |
| Unsmoked_1 | 3.7 | 1.0 | 2.0 | 0.2 | 1.1 | 3.0 | 2.4 | 1.2 | 4.8 |
| Unsmoked_2 | 2.1 | 1.0 | 0.6 | 0.4 | 1.5 | 3.2 | 2.8 | 2.0 | 3.9 |
| LSD-value | 0.8 | 0.5 | 0.5 | 0.4 | 0.9 | 0.9 | 1.0 | 1.1 | 1.0 |
| Wines | Dark fruit | Vanilla | Green | Smoky | Raisin/Prune | Leather | Hot | Astringent |
|---|---|---|---|---|---|---|---|---|
| AV_10.1 | 3.0 | 1.3 | 0.9 | 1.5 | 2.3 | 1.0 | 4.1 | 3.0 |
| AV_10.2 | 2.3 | 1.7 | 1.1 | 1.7 | 2.1 | 1.1 | 3.8 | 3.2 |
| AV_15.1 | 2.7 | 1.6 | 1.2 | 1.4 | 2.0 | 0.9 | 4.3 | 3.4 |
| AV_15.3 | 1.8 | 1.3 | 1.8 | 1.5 | 1.4 | 1.5 | 4.1 | 3.7 |
| AV_20.1 | 3.0 | 1.6 | 0.9 | 1.9 | 2.0 | 1.0 | 3.9 | 3.6 |
| AV_20.2 | 2.8 | 1.8 | 0.8 | 1.5 | 2.4 | 1.1 | 4.1 | 3.6 |
| AV_25.1 | 2.6 | 1.8 | 0.8 | 1.9 | 2.1 | 1.4 | 4.1 | 4.4 |
| AV_25.2 | 2.5 | 1.6 | 1.0 | 1.7 | 2.4 | 1.0 | 4.2 | 4.4 |
| NS-Control_1 | 2.7 | 2.4 | 0.8 | 0.9 | 2.5 | 0.6 | 4.6 | 5.0 |
| NS-Control_2 | 3.1 | 1.9 | 1.2 | 0.7 | 2.6 | 0.6 | 5.3 | 5.2 |
| S-Control_1 | 2.8 | 2.7 | 0.6 | 1.3 | 2.5 | 0.7 | 3.8 | 3.4 |
| S-Control_2 | 3.8 | 2.0 | 0.4 | 1.2 | 3.0 | 0.7 | 3.8 | 4.4 |
| ST_C1 | 3.7 | 1.4 | 0.9 | 1.4 | 2.7 | 1.0 | 4.3 | 5.1 |
| ST_C2 | 3.7 | 1.6 | 0.9 | 1.1 | 2.7 | 0.8 | 4.7 | 5.1 |
| ST_E+T_1 | 3.1 | 1.9 | 0.6 | 1.3 | 2.4 | 0.9 | 3.9 | 6.2 |
| ST_E+T_2 | 2.2 | 1.3 | 1.1 | 1.0 | 1.7 | 1.5 | 4.4 | 6.3 |
| LSD-value | 0.8 | 0.7 | 0.6 | 0.6 | 0.7 | 0.5 | 0.8 | 0.7 |
| Wine code | Vineyard | Treatment |
|---|---|---|
| OFV6 | Oakville block 6 | Standard SOP |
| EC1118 (Control) | Oakville block 9 | Standard SOP, treatment control fermented with yeast EC1118 |
| BDX | Oakville block 9 | Fermented with yeast BDX |
| D80 | Oakville block 9 | Fermented with yeast D80 |
| D254 | Oakville block 9 | Fermented with yeast D254 |
| NB | Oakville block 9 | Addition of Nobile Base oak chips |
| NF | Oakville block 9 | Addition of Nobile Fresh oak chips |
| QT | Oakville block 9 | Additions of ellagic oak tannin (Quertanin) |
| Unsmoked | Oakville block 9 | Standard SOP, fruit harvested before the wildfires, rootstock 110R |
| AV_25 | Alexander Valley | Standard SOP, treatment control fermented at 25 °C |
| AV_20 | Alexander Valley | Fermented at 20 °C |
| AV_15 | Alexander Valley | Fermented at 15 °C |
| AV_10 | Alexander Valley | Cold soak at 10 °C for 7 days, pressed, fermented without skins/seeds |
| ST_C | Stag’s Leap District | Standard SOP, treatment control |
| ST_E+T | Stag’s Leap District | Addition of enzymes and tannin |
| S-Control | Oakville block 9 | Standard SOP from smoke-exposed fruit |
| NS-Control | Oakville block 9 | Standard SOP from non-smoke-exposed fruit harvested before fires rootstock 420A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberholster, A.; Wen, Y.; Dominguez Suarez, S.; Erdmann, J.; Cauduro Girardello, R.; Rumbaugh, A.; Neupane, B.; Brenneman, C.; Cantu, A.; Heymann, H. Investigation of Different Winemaking Protocols to Mitigate Smoke Taint Character in Wine. Molecules 2022, 27, 1732. https://doi.org/10.3390/molecules27051732
Oberholster A, Wen Y, Dominguez Suarez S, Erdmann J, Cauduro Girardello R, Rumbaugh A, Neupane B, Brenneman C, Cantu A, Heymann H. Investigation of Different Winemaking Protocols to Mitigate Smoke Taint Character in Wine. Molecules. 2022; 27(5):1732. https://doi.org/10.3390/molecules27051732
Chicago/Turabian StyleOberholster, Anita, Yan Wen, Sandra Dominguez Suarez, Jesse Erdmann, Raul Cauduro Girardello, Arran Rumbaugh, Bishnu Neupane, Charles Brenneman, Annegret Cantu, and Hildegarde Heymann. 2022. "Investigation of Different Winemaking Protocols to Mitigate Smoke Taint Character in Wine" Molecules 27, no. 5: 1732. https://doi.org/10.3390/molecules27051732
APA StyleOberholster, A., Wen, Y., Dominguez Suarez, S., Erdmann, J., Cauduro Girardello, R., Rumbaugh, A., Neupane, B., Brenneman, C., Cantu, A., & Heymann, H. (2022). Investigation of Different Winemaking Protocols to Mitigate Smoke Taint Character in Wine. Molecules, 27(5), 1732. https://doi.org/10.3390/molecules27051732








