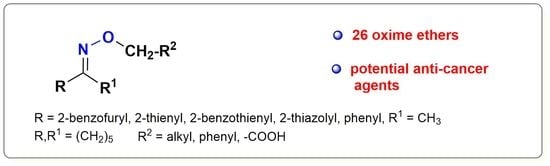
The Oxime Ethers with Heterocyclic, Alicyclic and Aromatic Moiety as Potential Anti-Cancer Agents
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
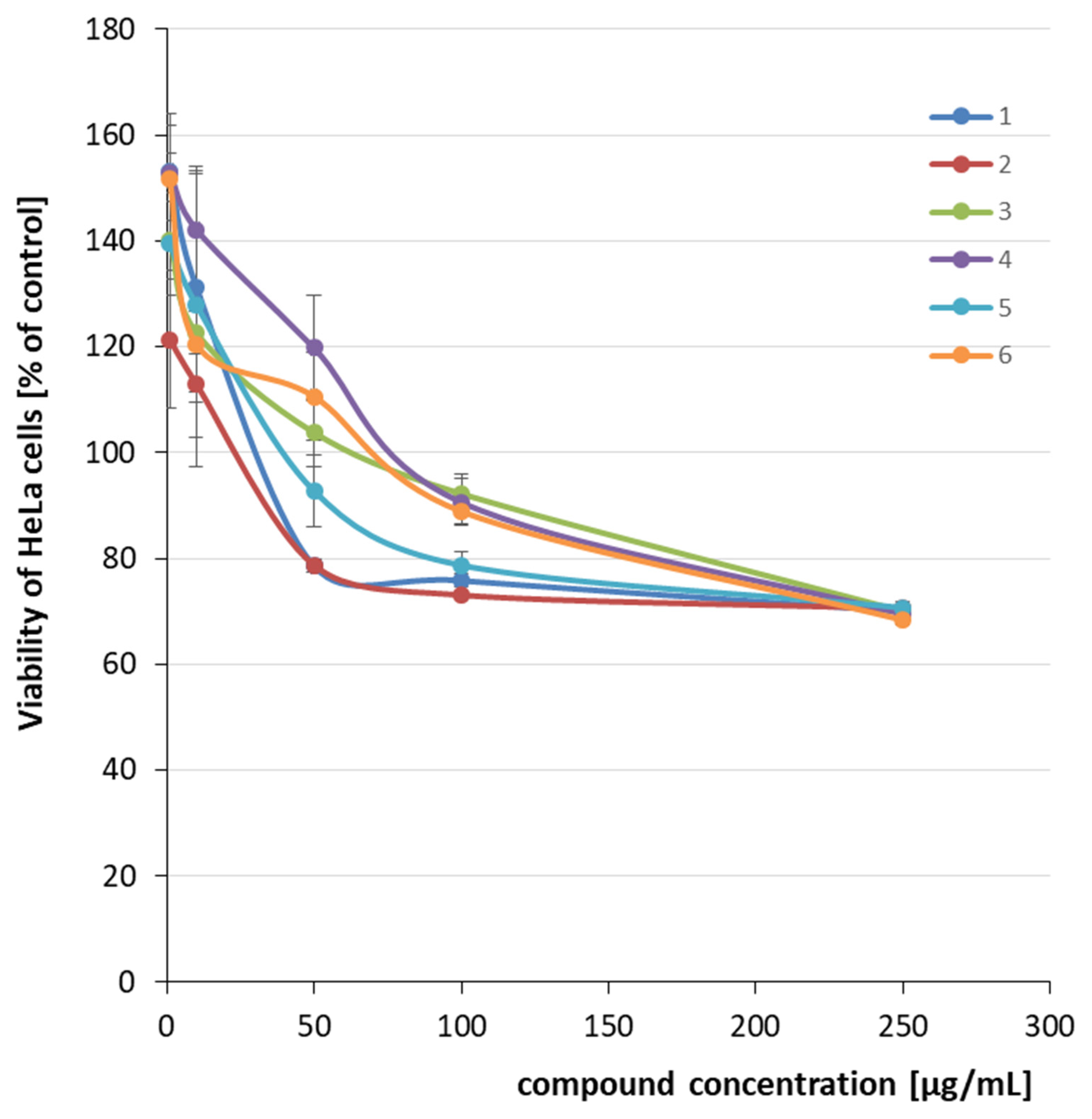
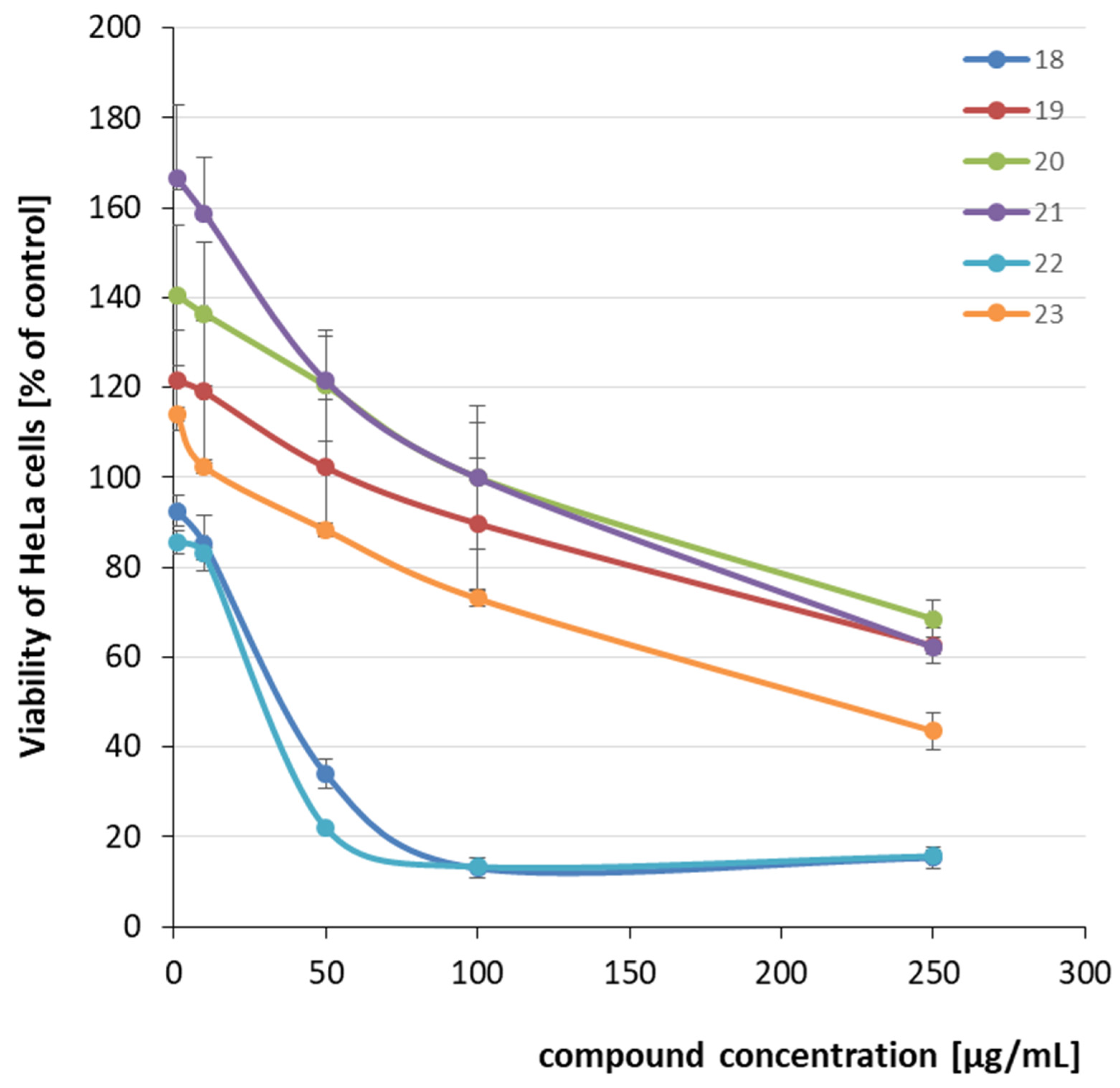
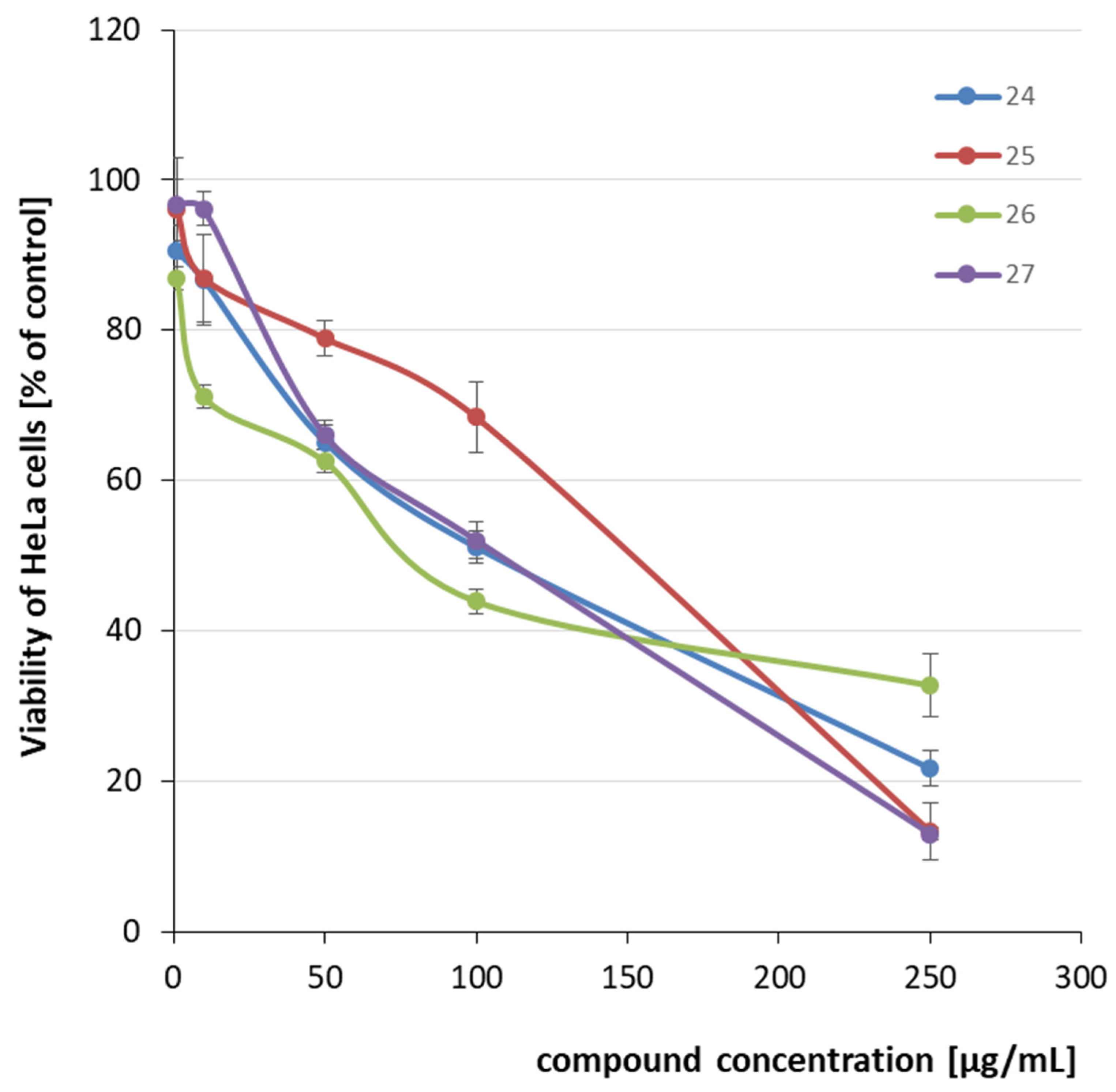
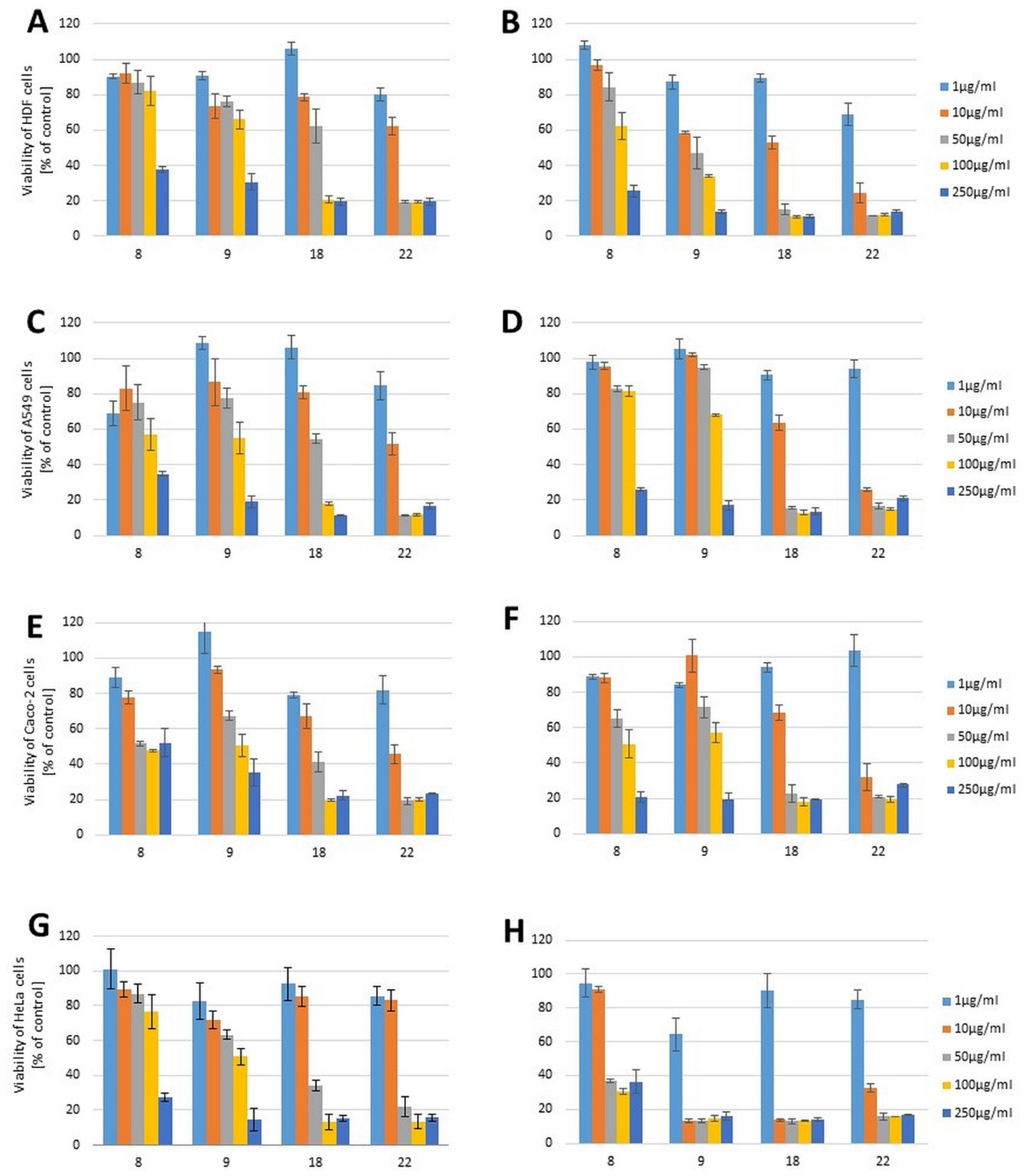
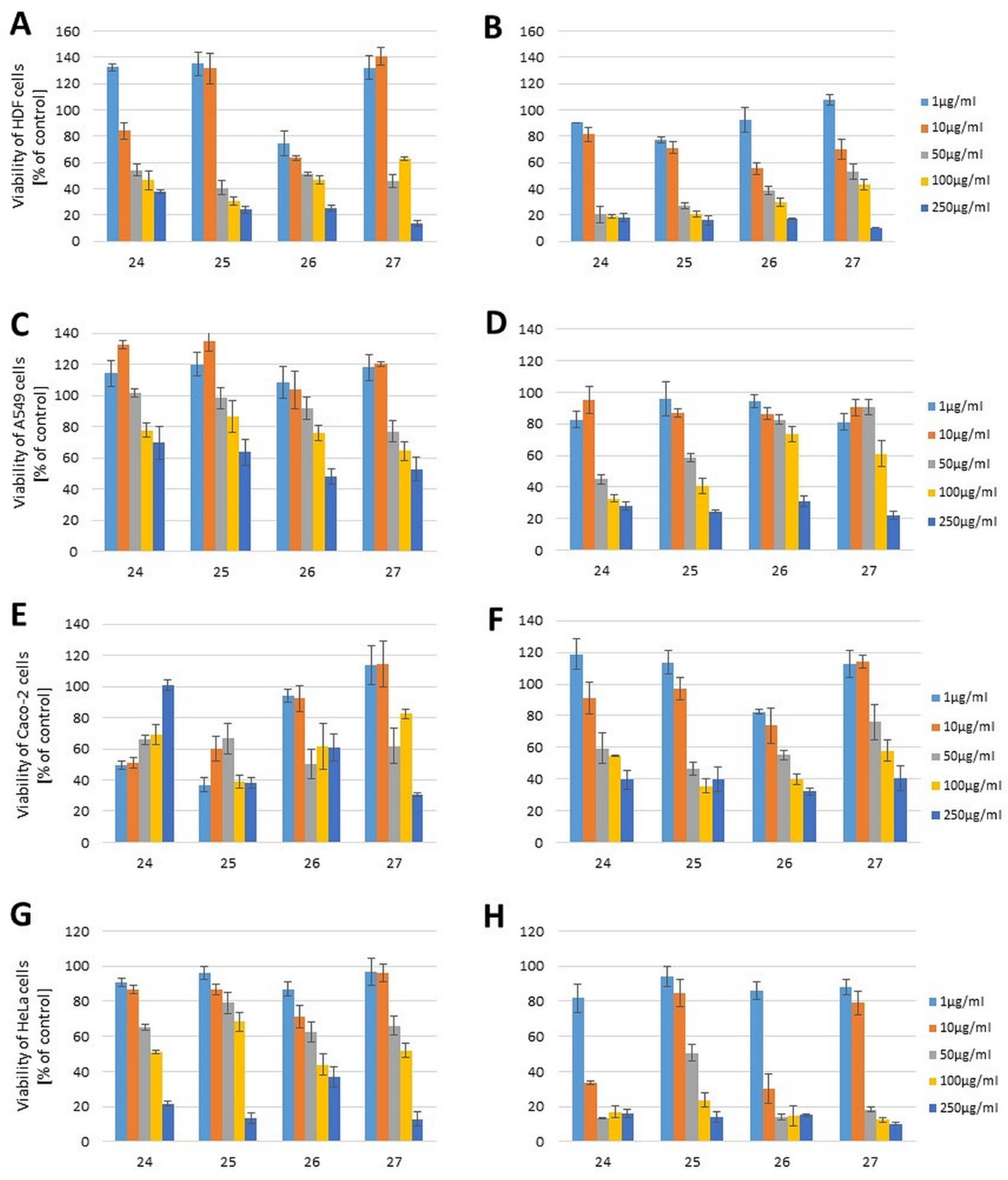
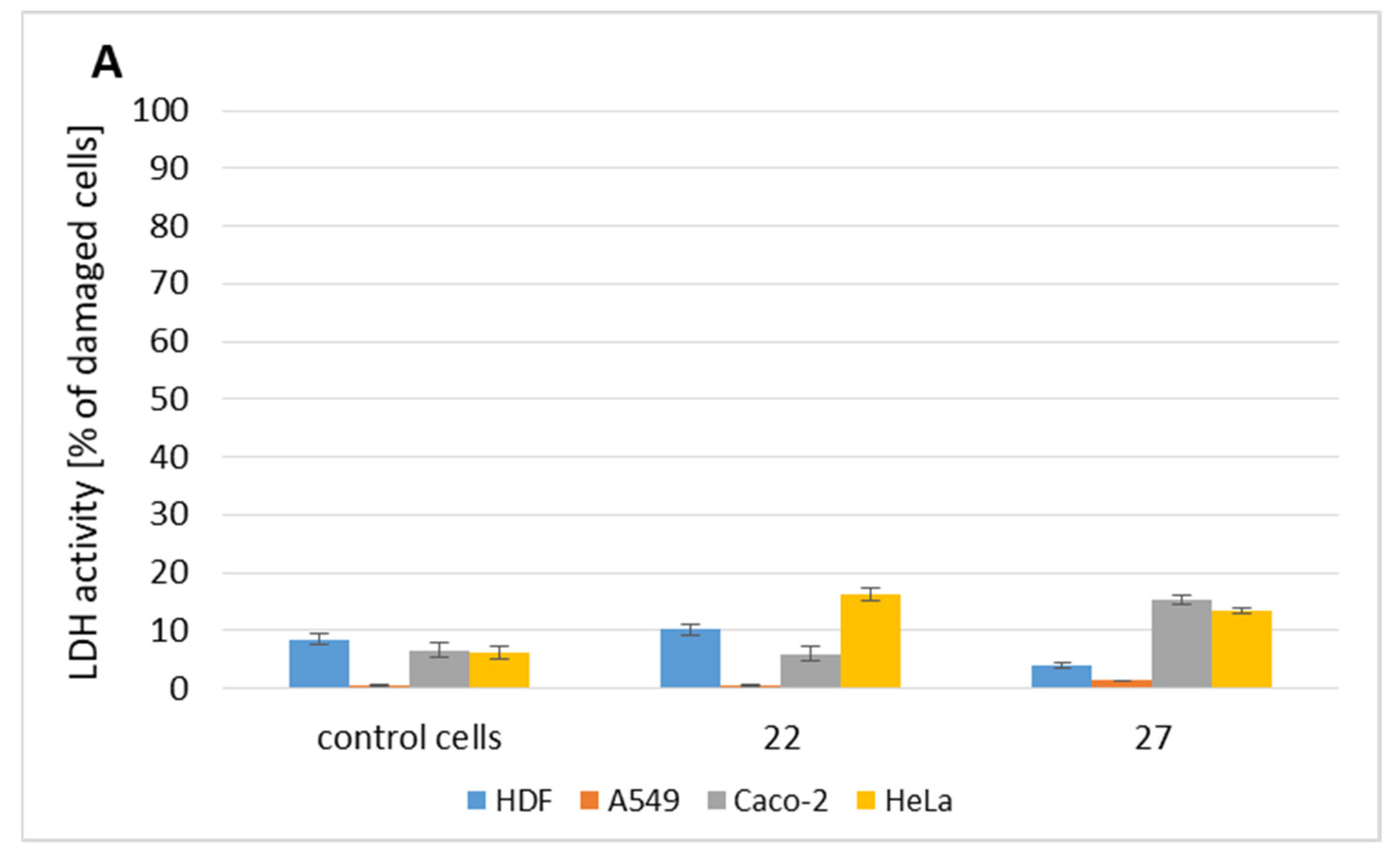
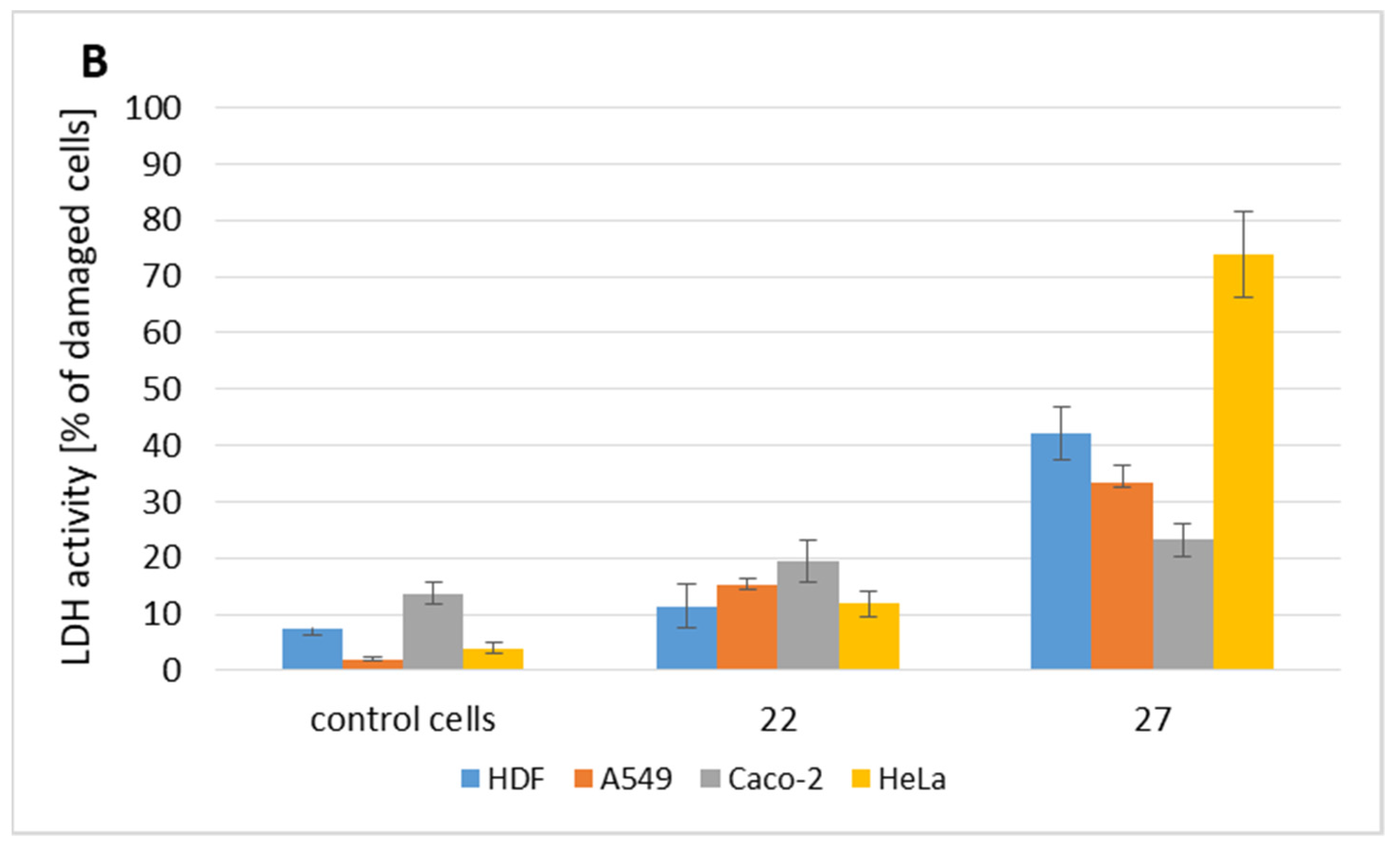
2.2. Cytotoxicity Evaluation
2.3. Bioavailability of Tested Compounds
3. Materials and Methods
3.1. General Information
3.2. Reagents and Solvents
3.3. Synthesis of Compounds 13–15, 24–25 and 26–27—General Procedure
3.4. Cytotoxicity Evaluation of Tested Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All. Available online: https://www.who.int/publications/i/item/who-report-on-cancer-setting-priorities-investing-wisely-and-providing-care-for-all (accessed on 1 March 2021).
- Meegan, M.J.; O’Boyle, N.M. Special Issue, Anticancer Drugs. Pharmaceuticals 2019, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, P.; Rathika, P.; Ramkumar, V.; Son, S.M.; Jeong, Y.T. Stereospecific synthesis of oximes and oxime ethers of 3-azabicycles: A SAR study towards antimicrobial agents. Bioorg. Med. Chem. Lett. 2010, 20, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-B.; Luo, H.-A.; Wang, X.-G.; Huang, M.-Z.; Huang, L.; Pang, H.-L.; Mao, C.-H.; Pei, H.; Huang, C.-Q.; Sun, J.; et al. Synthesis and evaluation O-benzyl oxime-ether derivatives containing β-methoxyacrylate moiety for insecticidal and fungicidal activities. Bull. Korean Chem. Soc. 2014, 35, 1073–1076. [Google Scholar] [CrossRef][Green Version]
- Özdemir, Z.; Sari, S.; Karakurt, A.; Dalkara, S. Synthesis, anticonvulsant screening, and molecular modeling studies of new arylalkylimidazole oxime ether derivatives. Drug Dev. Res. 2019, 80, 269–280. [Google Scholar] [CrossRef]
- Chakravarti, B.; Akhtar, T.; Rai, B.; Yadav, M.; Siddiqui, J.A.; Dwivedi, S.K.D.; Thakur, R.; Singh, A.K.; Kumar, H.; Khan, K.; et al. Thioaryl naphthylmethanone oxime ether analogs as novel anticancer agents. J. Med. Chem. 2014, 57, 8010–8025. [Google Scholar] [CrossRef]
- Díaz, J.E.; Martinez, D.C.; López, L.V.; Mendez, G.M.; Vera, R.; Loaiza, A.E. Synthesis and in vitro antiproliferative activity of flavone and 6-hydroxyflavone oxime ethers derivatives. J. Braz. Chem. Soc. 2018, 29, 177–184. [Google Scholar] [CrossRef]
- Latif, A.D.; Gonda, T.; Vágvölgyi, M.; Kúsz, N.; Kulmány, Á.; Ocsovszki, I.; Zomborszki, Z.P.; Zupkó, I.; Hunyadi, A. Synthesis and in vitro antitumor activity of naringenin oxime and oxime ether derivatives. Int. J. Mol. Sci. 2019, 20, 2184. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.-I.; An, J.-Y.; Lee, J.; Lee, N.-R.; Heo, J.; Kim, J.-E.; Yu, J.; Lee, J.S.; Inn, K.-S.; et al. Identification of novel estrogen receptor (ER) agonists that have additional and complementary anti-cancer activities via ER-independent mechanism. Bioorg. Med. Chem. Lett. 2016, 26, 1844–1848. [Google Scholar] [CrossRef]
- Hayakawa, I.; Shioya, R.; Agatsuma, T.; Furukawa, H.; Sugano, Y. Thienopyridine and benzofuran derivatives as potent antitumor agents possessing different structure-activity relationship. Bioorg. Med. Chem. Lett. 2004, 14, 3411–3414. [Google Scholar] [CrossRef]
- Coşkun, D.; Tekin, S.; Sandal, S.; Coşkun, M.F. Synthesis, Characterization, and Anticancer Activity of New Benzofuran Substituted Chalcones. J. Chem. 2016, 2016, 7678486. [Google Scholar] [CrossRef]
- Kamal, M.; Shakya, A.K.; Jawaid, T. Benzofurans: A New profile of biological activities. Int. J. Med. Pharm. Sci. 2011, 1, 1–15. [Google Scholar]
- Liu, Y.T.; Sun, J.; Wang, X.-J. Natural source, bioactivity and synthesis of benzofuran derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar]
- Petrou, A.; Fesaidou, M.; Geronikaki, A. Thiazole Ring—A Biologically Active Scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Chand, K.; Budagumpi, S.; Somappa, S.B.; Patil, S.A.; Nagaraja, B.M. An overview of benzo[b]thiophene-based medicinal chemistry. Eur. J. Med. Chem. 2017, 138, 1002–1033. [Google Scholar] [CrossRef]
- Demirayak, S.; Uçucu, U.; Benkli, K.; Gündoğdu-Karaburun, N.; Karaburun, A.C.; Akar, D.; Karabacak, M.; Kiraz, N. Synthesis and antifungal activities of some aryl(benzofuran-2-yl)ketoximes. Il Farmaco 2002, 57, 609–612. [Google Scholar] [CrossRef]
- Varache-Lembège, M.; Nuhrich, A.; Renard, P.; Duboudin, F.; Vercauteren, J.; Devaux, G. Platelet antiaggregant methoxyphenylthienyl ketoxime ethers. Synthesis and structure-activity relationships. Arch. Pharm. 1995, 328, 417–424. [Google Scholar] [CrossRef]
- Letafat, B.; Emami, S.; Mohammadhosseini, N.; Faramarzi, M.A.; Samadi, N.; Shafiee, A.; Foroumadi, A. Synthesis and antibacterial activity of new N-[2-(thiophen-3-yl)ethyl] piperazinyl quinolones. Chem. Pharm. Bull. 2007, 55, 894–898. [Google Scholar] [CrossRef]
- Abele, E.; Lukevics, E. Furan and thiophene oximes: Synthesis, reactions, and biological activity review. Chem. Heterocycl. Comp. 2001, 37, 141–167. [Google Scholar] [CrossRef]
- Matsumura, K.; Miyashita, O.; Shimadzu, H.; Hashimoto, N. Uracil Derivatives and Production Thereof. U.S. Patent 4190656, 26 February 1980. [Google Scholar]
- Pathania, A.S.; Chawla, P.A. Thiophene-based derivatives as anticancer agents: An overview on decade’s work. Bioorg. Chem. 2020, 101, 104026–104043. [Google Scholar]
- Bozdag, O.; Gümüse, B.; Demirdamar, R.; Büyükbingöl, E.; Rolland, Y.; Ertan, R. Synthesis of some novel oxime ether derivatives and their activity in the ‘behavioral despair test’. Eur. J. Med. Chem. 1998, 33, 133–141. [Google Scholar] [CrossRef]
- Kosmalski, T.; Kutkowska, J.; Gzella, A.K.; Nowakiewicz, A. New heterocyclic oxime ethers of 1-(benzofuran-2-yl)ethan-1-one and their antimicrobial activity. Acta Pol. Pharm. 2015, 72, 289–295. [Google Scholar] [PubMed]
- Kosmalski, T.; Kutkowska, J.; Dwojak, I.; Studzińska, R.; Sikora, A.; Modzelewska-Banachiewicz, B.; Gzella, A. Novel O-benzyl oxime ethers of 1-(thiophen-2-yl)ethan-1-one—Synthesis and antimicrobial activity. Heterocycles 2017, 94, 523–530. [Google Scholar] [CrossRef]
- Kosmalski, T.; Studzińska, R.; Daniszewska, N.; Ullrich, M.; Sikora, A.; Marszałł, M.; Modzelewska-Banachiewicz, B. Study of the room-temperature synthesis of oxime ethers by using a super base. ChemistryOpen 2018, 7, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Rebucci, M.; Michiels, C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem. Pharmacol. 2013, 85, 1219–1226. [Google Scholar] [CrossRef]
- Bai, X.; Ni, J.; Beretov, P.; Graham, P.; Li, Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat. Rev. 2018, 69, 152–163. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and premeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of DrugCandidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Molinspiration. Available online: https://www.molinspiration.com/ (accessed on 15 September 2021).
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application on the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Kelder, J.; Grootenhuis, P.D.J.; Bayada, M.D.; Delbressine, L.P.C.; Ploemen, J.P. Polar Molecular Surface as a Dominating Determinant for Oral Absorption and Brain Penetration of Drugs. Pharm. Res. 1999, 16, 1514–1519. [Google Scholar] [CrossRef]
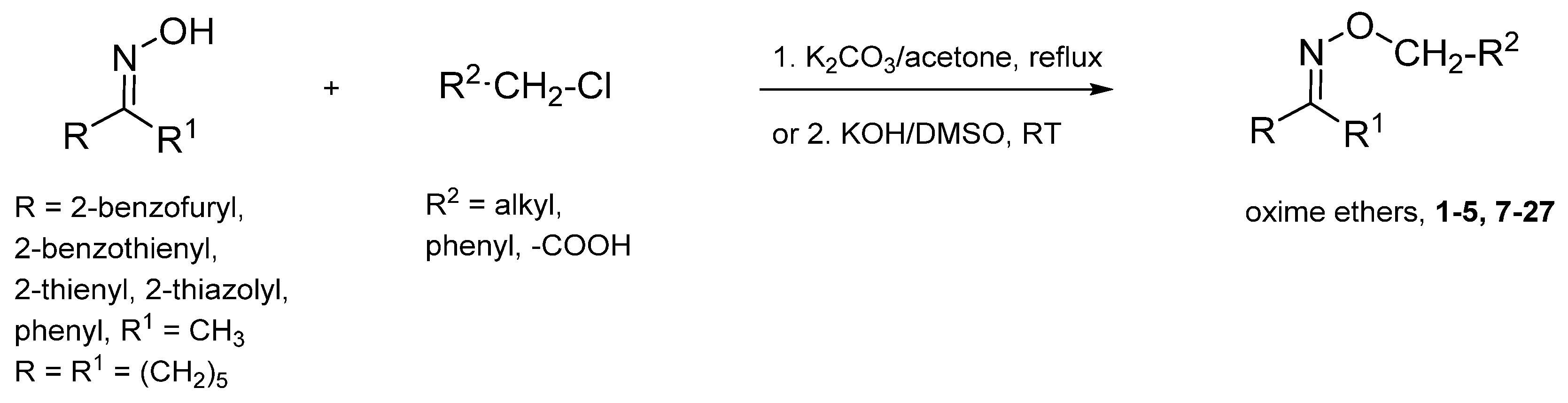

| No. | R | R1 | R2 | EC50 for HeLa Cells * | |
|---|---|---|---|---|---|
| μg/mL | mM | ||||
| 1 | 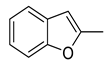 | CH3 | C6H3-2.4-diCl | >250 | >0.75 |
| 2 | C6H4-4-CF3 | >250 | >0.75 | ||
| 3 | C6H4-4-Br | >250 | >0.73 | ||
| 4 | C6H4-4-NO2 | >250 | >0.81 | ||
| 5 | C6H3-2.6-diF | >250 | >0.83 | ||
| 6 | H (oxime) | >250 | >1.43 | ||
| 7 | 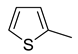 | CH3 | C6H3-2.4-diCl | >250 | >0.83 |
| 8 | C6H4-4-Br | 188.12 | 0.61 | ||
| 9 | C6H4-4-NO2 | 126.30 | 0.46 | ||
| 10 | C6H3-5-MeO-2-NO2 | >250 | >0.82 | ||
| 11 | CH2-NMe2 | >250 | >1.18 | ||
| 12 | 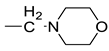 | >250 | >0.98 | ||
| 13 | CH2-NH2 | >250 | >1.36 | ||
| 14 | CH2-NEt2 | >250 | >1.04 | ||
| 15 | CH2CH2-NH2 | >250 | >1.10 | ||
| 16 | CH2CH2-NMe2 | >250 | >1.10 | ||
| 17 | COOH | >250 | >1.25 | ||
| 18 | (CH2)5 | COOH | 32.26 | 0.19 | |
| 19 | CH2-NMe2 | >250 | >1.36 | ||
| 20 | 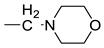 | >250 | >1.10 | ||
| 21 | 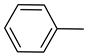 | CH3 |  | >250 | >0.67 |
| 22 |  | 23.15 | 0.09 | ||
| 23 | CH2-NMe2 | 233.14 | 1.13 | ||
| 24 |  | CH3 | C6H4-4-OMe | 110.30 | 0.42 |
| 25 | C6H4-4-Br | 130.10 | 0.42 | ||
| 26 | 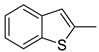 | CH3 | C6H3-2.4-diCl | 121.23 | 0.35 |
| 27 | C6H4-4-OMe | 93.80 | 0.30 | ||
| EC50 Value * in μg/mL (mM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cells | HDF | A549 | Caco-2 | HeLa | ||||
| Time No. | 24 h | 72 h | 24 h | 72 h | 24 h | 72 h | 24 h | 72 h |
| 8 | 126.70 (0.41) | 118.21 (0.38) | 193.59 (0.62) | 228.30 (0.74) | 229.79 (0.74) | 118.43 (0.38) | 188.12 (0.61) | 40.00 (0.13) |
| 9 | 171.57 (0.62) | 44.74 (0.16) | 106.70 (0.39) | 130.75 (0.47) | 77.96 (0.28) | 104.88 (0.38) | 126.30 (0.46) | 6.90 (0.02) |
| 18 | 43.66 (0.26) | 14.69 (0.09) | 47.79 (0.28) | 17.88 (0.10) | 44.36 (0.26) | 17.46 (0.10) | 32.26 (0.19) | 4.90 (0.03) |
| 22 | 27.31 (0.11) | 8.04 (0.03) | 10.19 (0.04) | 6.72 (0.03) | 9.45 (0.04) | 6.79 (0.03) | 23.15 (0.09) | 7.00 (0.03) |
| 24 | 35.26 (0.13) | 30.65 (0.12) | >250 (>0.95) | 35.74 (0.14) | >250 (>0.95) | 69.10 (0.26) | 110.30 (0.42) | 8.70 (0.03) |
| 25 | 37.88 (0.12) | 25.66 (0.08) | >250 (>0.80) | 79.86 (0.26) | 70.85 (0.23) | 50.47 (0.16) | 130.10 (0.42) | 47.33 (0.15) |
| 26 | 134.78 (0.38) | 25.66 (0.07) | 199.12 (0.57) | 176.95 (0.51) | >250 (>0.71) | 50.08 (0.14) | 121.23 (0.35) | 8.18 (0.02) |
| 27 | 149.64 (0.48) | 139.57 (0.45) | >250 (>0.80) | 142.64 (0.46) | 122.00 (0.39) | 116.47 (0.37) | 93.80 (0.30) | 27.9 (0.09) |
| No. | miLog P a | tPSA [Å2] a | MW a | nOH a | nOHNH a | Nrotb a | Lipinski Violations a | Veber Violations a |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.62 | 34.74 | 334.20 | 3 | 0 | 4 | 1 | 0 |
| 2 | 5.23 | 37.74 | 333.31 | 3 | 0 | 5 | 1 | 0 |
| 3 | 5.15 | 34.74 | 344.21 | 3 | 0 | 4 | 1 | 0 |
| 4 | 4.30 | 80.56 | 310.31 | 6 | 0 | 5 | 0 | 0 |
| 5 | 4.57 | 34.74 | 301.29 | 3 | 0 | 4 | 0 | 0 |
| 6 | 2.47 | 45.73 | 175.19 | 3 | 1 | 1 | 0 | 0 |
| 7 | 4.96 | 21.60 | 300.21 | 2 | 0 | 4 | 0 | 0 |
| 8 | 4.48 | 21.60 | 310.22 | 2 | 0 | 4 | 0 | 0 |
| 9 | 3.63 | 67.42 | 276.32 | 5 | 0 | 5 | 0 | 0 |
| 10 | 3.62 | 76.66 | 306.34 | 6 | 0 | 6 | 0 | 0 |
| 11 | 2.11 | 24.84 | 212.32 | 3 | 0 | 5 | 0 | 0 |
| 12 | 1.95 | 34.07 | 254.35 | 4 | 0 | 5 | 0 | 0 |
| 13 | 0.88 | 47.62 | 184.26 | 3 | 2 | 4 | 0 | 0 |
| 14 | 2.86 | 24.84 | 240.37 | 3 | 0 | 7 | 0 | 0 |
| 15 | 1.15 | 47.62 | 198.29 | 3 | 2 | 5 | 0 | 0 |
| 16 | 2.38 | 24.84 | 226.34 | 3 | 0 | 6 | 0 | 0 |
| 17 | 1.32 | 58.90 | 199.23 | 4 | 1 | 4 | 0 | 0 |
| 18 | 1.37 | 58.90 | 171.20 | 4 | 1 | 3 | 0 | 0 |
| 19 | 2.16 | 24.84 | 184.28 | 3 | 0 | 4 | 0 | 0 |
| 20 | 2.00 | 34.07 | 226.32 | 4 | 0 | 4 | 0 | 0 |
| 21 | 4.93 | 21.60 | 375.35 | 2 | 0 | 4 | 0 | 0 |
| 22 | 2.05 | 34.07 | 248.33 | 4 | 0 | 5 | 0 | 0 |
| 23 | 2.21 | 24.84 | 206.27 | 3 | 0 | 5 | 0 | 0 |
| 24 | 2.49 | 43.72 | 262.33 | 4 | 0 | 5 | 0 | 0 |
| 25 | 3.25 | 34.49 | 311.20 | 3 | 0 | 4 | 0 | 0 |
| 26 | 6.26 | 21.60 | 350.27 | 2 | 0 | 4 | 1 | 0 |
| 27 | 5.04 | 30.83 | 311.41 | 3 | 0 | 5 | 1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmalski, T.; Hetmann, A.; Studzińska, R.; Baumgart, S.; Kupczyk, D.; Roszek, K. The Oxime Ethers with Heterocyclic, Alicyclic and Aromatic Moiety as Potential Anti-Cancer Agents. Molecules 2022, 27, 1374. https://doi.org/10.3390/molecules27041374
Kosmalski T, Hetmann A, Studzińska R, Baumgart S, Kupczyk D, Roszek K. The Oxime Ethers with Heterocyclic, Alicyclic and Aromatic Moiety as Potential Anti-Cancer Agents. Molecules. 2022; 27(4):1374. https://doi.org/10.3390/molecules27041374
Chicago/Turabian StyleKosmalski, Tomasz, Anna Hetmann, Renata Studzińska, Szymon Baumgart, Daria Kupczyk, and Katarzyna Roszek. 2022. "The Oxime Ethers with Heterocyclic, Alicyclic and Aromatic Moiety as Potential Anti-Cancer Agents" Molecules 27, no. 4: 1374. https://doi.org/10.3390/molecules27041374
APA StyleKosmalski, T., Hetmann, A., Studzińska, R., Baumgart, S., Kupczyk, D., & Roszek, K. (2022). The Oxime Ethers with Heterocyclic, Alicyclic and Aromatic Moiety as Potential Anti-Cancer Agents. Molecules, 27(4), 1374. https://doi.org/10.3390/molecules27041374