Unique Properties of Heme Binding of the Porphyromonas gingivalis HmuY Hemophore-like Protein Result from the Evolutionary Adaptation of the Protein Structure
Abstract
:1. Introduction
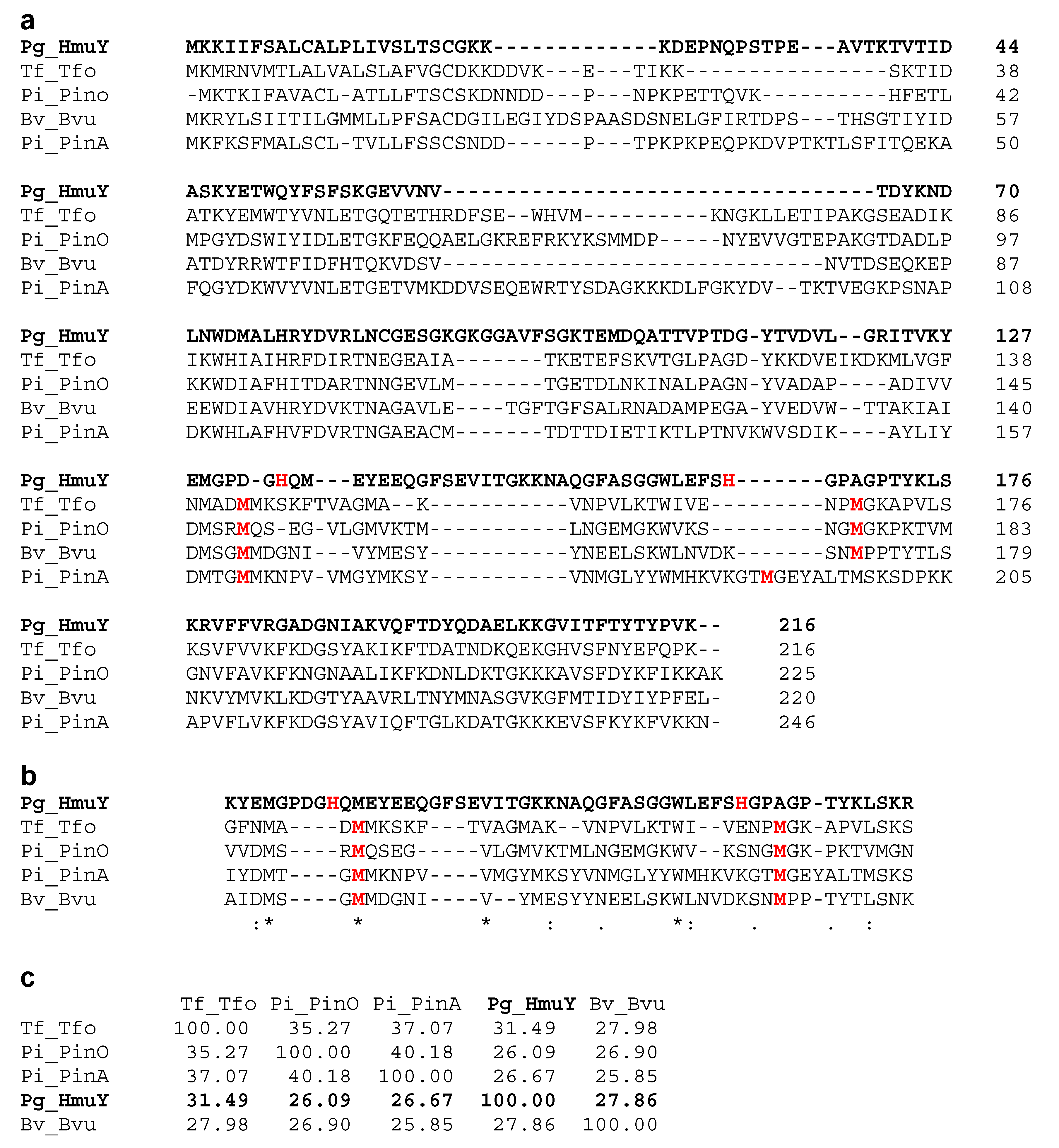
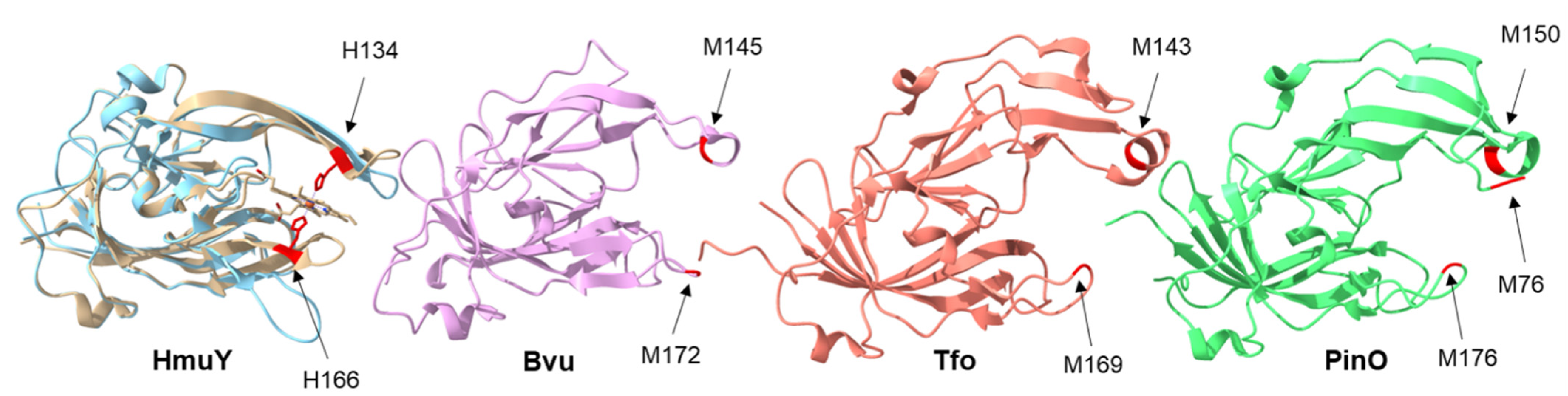
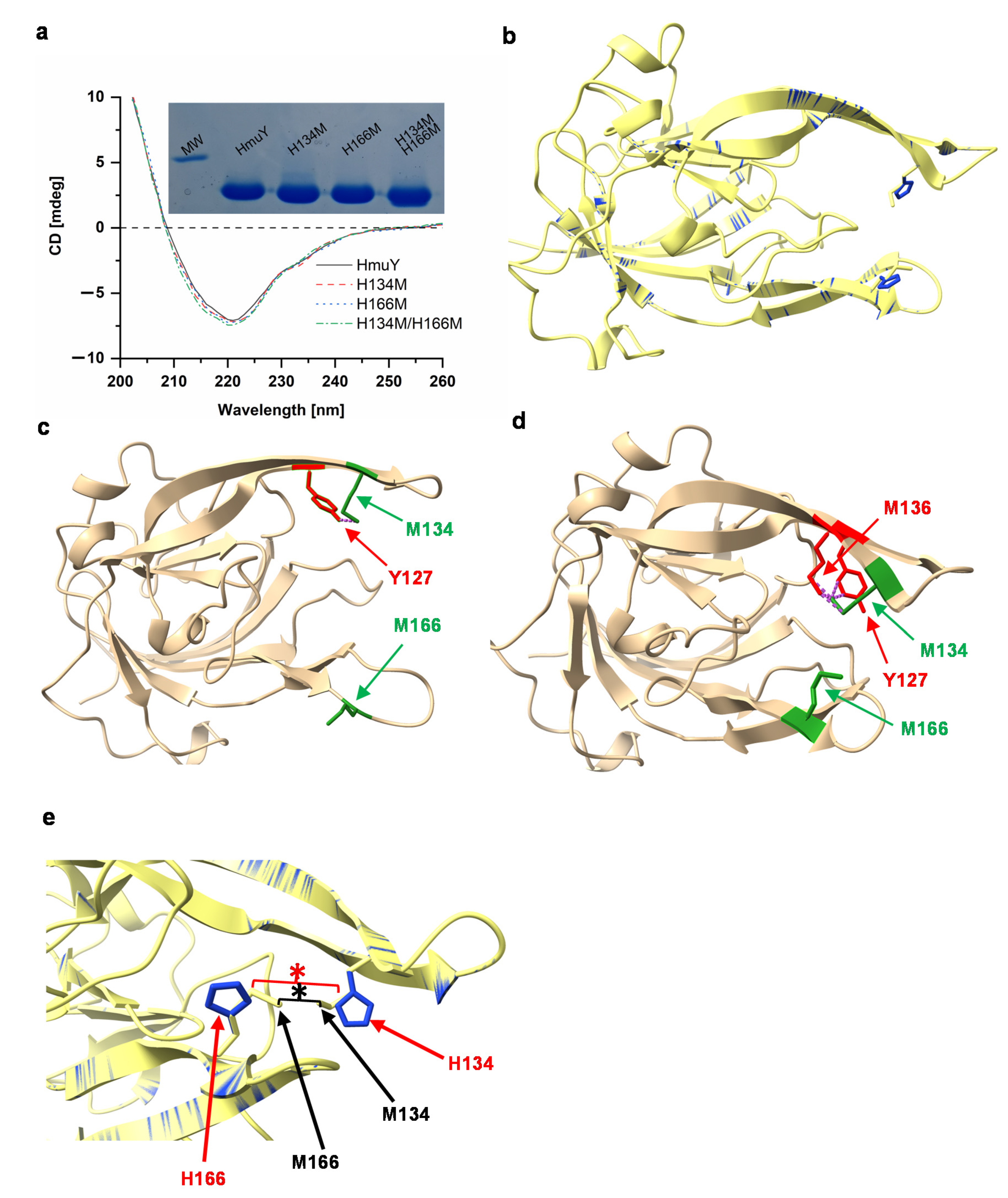
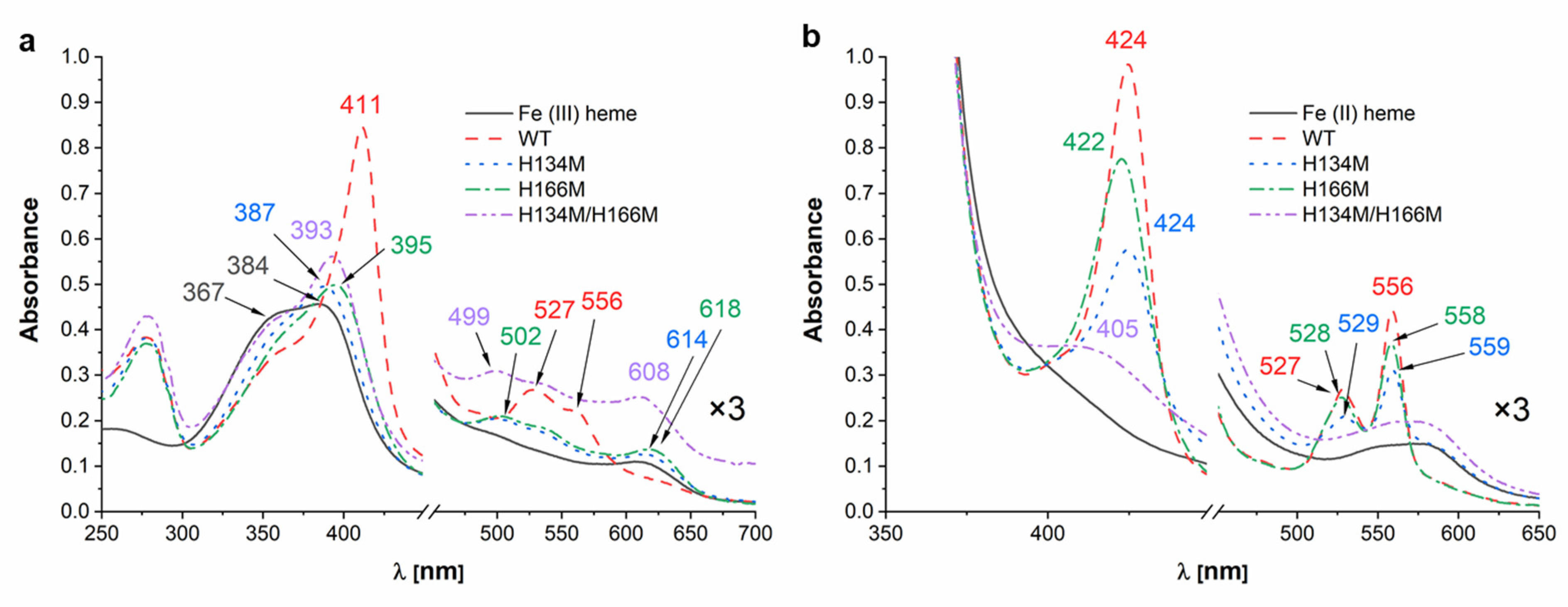
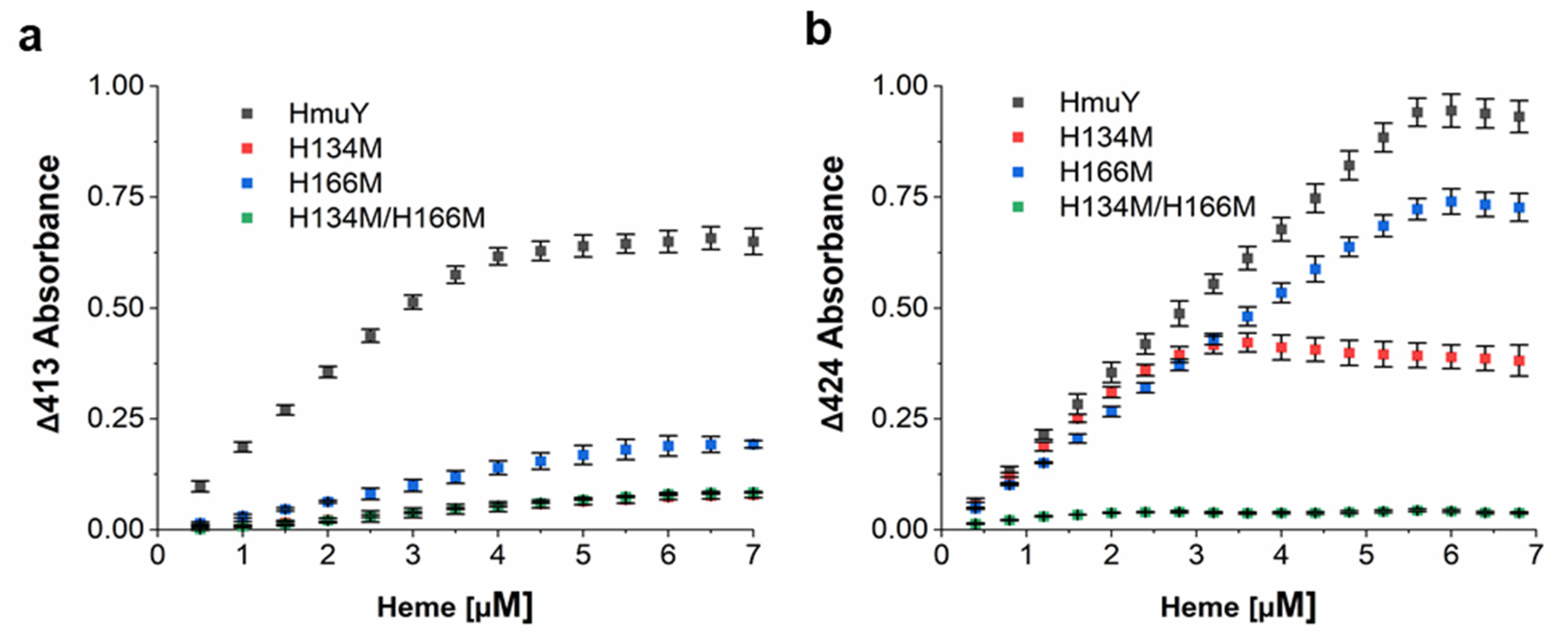
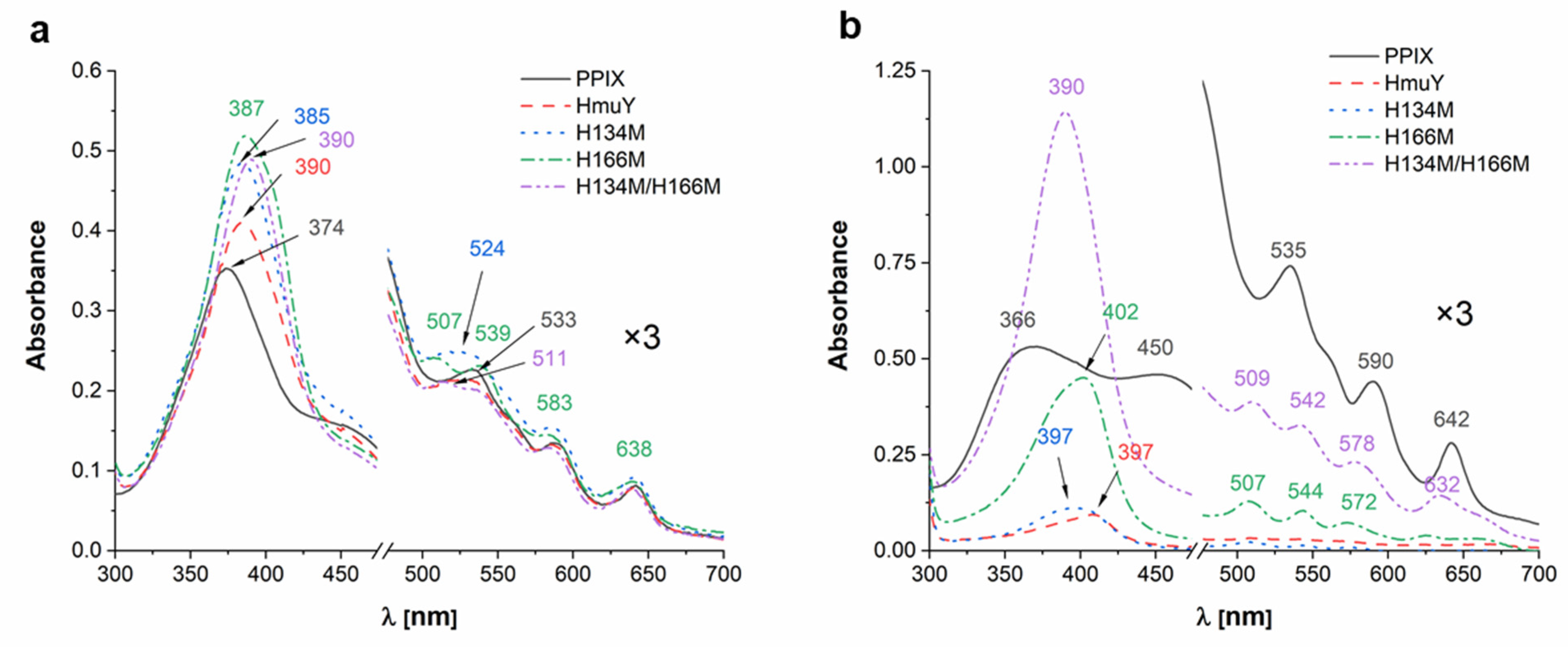
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Site-Directed Mutagenesis
4.2. Protein Overexpression and Purification
4.3. Heme and PPIX Binding Experiments
4.4. Far-UV Circular Dichroism (Far UV CD) Spectroscopy
4.5. Bioinformatic and Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Socransky, S.S.; Hafajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Holt, S.C.; Ebersole, J.L. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol. 2000 2005, 38, 72–122. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamaguchi, A.; Ohyama, T.; Sakai, E.; Nakamura, R.; Watanabe, T.; Baba, H.; Nakayama, K. Adhesins encoded by the gingipain genes of Porphyromonas gingivalis are responsible for co-aggregation with Prevotella intermedia. Microbiology 2003, 149, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.L.; Sztajer, H.; Jarek, M.; Bhuju, S.; Wagner-Dobler, I. Worlds apart–transcriptome profiles of key oral microbes in the periodontal pocket compared to single laboratory culture reflect synergistic interactions. Front. Microbiol. 2018, 9, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llambes, F.; Arias-Herrera, S.; Caffesse, R. Relationship between diabetes and periodontal infection. World J. Diabetes 2015, 6, 927–935. [Google Scholar] [CrossRef]
- Bansal, M.; Khatri, M.; Taneja, V. Potential role of periodontal infection in respiratory diseases–a review. J. Med. Life 2013, 6, 244–248. [Google Scholar]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedyk, M.; Byrne, D.P.; Glowczyk, I.; Potempa, J.; Olczak, M.; Olczak, T.; Smalley, J.W. Pyocyanin: A contributory factor in haem acquisition and virulence enhancement of Porphyromonas gingivalis in the lung. PLoS ONE 2015, 10, e0118319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smiga, M.; Smalley, J.W.; Slezak, P.; Brown, J.L.; Sieminska, K.; Rosalind, R.E.; Yates, E.A.; Olczak, T. Glycation of host proteins increases pathogenic potential of Porphyromonas gingivalis. Int J. Mol. Sci 2021, 22, 12084. [Google Scholar] [CrossRef] [PubMed]
- Bregaint, S.; Boyer, E.; Fong, S.B.; Meuric, V.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Porphyromonas gingivalis outside the oral cavity. Odontol 2021. [Google Scholar] [CrossRef]
- Aguayo, S.; Schuh, C.M.A.P.; Vicente, B.; Aguayo, L.G. Association between Alzheimer’s disease and oral and gut microbiota: Are pore forming proteins the missing link ? J. Alzheimers Dis. 2018, 65, 29–46. [Google Scholar] [CrossRef]
- Carter, C.J.; France, J.; Crean, S.; Singhrao, S.K. The Porphyromonas gingivalis/host interactome shows enrichment in GWASdb genes related to Alzheimer’s disease, diabetes and cardiovascular diseases. Front. Aging Neurosci. 2017, 9, 408. [Google Scholar] [CrossRef] [Green Version]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and its systemic impact: Current status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Olczak, T. Haem acquisition mechanisms of Porphyromonas gingivalis-strategies used in polymicrobial community in a haem-limited host environment. Mol. Oral Microbiol. 2017, 32, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Smalley, J.W.; Byrne, D.P.; Birss, A.J.; Wojtowicz, H.; Sroka, A.; Potempa, J.; Olczak, T. HmuY haemophore and gingipain proteases constitute a unique syntrophic system of haem acquisition by Porphyromonas gingivalis. PLoS ONE 2011, 6, e17182. [Google Scholar] [CrossRef]
- Hargrove, M.S.; Barrick, D.; Olson, J.S. The association rate constant for heme binding to globin is independent of protein structure. Biochemistry 1996, 35, 11293–11299. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.P.; Potempa, J.; Olczak, T.; Smalley, J.W. Evidence of mutualism between two periodontal pathogens: Co-operative haem acquisition by the HmuY haemophore of Porphyromonas gingivalis and the cysteine protease interpain A (InpA) of Prevotella intermedia. Mol. Oral Microbiol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smalley, J.W.; Birss, A.J.; Szmigielski, B.; Potempa, J. Sequential action of R and K-specific gingipains of Porphyromonas gingivalis in the generation of the haem containing pigment from oxyhaemoglobin. Arch. Biochem. Biophys. 2007, 465, 44–49. [Google Scholar] [CrossRef]
- Smalley, J.W.; Birss, A.J.; Szmigielski, B.; Potempa, J. Mechanism of methaemoglobin breakdown by the lysine-specific gingipain of the periodontal pathogen Porphyromonas gingivalis. Biol. Chem. 2008, 389, 1235–1238. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L.; Yates, E.A.; Bielecki, M.; Olczak, T.; Smalley, J.W. Potential role for Streptococcus gordonii-derived hydrogen peroxide in heme acquisition by Porphyromonas gingivalis. Mol. Oral Microbiol. 2018, 33, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Slezak, P.; Smiga, M.; Smalley, J.W.; Sieminska, K.; Olczak, T. Porphyromonas gingivalis HmuY and Streptococcus gordonii GAPDH–novel heme acquisition strategy in the oral microbiome. Int J. Mol. Sci 2020, 21, 4150. [Google Scholar] [CrossRef] [PubMed]
- Smiga, M.; Bielecki, M.; Olczak, M.; Smalley, J.W.; Olczak, T. Anti-HmuY antibodies specifically recognize Porphyromonas gingivalis HmuY protein but not homologous proteins in other periodontopathogens. PLoS ONE 2015, 10, e0117508. [Google Scholar] [CrossRef] [PubMed]
- Gmiterek, A.; Wojtowicz, H.; Mackiewicz, P.; Radwan-Oczko, M.; Kantorowicz, M.; Chomyszyn-Gajewska, M.; Fraszczak, M.; Bielecki, M.; Olczak, M.; Olczak, T. The unique hmuY gene sequence as a specific marker of Porphyromonas gingivalis. PLoS ONE 2013, 8, e67719. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, M.; Antonyuk, S.; Strange, R.W.; Smalley, J.W.; Mackiewicz, P.; Smiga, M.; Stepien, P.; Olczak, M.; Olczak, T. Tannerella forsythia Tfo belongs to Porphyromonas gingivalis HmuY-like family of proteins but differs in heme-binding properties. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Bielecki, M.; Antonyuk, S.; Strange, R.W.; Sieminska, K.; Smalley, J.W.; Mackiewicz, P.; Smiga, M.; Cowan, M.; Capper, M.J.; Slezak, P.; et al. Prevotella intermedia produces two proteins homologous to Porphyromonas gingivalis HmuY but with different heme coordination mode. Biochem. J. 2020, 477, 381–405. [Google Scholar] [CrossRef]
- Sieminska, K.; Cierpisz, P.; Smiga, M.; Olczak, T. Porphyromonas gingivalis HmuY and Bacteroides vulgatus Bvu–a novel competitive heme acquisition strategy. Int. J. Mol. Sci. 2021, 22, 2237. [Google Scholar] [CrossRef]
- Wojtowicz, H.; Guevara, T.; Tallant, C.; Olczak, M.; Sroka, A.; Potempa, J.; Sola, M.; Olczak, T.; Gomis-Ruth, F.X. Unique structure and stability of HmuY, a novel heme-binding protein of Porphyromonas gingivalis. PLoS Pathog. 2009, 5, e1000419. [Google Scholar] [CrossRef] [Green Version]
- Benson, D.R.; Rivera, M. Heme uptake and metabolism in bacteria. Met. Ions Life Sci. 2013, 12, 279–332. [Google Scholar] [CrossRef] [PubMed]
- Olczak, T.; Wojtowicz, H.; Ciuraszkiewicz, J.; Olczak, M. Species specificity, surface exposure, protein expression, immunogenicity, and participation in biofilm formation of Porphyromonas gingivalis HmuY. BMC Microbiol. 2010, 10, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojtowicz, H.; Wojaczynski, J.; Olczak, M.; Kroliczewski, J.; Latos-Grazynski, L.; Olczak, T. Heme environment of Porphyromonas gingivalis HmuY heme-binding protein. Biochem. Biophys. Res. Commun. 2009, 382, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Vouch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.K.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modelling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 22, 3533–3539. [Google Scholar] [CrossRef]
- Schejter, A.; Plotkin, B.; Vig, I. The reactivity of cytochrome c with soft ligands. FEBS Lett. 1991, 280, 199–201. [Google Scholar] [CrossRef] [Green Version]
- Ayers, P.W.; Parr, R.G.; Pearson, R.G. Elucidating the hard/soft acid/base principle: A perspective based on half-reactions. J. Chem. Phys. 2006, 124, 194107. [Google Scholar] [CrossRef]
- Kozlowski, P.; Bingham, J.R.; Jarzecki, A. Theoretical analysis of core size effect in metalloporphyrins. J. Phys. Chem. A 2008, 112, 12781–12788. [Google Scholar] [CrossRef]
- Wojtowicz, H.; Bielecki, M.; Wojaczynski, J.; Olczak, M.; Smalley, J.W.; Olczak, T. Porphyromonas gingivalis HmuY haemophore binds gallium(III), zinc(II), cobalt(III), manganese(III), nickel(II), and copper(II) protoporphyrin IX but in a manner different to iron(III) protoporphyrin IX. Metallomics 2013, 5, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojiljkovic, I.; Evavold, B.D.; Kumar, V. Antimicrobial properties of porphyrins. Expert Opin. Investig. Drugs 2002, 10, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, I.; Kumar, V.; Srinivasan, N. Non-iron metalloporphyrins: Potent antibacterial compounds that exploit haem/Hb uptake systems pf pathogenic bacteria. Mol. Microbiol. 1999, 31, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Shisaka, Y.; Iwai, Y.; Yamada, S.; Uehara, H.; Tosha, T.; Sugimoto, H.; Shiro, Y.; Stanfield, J.K.; Ogawa, K.; Watanabe, Y.; et al. Hijacking the heme acquisition system of Pseudomonas aeruginosa for the delivery of phtalocyanine as an antimicrobial. ACS Chem. Biol. 2019, 14, 1637–1642. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosno, J.; Siemińska, K.; Olczak, T. Unique Properties of Heme Binding of the Porphyromonas gingivalis HmuY Hemophore-like Protein Result from the Evolutionary Adaptation of the Protein Structure. Molecules 2022, 27, 1703. https://doi.org/10.3390/molecules27051703
Kosno J, Siemińska K, Olczak T. Unique Properties of Heme Binding of the Porphyromonas gingivalis HmuY Hemophore-like Protein Result from the Evolutionary Adaptation of the Protein Structure. Molecules. 2022; 27(5):1703. https://doi.org/10.3390/molecules27051703
Chicago/Turabian StyleKosno, Joanna, Klaudia Siemińska, and Teresa Olczak. 2022. "Unique Properties of Heme Binding of the Porphyromonas gingivalis HmuY Hemophore-like Protein Result from the Evolutionary Adaptation of the Protein Structure" Molecules 27, no. 5: 1703. https://doi.org/10.3390/molecules27051703
APA StyleKosno, J., Siemińska, K., & Olczak, T. (2022). Unique Properties of Heme Binding of the Porphyromonas gingivalis HmuY Hemophore-like Protein Result from the Evolutionary Adaptation of the Protein Structure. Molecules, 27(5), 1703. https://doi.org/10.3390/molecules27051703





