MiodesinTM Positively Modulates the Immune Response in Endometrial and Vaginal Cells
Abstract
:1. Introduction
2. Results
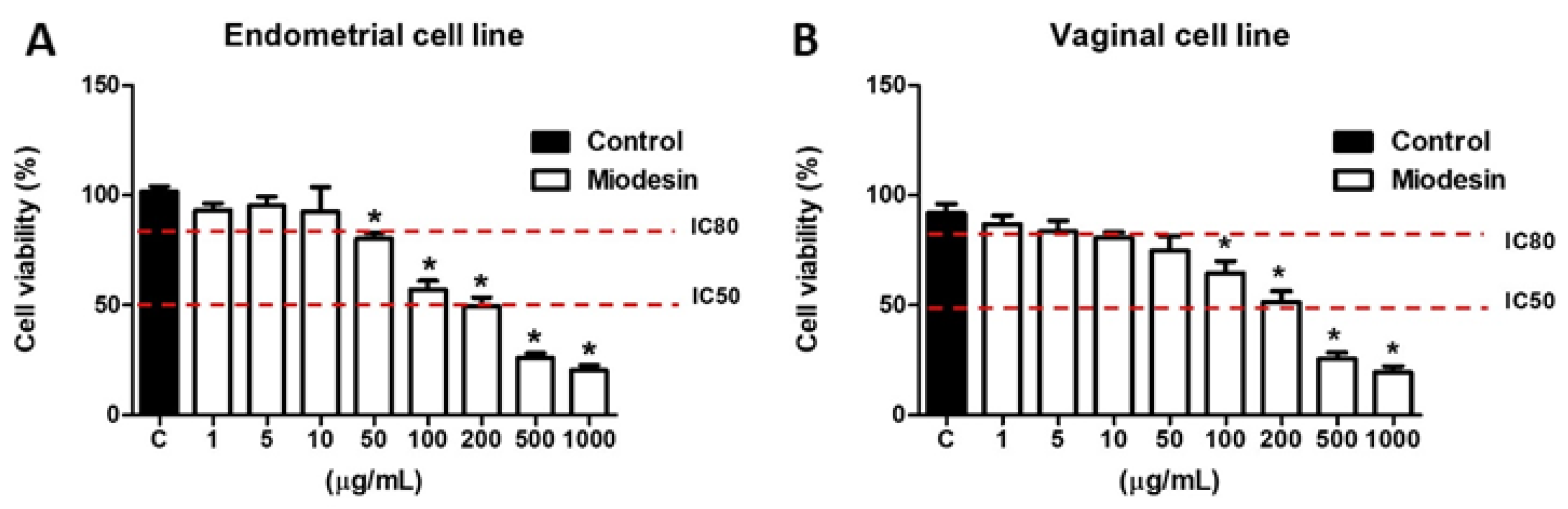
2.1. Effects of MiodesinTM on Cell Line Viability
2.2. Pro-Inflammatory Response Induced by Candida Albicans in Vaginal Cell Line VK2 E6/E7 Is Inhibited by MiodesinTM
2.3. Expression of Cytokine and Inflammatory Mediators at mRNA Levels in Vaginal Cell Line (VK2 E6/E7)
2.4. MiodesinTM Down-Regulates LPS-Induced Inflammatory Cytokine and Chemokine Release by Endometrial Cell Line (KLE)
2.5. MiodesinTM Regulates MMP-2, MMP-9, and VEGF in Endometrial Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Culture and Cytotoxicity Evaluation of Miodesin by MTT Assay
4.3. Candida Albicans (Stimulator Cells)
4.4. Coculture Supernatant Collection for Examination of Cytokines and Chemokines
4.5. Cytokine and Chemokine Analysis of Coculture and Endometrial Cells Supernatants
4.6. Reverse Transcription-Quantitative PCR (RT-qPCR)
| Gene | Primer sequences |
| NF-κβ | Forward 5′-ATGGCTTCTATGAGGCTGAG- 3′ |
| Reverse 5′-GTTGTTGTTGGTCTGGATGC- 3′ | |
| COX-1 | Forward 5′-AGGAGATGGCTGCTGAGTTGG-3′ |
| Reverse 5′-AATCTGACTTTCTGAGTTGCC-3′ | |
| COX-2 | Forward 5′-ACACCTTCAACATTGAAGACC-3′ |
| Reverse 5′-ATCCCTTCACTAAATGCCCTC-3′ | |
| PLA2 | Forward 5′-AAAGAACACTATAGGGAGAG-3′ |
| Reverse 5´-AAAGAGGTAAAGGGCATTGT-3′ | |
| CCL2 | Forward 5′-GATCCCAATGAGTAGGCTGG-3′ |
| Reverse 5′-CGGGTCAACTTCACATTCAAAG-3′ | |
| CCL3 | Forward 5′-ACACCAGAAGGATACAAGCAG-3′ |
| Reverse 5′-CGATGAATTGGCGTGGAATC-3′ | |
| CCL5 | Forward 5′-CCCACGTCAAGGAGTATTTCTAC-3′ |
| Reverse 5′-CTAGGACTAGAGCAAGCGATG-3′ | |
| MMP-2 | Forward 5′-ACCGCGACAAGAAGTATGGC-3′ |
| Reverse 5′-CCACTTGCGGTCA TCATCGT-3´ | |
| MMP-9 | Forward 5′-CGATGACGAGTTGTGGTCCC-3′ |
| Reverse 5′-TCGTAGTTG GCCGTGGTACT-3′ | |
| VEGF | Forward 5′-TGCAGATTATGCGGATCAAACC-3′ |
| Reverse 5′-TGCATTCACATTTGTTGTGCTGTAG-3′ | |
| GAPDH | Forward 5′-CGGTGTGAACGGATTTGGC-3′ |
| Reverse 5′-GTGAGTGGAGTCATACTGGAAC-3′ |
4.7. Enzyme-Linked Immunosorbent Assay for the Determination of MMP2 and MMP9
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Murji, A.; Bedaiwy, M.; Singh, S.S.; Bougie, O.; Committee, C.R.S. Influence of Ethnicity on Clinical Presentation and Quality of Life in Women with Uterine Fibroids: Results from a Prospective Observational Registry. J. Obstet. Gynaecol. Can. 2020, 42, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhong, Q.; Xia, Y.; Li, E.; Wang, S.; Ren, R. MicroRNA-2861 Targets STAT3 and MMP2 to Regulate the Proliferation and Apoptosis of Ectopic Endometrial Cells in Endometriosis. Die Pharm.-Int. J. Pharm. Sci. 2019, 74, 243–249. [Google Scholar]
- Bostanci Durmus, A.; Dincer Cengiz, S.; Yılmaz, H.; Candar, T.; Gursoy, A.Y.; Sinem Caglar, G. The Levels of Matrix Metalloproteinase-9 and Neutrophil Gelatinase-Associated Lipocalin in Different Stages of Endometriosis. J. Obstet. Gynaecol. 2019, 39, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, X.; Wang, W.; Dong, H.; Xia, Y.; Ruan, L.; Liu, L. Expression of MMIF, HIF-1α and VEGF in Serum and Endometrial Tissues of Patients with Endometriosis. Curr. Med. Sci. 2018, 38, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, N.; Babaei, S.; Moini, A.; Eftekhari-Yazdi, P. Controlling Semi-Invasive Activity of Human Endometrial Stromal Cells by Inhibiting NF-kB Signaling Pathway Using Aloe-emodin and Aspirin. J. Reprod. Infertil. 2021, 22, 227–240. [Google Scholar] [CrossRef]
- Huang, F.; Cao, J.; Liu, Q.; Zou, Y.; Li, H.; Yin, T. MAPK/ERK signal pathway involved expression of COX-2 and VEGF by IL-1β induced in human endometriosis stromal cells in vitro. Int. J. Clin. Exp. Pathol. 2013, 6, 2129–2136. [Google Scholar]
- Sano, M.; Morishita, T.; Nozaki, M.; Yokoyama, M.; Watanabe, Y.; Nakano, H. Elevation of the phospholipase A2 activity in peritoneal fluid cells from women with endometriosis. Fertil. Steril. 1994, 61, 657–662. [Google Scholar] [CrossRef]
- Kowalczyk-Zieba, I.; Woclawek-Potocka, I.; Wasniewski, T.; Boruszewska, D.; Grycmacher, K.; Sinderewicz, E.; Staszkiewicz, J.; Wolczynski, S. LPAR2 and LPAR4 are the Main Receptors Responsible for LPA Actions in Ovarian Endometriotic Cysts. Reprod. Sci. 2019, 26, 139–150. [Google Scholar] [CrossRef]
- Nicolaus, K.; Bräuer, D.; Sczesny, R.; Lehmann, T.; Diebolder, H.; Runnebaum, I.B. Unexpected Coexistent Endometriosis in Women with Symptomatic Uterine Leiomyomas Is Independently Associated with Infertility, Nulliparity and Minor Myoma Size. Arch. Gynecol. Obstet. 2019, 300, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Maia, H.; Saback, W.; Haddad, C.; Sitya, P.R. Treatment of Endometriosis and Leiomyoma with the Association of Miodesin and Gestrinone in Pentravan Through the Vaginal Route. J. Clin. Rev. Case Rep. 2018, 3, 1–5. [Google Scholar] [CrossRef]
- Florence, A.M.; Fatehi, M. Leiomyoma. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430685/ (accessed on 20 December 2021).
- Brown, J.; Crawford, T.J.; Allen, C.; Hopewell, S.; Prentice, A. Nonsteroidal Anti-inflammatory Drugs for Pain in Women with Endometriosis. Cochrane Database Syst. Rev. 2017, 1, CD004753. [Google Scholar] [CrossRef] [PubMed]
- Sabry, M.; Al-Hendy, A. Medical Treatment of Uterine Leiomyoma. Reprod. Sci. 2012, 19, 339–353. [Google Scholar] [CrossRef] [Green Version]
- Valerio, L.G.; Gonzales, G.F. Toxicological Aspects of the South American Herbs Cat’s Claw (Uncaria Tomentosa) and Maca (Lepidium Meyenii). Toxicol. Rev. 2005, 24, 11–35. [Google Scholar] [CrossRef]
- Silva, L.R.; Teixeira, R. Phenolic Profile and Biological Potential of Endopleura Uchi Extracts. Asian Pac. J. Trop. Med. 2015, 8, 889–897. [Google Scholar] [CrossRef] [Green Version]
- Muniz, M.P. Estudo Fitoquímico e Da Atividade Biológica de Endopleura Uchi Huber Cuatrecasas. Ph.D. Thesis, Federal University of Amazonas, Manaus, AM, Brazil, 2013. [Google Scholar]
- da Silva, S.L.; de Oliveira, V.G.; Yano, T.; Nunomura, R.d.C.S. Antimicrobial Activity of Bergenin from Endopleura Uchi (Huber) Cuatrec. Acta Amaz. 2009, 39, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Correa, M.; Penna, L. Dicionário Das Plantas Úteis Do Brasil e Das Exóticas Cultivadas; Instituto Brasileiro de Desenvolvimento Florestal: Rio de Janeiro, Brazil, 1974; Volume 5. [Google Scholar]
- Rao, A.R.; Reddy, A.H.; Aradhya, S.M. Antibacterial Properties of Spirulina Platensis, Haematococcus Pluvialis, Botryococcus Braunii Micro Algal Extracts. Curr. Trends Biotechnol. Pharm. 2010, 4, 809–819. [Google Scholar]
- Shah, M.; Mahfuzur, R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus Pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Chew, B.P. Astaxanthin Stimulates Cell-Mediated and Humoral Immune Responses in Cats. Vet. Immunol. Immunopathol. 2011, 144, 455–461. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin Decreased Oxidative Stress and Inflammation and Enhanced Immune Response in Humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, B.P.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Park, J.S. Dietary Astaxanthin Enhances Immune Response in Dogs. Vet. Immunol. Immunopathol. 2011, 140, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Maia, H.; Saback, W.; Haddad, C.; Sitya, P.R. Effect of Vaginal MiodesinTM in PentravanTM on the Response to Progestin Therapy in Patients with Deep Endometriosis and Adenomyosis. J. Clin. Rev. Case Rep. 2019, 4, 1–5. [Google Scholar]
- Maia, H.; Saback, W.; Coutinho, E. Short Term Effects of the Vaginal Administration of Gestrinone and MiodesinTM on Endometriosis Pain. J. Clin. Rev. Case Rep. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Luckow Invitti, A.; Schor, E.; Martins Parreira, R.; Kopelman, A.; Kamergorodsky, G.; Gonçalves, G.A.; Batista Castello Girão, M.J. Inflammatory Cytokine Profile of Co-cultivated Primary Cells from the Endometrium of Women with and without Endometriosis. Mol. Med. Rep. 2018, 18, 1287–1296. [Google Scholar] [CrossRef] [Green Version]
- Ilie, I.; Ilie, R. Cytokines and Endometriosis-the Role of Immunological Alterations. Biotechnol. Mol. Biol. Nanomed. 2013, 1, 8–19. [Google Scholar]
- Gazvani, R.; Templeton, A. Peritoneal Environment, Cytokines and Angiogenesis in the Pathophysiology of Endometriosis. Reproduction 2002, 123, 217–226. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Chen, H.-Y.; Chen, W.; Liu, Y.-N.; Fu, Y.; Wang, L.-N. Expression of Inflammatory Cytokines in Serum and Peritoneal Fluid from Patients with Different Stages of Endometriosis. Gynecol. Endocrinol. 2018, 34, 507–512. [Google Scholar] [CrossRef]
- Kalu, E.; Sumar, N.; Giannopoulos, T.; Patel, P.; Croucher, C.; Sherriff, E.; Bansal, A. Cytokine Profiles in Serum and Peritoneal Fluid from Infertile Women with and without Endometriosis. J. Obstet. Gynaecol. Res. 2007, 33, 490–495. [Google Scholar] [CrossRef]
- Jørgensen, H.; Hill, A.S.; Beste, M.T.; Kumar, M.P.; Chiswick, E.; Fedorcsak, P.; Isaacson, K.B.; Lauffenburger, D.A.; Griffith, L.G.; Qvigstad, E. Peritoneal Fluid Cytokines Related to Endometriosis in Patients Evaluated for Infertility. Fertil. Steril. 2017, 107, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.-E.; Nothnick, W.B. The Relevancy of the Matrix Metalloproteinase System to the Pathophysiology of Endometriosis. Front. Biosci. 2005, 10, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Arici, A. Interleukin-8 Stimulates the Adhesion of Endometrial Stromal Cells to Fibronectin. Fertil. Steril. 1999, 72, 336–340. [Google Scholar] [CrossRef]
- Herington, J.L.; Bruner-Tran, K.L.; Lucas, J.A.; Osteen, K.G. Immune Interactions in Endometriosis. Expert Rev. Clin. Immunol. 2011, 7, 611–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, V.J.; Brown, J.K.; Saunders, P.T.K.; Horne, A.W. The Role of the Peritoneum in the Pathogenesis of Endometriosis. Hum. Reprod. Update 2013, 19, 558–569. [Google Scholar] [CrossRef]
- Lebovic, D.I.; Baldocchi, R.A.; Mueller, M.D.; Taylor, R.N. Altered Expression of a Cell-Cycle Suppressor Gene, Tob-1, in Endometriotic Cells by CDNA Array Analyses. Fertil. Steril. 2002, 78, 849–854. [Google Scholar] [CrossRef]
- Sikora, J.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Association of the Precursor of Interleukin-1β and Peritoneal Inflammation—Role in Pathogenesis of Endometriosis. J. Clin. Lab. Anal. 2016, 30, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, Y.; Harada, T.; Horie, S.; Iba, Y.; Taniguchi, F.; Yoshida, S.; Iwabe, T.; Terakawa, N. Tumor Necrosis Factor-α-Induced Interleukin-8 (IL-8) Expression in Endometriotic Stromal Cells, Probably through Nuclear Factor-ΚB Activation: Gonadotropin-Releasing Hormone Agonist Treatment Reduced IL-8 Expression. J. Clin. Endocrinol. Metab. 2003, 88, 730–735. [Google Scholar] [CrossRef]
- McKinnon, B.D.; Kocbek, V.; Nirgianakis, K.; Bersinger, N.A.; Mueller, M.D. Kinase Signalling Pathways in Endometriosis: Potential Targets for Non-Hormonal Therapeutics. Hum. Reprod. Update 2016, 22, 382–403. [Google Scholar] [CrossRef] [Green Version]
- Steele, C.; Fidel Jr, P.L. Cytokine and Chemokine Production by Human Oral and Vaginal Epithelial Cells in Response to Candida Albicans. Infect. Immun. 2002, 70, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Fichorova, R.N.; Anderson, D.J. Differential Expression of Immunobiological Mediators by Immortalized Human Cervical and Vaginal Epithelial Cells. Biol. Reprod. 1999, 60, 508–514. [Google Scholar] [CrossRef]
- Borrelli, G.; Carvalho, K.; Kallas, E.; Mechsner, S.; Baracat, E.; Abrao, M. Chemokines in the Pathogenesis of Endometriosis and Infertility. J. Reprod. Immunol. 2013, 98, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Złotkowska, A.; Andronowska, A. Chemokines as the Modulators of Endometrial Epithelial Cells Remodelling. Sci. Rep. 2019, 9, 12968. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Nasu, K.; Narahara, H. Role of Chemokines in the Pathogenesis of Endometriosis. Front. Biosci. Sch. Ed. 2011, 3, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Chegini, N.; Roberts, M.; Ripps, B. Differential Expression of Interleukins (IL)-13 and IL-15 in Ectopic and Eutopic Endometrium of Women with Endometriosis and Normal Fertile Women. Am. J. Reprod. Immunol. 2003, 49, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Bi, K.; Lu, Z.; Wang, K.; Xu, Y.; Wu, H.; Cao, Y.; Jiang, H. CCR5/CCR5 Ligand-Induced Myeloid-Derived Suppressor Cells Are Related to the Progression of Endometriosis. Reprod. BioMedicine Online 2019, 39, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-F.; Hong, L.-H.; Tan, Y.; Sheng, J.-Z. Matrix Metalloproteinase 2 Is Associated with Changes in Steroid Hormones in the Sera and Peritoneal Fluid of Patients with Endometriosis. Fertil. Steril. 2004, 81, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-W.; Wen, Y.; Chun, S.-H.; Nezhat, C.; Woo, B.-H.; Lake Polan, M. Matrix Metalloproteinase-9 and Tissue Inhibitor of Metalloproteinase-3 MRNA Expression in Ectopic and Eutopic Endometrium in Women with Endometriosis: A Rationale for Endometriotic Invasiveness. Fertil. Steril. 2001, 75, 152–159. [Google Scholar] [CrossRef]
- Schadendorf, D.; Möller, A.; Algermissen, B.; Worm, M.; Sticherling, M.; Czarnetzki, B.M. IL-8 Produced by Human Malignant Melanoma Cells in vitro Is an Essential Autocrine Growth Factor. J. Immunol. 1993, 151, 2667–2675. [Google Scholar]
- Iwabe, T.; Harada, T.; Tsudo, T.; Nagano, Y.; Yoshida, S.; Tanikawa, M.; Terakawa, N. Tumor Necrosis Factor-Alpha Promote Proliferation of Endometriotic Stromal Cells by Inducing Gene and Protein Expression. J. Clin. Endocrinol. Metab. 2000, 85, 824–829. [Google Scholar] [CrossRef] [Green Version]




| Cytokine | Stimulus | Cytokine Concentration (Mean pg/mL ± SEM) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| IL-6 | Medium | 4.9 ± 0.8 | 7.8 ± 2.4 | 8.2 ± 2.6 |
| C. albicans | 17.1 ± 1.0 # | 31.0 ± 2.9 # | 64.3 ± 3.7 # | |
| C. albicans + MiodesinTM (10 µg/mL) | 6.2 ± 2.3 * | 12.9 ± 3.8 * | 28.2 ± 3.9 * | |
| Medium | 3.4 ± 0.5 | 12.6 ± 2.8 | 26.0 ± 3.7 | |
| C. albicans | 19.0 ± 0.9 # | 39.0 ± 3.7 # | 68.0± 4.5 # | |
| C. albicans + MiodesinTM (200 µg/mL) | 11.0 ± 2.9 * | 15.0 ± 3.5 * | 29.0 ± 3.2 * | |
| IL-8 | Medium | 31.4 ± 2.4 | 35.2 ± 2.9 | 32.1 ± 3.2 |
| C. albicans | 63.0 ± 4.0 # | 76.6 ± 3.0 # | 102.4 ± 3.7 # | |
| C. albicans +MiodesinTM (10 µg/mL) | 33.9 ± 2.7 * | 26.8 ± 1.9 * | 29.7 ± 3.1 * | |
| Medium | 30.0 ± 3.7 | 31.3 ± 1.6 | 29.6 ± 3.8 | |
| C. albicans | 69.0 ± 5.1 # | 87 ± 2.5 # | 107.6 ± 3.4 # | |
| C. albicans + MiodesinTM (200 µg/mL) | 36.0 ± 3.5 * | 21 ± 2.9 * | 20.3 ± 1.7 * | |
| IL-1β | Medium | 4.7 ± 2.6 | 5.8 ± 2.1 | 5.7 ± 1.4 |
| C. albicans | 15.3 ± 2.3 # | 23.2 ± 2.8 # | 31.5 ± 3.3 # | |
| C. albicans + MiodesinTM (10 µg/mL) | 9.0 ± 1.6 * | 11.5 ± 2.5 * | 17.3 ± 1.1 * | |
| Medium | 3.3 ± 2.1 | 3.9 ± 1.8 | 6.0 ± 2.3 | |
| C. albicans | 18.0 ± 1.9 # | 22.0 ± 3.9 # | 32.0 ± 3.1 # | |
| C. albicans + MiodesinTM (200 µg/mL) | 12.0 ± 1.1 * | 17.0 ± 3.5 * | 18.0 ± 2.1 * | |
| TNF-α | Medium | 7.1 + 0.9 | 9.2 + 3.2 | 8.6 + 1.7 |
| C. albicans | 25.2 + 2.1 # | 29.9 + 4.1 # | 37.0 + 3.2 # | |
| C. albicans + MiodesinTM (10 µg/mL) | 11.1 + 1.9 * | 13.2 + 2.1 * | 14.9 + 4,2 * | |
| Medium | 5.7 ± 0.2 | 12.6 ± 2.8 | 18.6 ± 3.7 | |
| C. albicans | 21.5 ± 0.5 # | 31.9 ± 3.7 # | 67.2 ± 2.5 # | |
| C. albicans + MiodesinTM (200 µg/mL) | 11.0 ± 2.7 * | 16.0 ± 3.5 * | 31.0 ± 3.2 * | |
| Cytokine | Stimulus | Cytokine Concentration (Mean pg/mL ± SEM) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| CCL2 (MCP-1) | Medium | 3.3 ± 2.9 | 4.7 ± 1.2 | 6.5 ± 2.8 |
| C. albicans | 10.1 ± 2.2 # | 14.2 ± 2.9 # | 21.5 ± 1.5 # | |
| C. albicans + MiodesinTM (10 µg/mL) | 6.7 ± 3.2 * | 8.9 ± 3.5 * | 10.1 ± 1.1 * | |
| Medium | 2.1 ± 3.2 | 4.9 ± 2.2 | 8.10 ± 1.7 | |
| C. albicans | 9.8 ± 1.2 # | 16.0 ± 3.2 # | 31.0 ± 2.5 # | |
| C. albicans + MiodesinTM (200 µg/mL) | 5.2 ± 1.1 * | 12.6 ± 1.5 * | 19.8 ± 1.7 * | |
| CCL3 (MIP-1α) | Medium | 33.0 ± 2.8 | 35.2 ± 3.9 | 31.4 ± 2.3 |
| C. albicans | 58.6 ± 3.3 # | 69.8 ± 4.1 # | 75.2 ± 2.8 # | |
| C. albicans + MiodesinTM (10 µg/mL) | 27.1 ± 2.5 * | 31.6 ± 1.9 * | 25.2 ± 3.2 * | |
| Medium | 30.0 ± 3.7 | 32.3 ± 1.6 | 29.6 ± 3.8 | |
| C. albicans | 59.0 ± 3.1 # | 67 ± 2.5 # | 81.7 ± 3.4 # | |
| C. albicans + MiodesinTM (200 µg/mL) | 26.0 ± 3.5 * | 29 ± 2.9 * | 24.0 ± 1.7 * | |
| CCL5 (RANTES) | Medium | 3.7 ± 1.9 | 3.1 ± 2.8 | 3.6 ± 1.7 |
| C. albicans | 19.1 ± 3.7 # | 24.2 ± 2.7 # | 29.9 ± 2.9 # | |
| C. albicans + MiodesinTM (10 µg/mL) | 9.2 ± 4.5 | 11.9 ± 2.5 | 12.8 ± 5.8 | |
| Medium | 3.3 ± 2.1 | 3.9 ± 1.8 | 4.0 ± 2.3 | |
| C. albicans | 16.0 ± 2.9 # | 22.0 ± 3.9 # | 32.0 ± 3.1 # | |
| C. albicans + MiodesinTM (200 µg/mL) | 9.3 ± 4.1 | 19.9 ± 3.9 | 28.0 ± 5.5 | |
| Cytokine | Stimulus | Cytokine Concentration (Mean pg/mL ± SEM) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| CCL2 (MCP-1) | Medium | 7.1 ± 2.1 | 6.2 ± 2.2 | 7.9 ± 4.1 |
| LPS | 15.2 ± 3.1 # | 17.2 ± 3.6 # | 21.3 ± 3.7 # | |
| LPS + MiodesinTM (10 µg/mL) | 6.1 ± 1.4 * | 8.9 ± 3.3 * | 9.2 ± 1.1 * | |
| Medium | 4.2 ± 2.9 | 6.4 ± 3.9 | 11.3 ± 3.2 | |
| LPS | 13.3 ± 3.9 # | 21.4 ± 5.1 # | 42.2 ± 3.5 # | |
| LPS + MiodesinTM (200 µg/mL) | 6.9 ± 2.4 * | 12.3 ± 2.3 * | 19.1 ± 2.1 * | |
| CCL3 (MIP-1α) | Medium | 33.2 ± 1.7 | 39.4 ± 2.2 | 36.3 ± 3.7 |
| LPS | 67.2 ± 3.8 # | 75.2 ± 2.9 # | 81.1 ± 6.2 # | |
| LPS + MiodesinTM (10 µg/mL) | 31.2 ± 4.2 * | 33.8 ± 5.2 * | 29.8 ± 11.1 * | |
| Medium | 31.1 ± 2.2 | 42.1 ± 3.7 | 31.2 ± 5.8 | |
| LPS | 69.1 ± 2.9 # | 77.8 ± 3.8 # | 93.2 ± 8.9 # | |
| LPS + MiodesinTM (200 µg/mL) | 21.1 ± 4.5 * | 31.2 ± 6.9 * | 20.4 ± 10.7 * | |
| CCL5 (RANTES) | Medium | 5.2 ± 4.1 | 8.4 ± 2.5 | 7.4 ± 2.8 |
| LPS | 30.3 ± 3.1 # | 36.2 ± 4.1 # | 39.6 ± 3.7 # | |
| LPS + MiodesinTM (10 µg/mL) | 9.4 ± 6.2 * | 11.3 ± 4.1 * | 12.9 ± 4.7 * | |
| Medium | 4.4 ± 7.9 | 3.9 ± 1.8 | 6.7 ± 2.3 | |
| LPS | 29.9 ± 5.1 # | 32.0 ± 3.9 # | 41.0 ± 3.1 # | |
| LPS + MiodesinTM (200 µg/mL) | 13.5 ± 8.1 * | 18.1 ± 5.2 * | 21.8 ± 4.9 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, C.R.; Polonini, H.; Marcucci, M.C.; Vieira, R.P. MiodesinTM Positively Modulates the Immune Response in Endometrial and Vaginal Cells. Molecules 2022, 27, 782. https://doi.org/10.3390/molecules27030782
Oliveira CR, Polonini H, Marcucci MC, Vieira RP. MiodesinTM Positively Modulates the Immune Response in Endometrial and Vaginal Cells. Molecules. 2022; 27(3):782. https://doi.org/10.3390/molecules27030782
Chicago/Turabian StyleOliveira, Carlos Rocha, Hudson Polonini, Maria Cristina Marcucci, and Rodolfo P. Vieira. 2022. "MiodesinTM Positively Modulates the Immune Response in Endometrial and Vaginal Cells" Molecules 27, no. 3: 782. https://doi.org/10.3390/molecules27030782
APA StyleOliveira, C. R., Polonini, H., Marcucci, M. C., & Vieira, R. P. (2022). MiodesinTM Positively Modulates the Immune Response in Endometrial and Vaginal Cells. Molecules, 27(3), 782. https://doi.org/10.3390/molecules27030782








