LC-MS/MS-Based Metabolomic Profiling of Constituents from Glochidion velutinum and Its Activity against Cancer Cell Lines
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis
2.1.1. Total Phenolic Contents
2.1.2. Total Flavonoid Contents
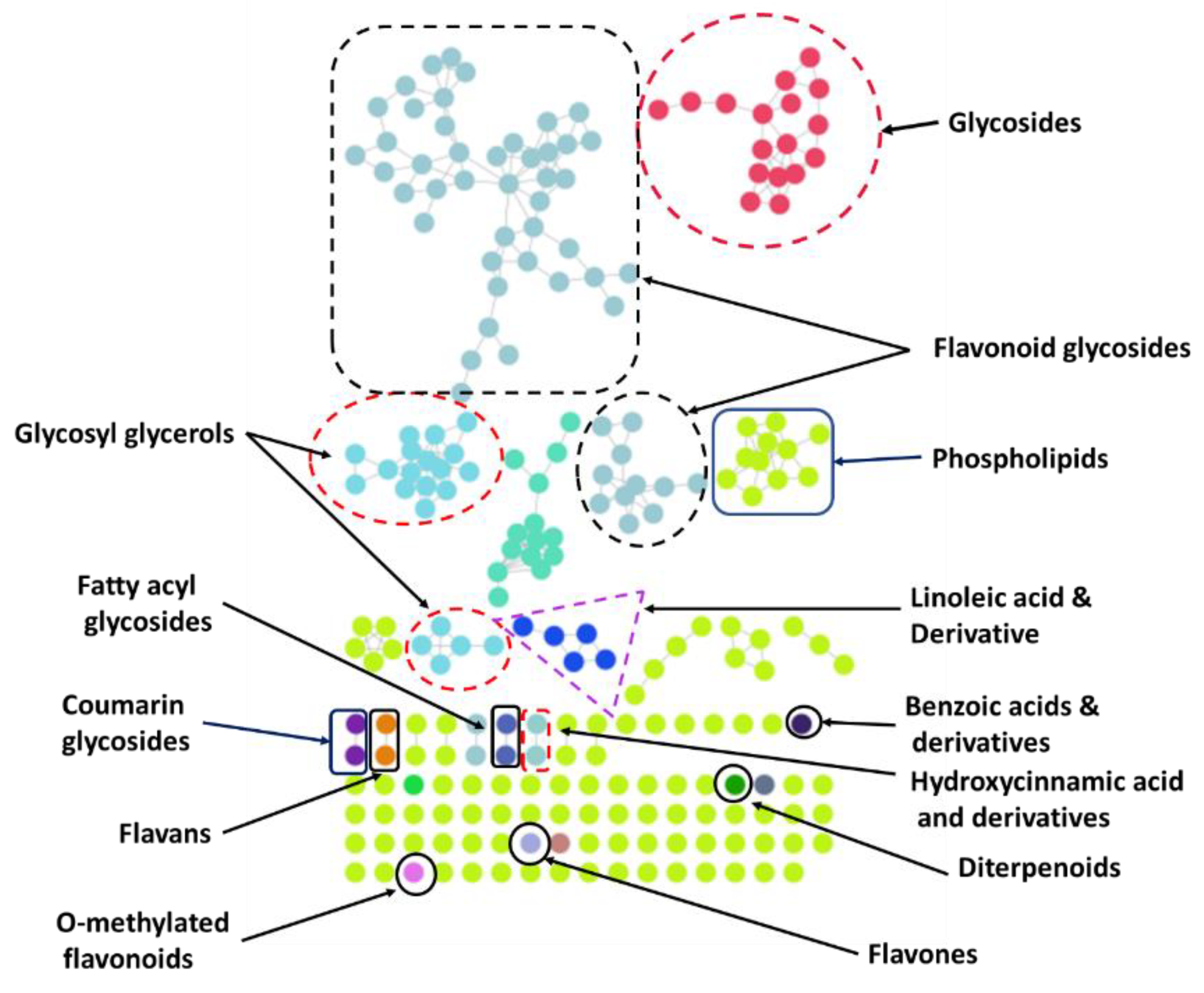
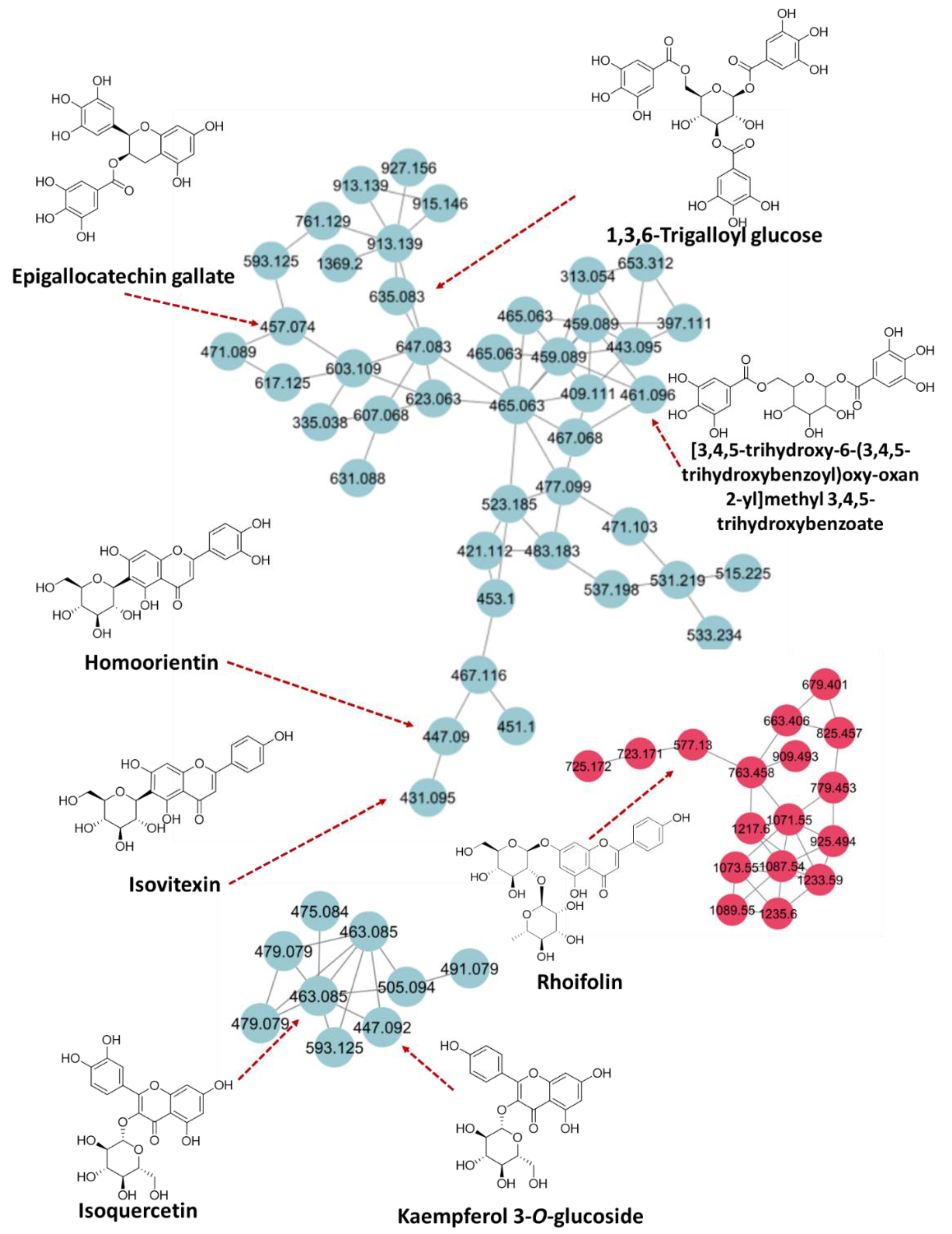
2.1.3. LC-MS/MS-Based Chemical Profiling
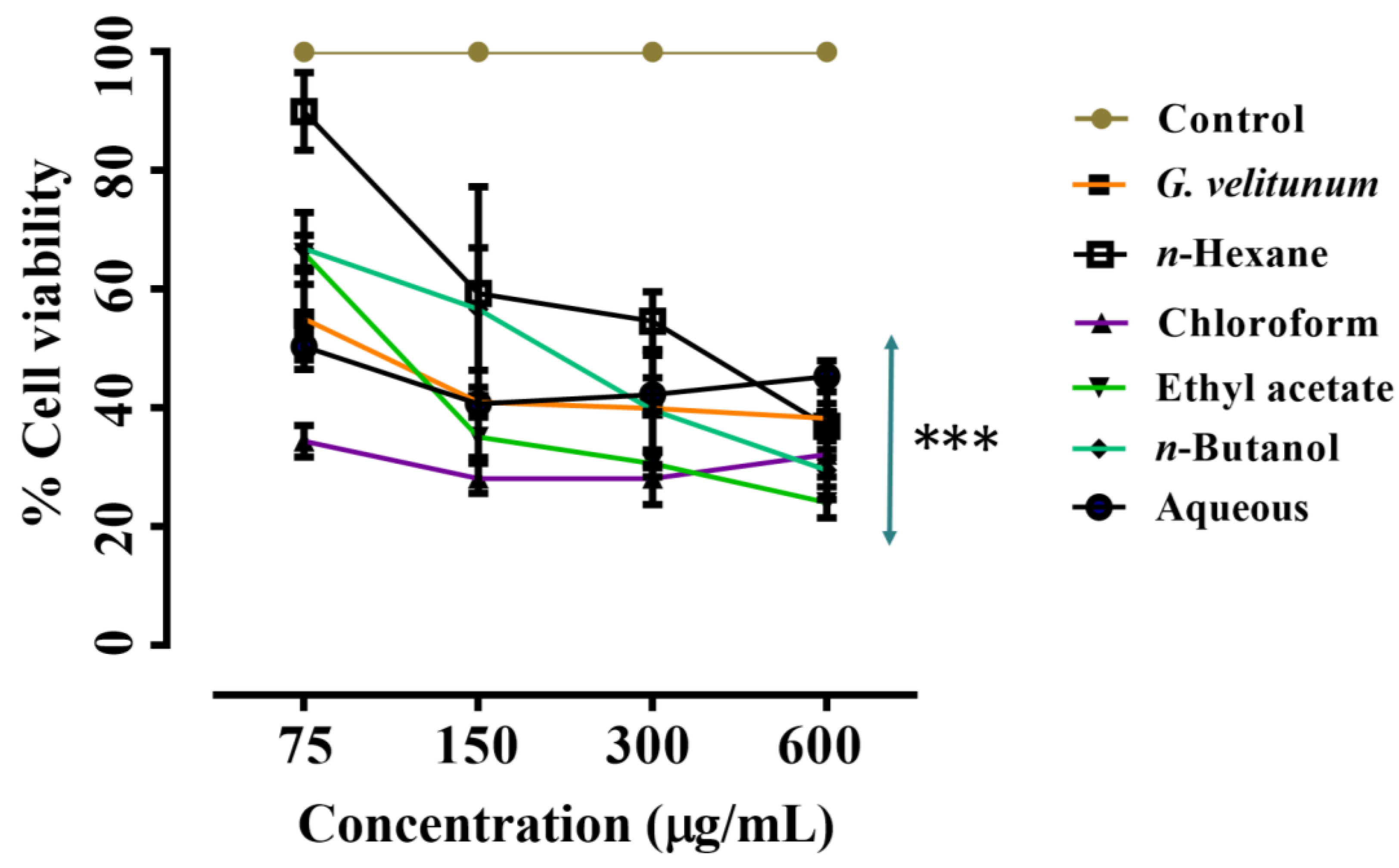
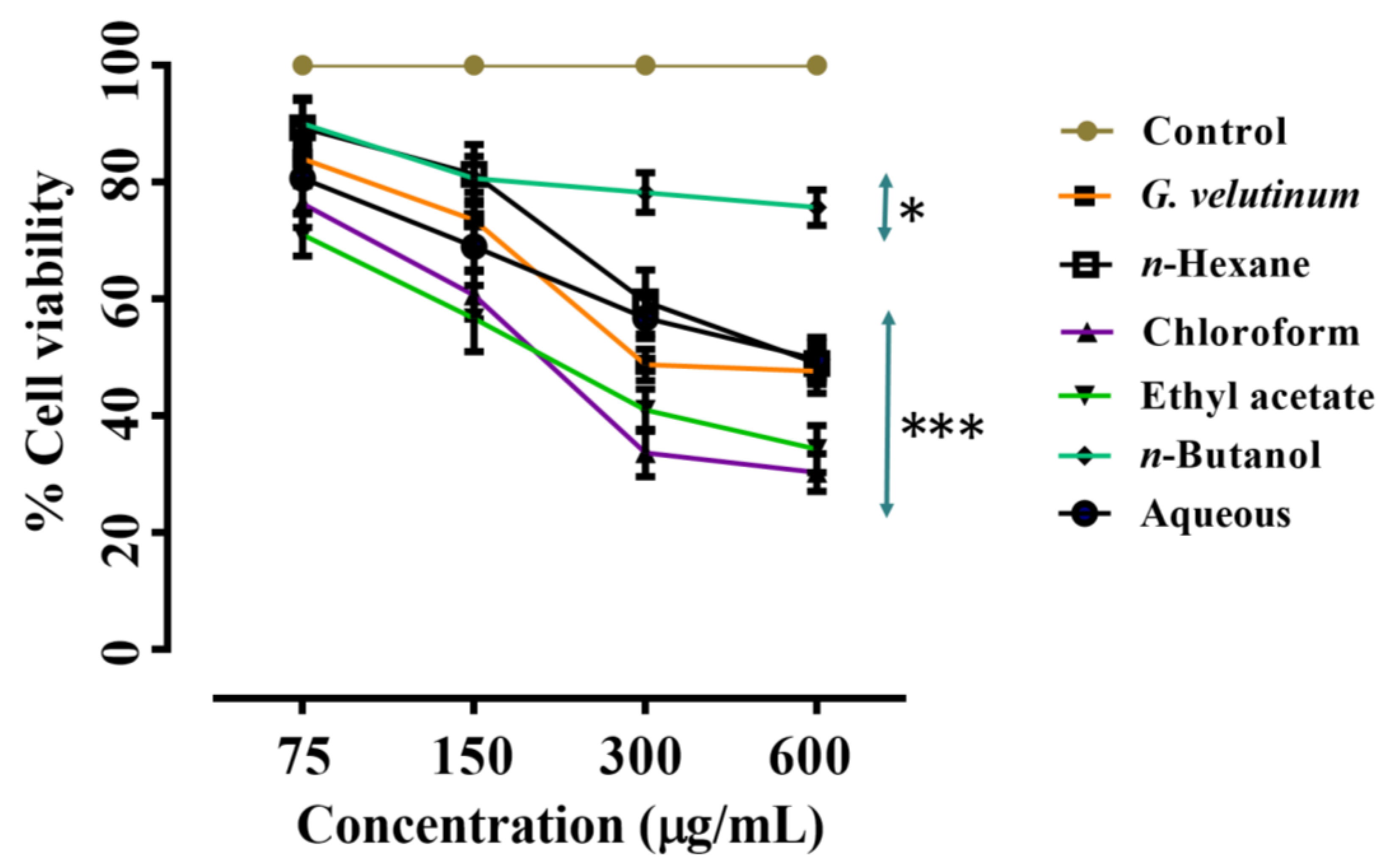
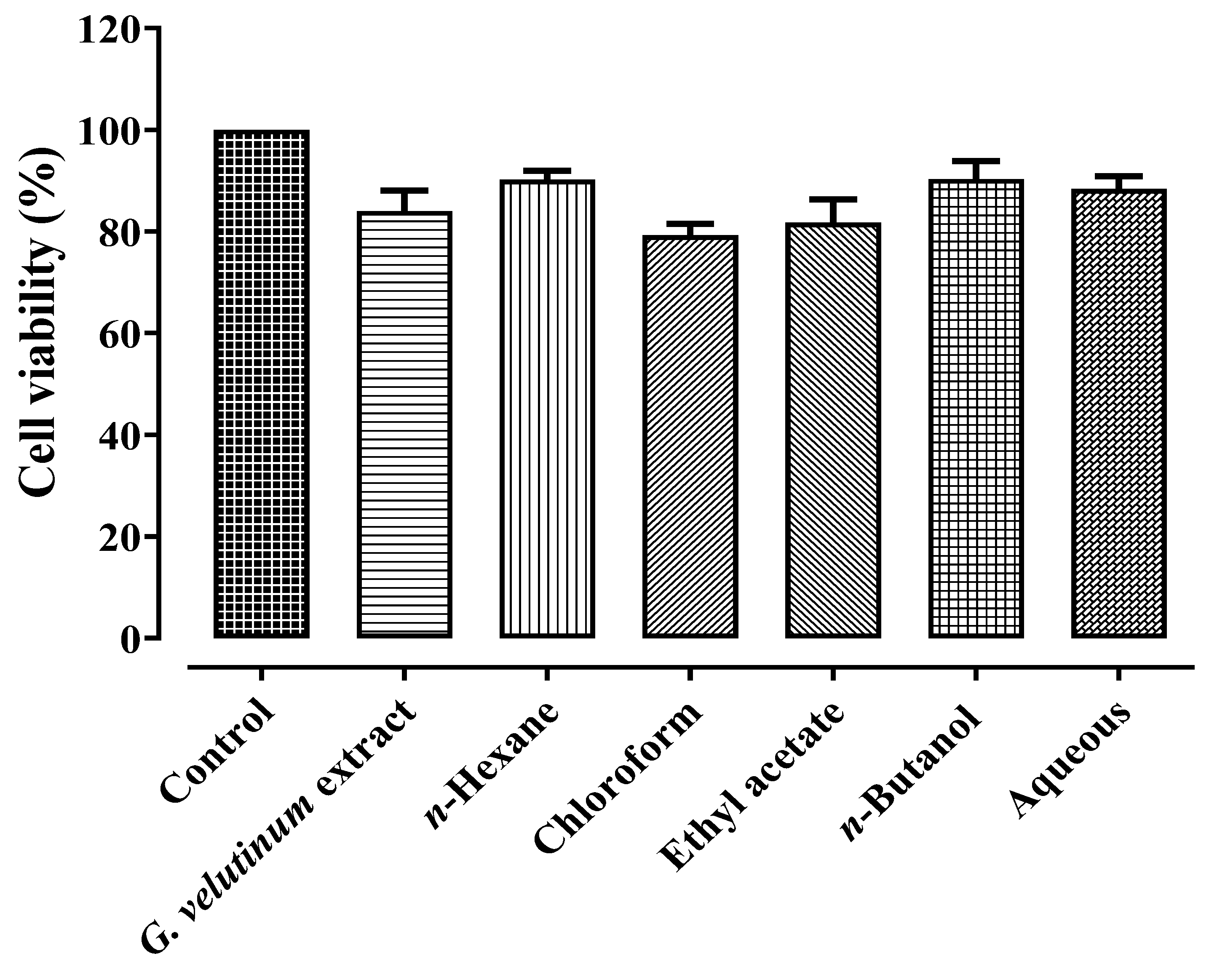
2.1.4. Anticancer Activity
3. Materials and Methods
3.1. Collection and Identification of the Selected Plant
3.2. Processing of Plant Material and Extraction
3.3. Fractionation of Crude Extract
3.4. Phytochemical Evaluation
3.4.1. Total Phenolic Contents (TPC)
3.4.2. Total Flavonoid Contents (TFC)
3.4.3. LC-MS/MS-based Metabolomic Profiling
3.5. Evaluation of the Anticancer Activity
3.5.1. Cell Culture
3.5.2. MTT Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Rong, D.; Li, Z.; Sun, G.; Wu, F.; Li, X.; Cao, H.; Cheng, Y.; Tang, W.; Sun, Y. Role of small molecule targeted compounds in cancer: Progress, opportunities, and challenges. Front. Cell Dev. Biol. 2021, 9, 694363. [Google Scholar] [CrossRef] [PubMed]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Seigel, R.; Lavarsanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 2021. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Komura, K.; Sweeney, C.J.; Inamoto, T.; Ibuki, N.; Azuma, H.; Kantoff, P.W. Current treatment strategies for advanced prostate cancer. Int. J. Urol. 2018, 25, 220–231. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Rasheed, H.M.; Wahid, F.; Ikram, M.; Qaisar, M.; Shah, A.J.; Khan, T. Chemical profiling and anti-breast cancer potential of hexane fraction of Sphaeranthus indicus flowers. Trop. J. Pharm. Res. 2021, 20, 1931–1939. [Google Scholar] [CrossRef]
- Oliveira, P.G.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Corral, M.F.; Gandara, J.S.; Prieto, M.A. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Babaei, G.; Aliarab, A.; Abroon, S.; Rasmi, Y.; Aziz, S.G.G. Application of sesquiterpene lactone: A new promising way for cancer therapy based on anticancer activity. Biomed. Pharmacother. 2018, 106, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Benny, S.; Krishnakumar, K.; Sandhya, A.S. Glochidion velutinum: An overview. J. Bio Innov. 2019, 8, 419–430. [Google Scholar]
- Deb, J.; Saha, S.; Deb, N.K. Review on Glochidion velutinum Wight, (Euphorbiaceae): A medicinal plant. Int. J. Herb. Med. 2019, 7, 1–3. [Google Scholar]
- Chaitnya, R.; Sandhya, S.; Banji, D. HRBC membrane stabilizing property of root, stem and leaf of Glochidion velutinum. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 256–259. [Google Scholar]
- Sandhya, S.; Chaitanya, R.; Vinod, K.R.; Rao, K.N.V.; Banji, D.; Sudhakar, K.; Swetha, R. An updated review on the genus Glochidion plant. Arch. Appl. Sci. Res. 2010, 2, 309–322. [Google Scholar]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2010, 62, 1–20. [Google Scholar] [CrossRef]
- Fernandez, J.; Silvan, B.; Entrialgo-Cadierno, R.; Villar, C.J.; Capasso, R.; Uranga, J.A.; Lombo, F.; Abalo, R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021, 143, 112241. [Google Scholar] [CrossRef]
- Allard, P.-M.; Péresse, T.; Bisson, J.; Gindro, K.; Marcourt, L.; Pham, V.C.; Roussi, F.; Litaudon, M.; Wolfender, J.-L. Integration of molecular networking and in-silico ms/ms fragmentation for natural products dereplication. Anal. Chem. 2016, 88, 3317–3323. [Google Scholar] [CrossRef]
- Olivon, F.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MZmine 2 data-preprocessing to enhance molecular networking reliability. Anal. Chem. 2017, 89, 7836–7840. [Google Scholar] [CrossRef]
- Srivastava, R.; Kulshreshtha, D.K. Triterpenoids from Glochidion heyneanum. Phytochemistry 1988, 27, 3575–3578. [Google Scholar] [CrossRef]
- Saeed, A.; Bashir, K.; Shah, A.J.; Qayyum, R.; Khan, T. Antihypertensive activity in high salt-induced hypertensive rats and lc-ms/ms-based phytochemical profiling of Melia azedarach L. (Meliaceae) leaves. BioMed Res. Int. 2022, 2022, 2791874. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Naz, S.; Rasheed, H.M.; Farooq, U.; Shah, A.J.; McCauley, E.P.; Crews, P.; Khan, T. Tandem high resolution mass spectrometry based phytochemical composition of Sauromatum guttatum tubers and its enzyme inhibitory potential with molecular docking. J. Chromatogr. A 2022, 1672, 463055. [Google Scholar] [CrossRef]
- Bashir, K.; Naz, S.; Farooq, U.; Wahid, F.; Shah, A.J.; McCauley, E.P.; Crews, P.; Khan, T. Assessing the ethnobotanical potential of Carissa opaca berries by merging outcomes from metabolomics profiling, enzyme assays, and in silico docking studies. Food Chem. 2021, 363, 130259. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Oyenihi, A.B.; Smith, C. Are polyphenol antioxidants at the root of medicinal plant anti-cancer success? J. Ethnopharmacol. 2019, 229, 54–72. [Google Scholar] [CrossRef]
- Roleira, F.M.F.; Tavares-da-Silva, E.J.; Varela, C.L.; Costa, S.C.; Silva, T.; Garrido, J.; Borges, F. Plant derived and dietary phenolic antioxidants: Anticancer properties. Food Chem. 2015, 183, 235–258. [Google Scholar] [CrossRef]
- Johnson, J.J.; Bailey, H.H.; Mukhtar, H. Green tea polyphenols for prostate cancer chemoprevention: A translational perspective. Phytomedicine 2010, 17, 3–13. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Clubbs, E.A.; Ferruzzi, M.; Bomser, J.A. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem.-Biol. Interact. 2008, 171, 89–95. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Yamthe, L.R.T.; Tali, B.T.; Adetunji, C.O.; Rahavian, A.; Mudau, F.N.; Martorell, M.; Setzer, W.N.; Rodrigues, R.M.; et al. Phytochemicals in prostate cancer: From bioactive molecules to upcoming therapeutic agents. Nutrients 2019, 11, 1483. [Google Scholar] [CrossRef]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of carbon quantum dots-quinic acid for drug delivery of gemcitabine to breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, M.; Mireskandari, E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomed. 2019, 9, 574–586. [Google Scholar] [PubMed]
- Ahmed, S.; Khan, H.; Fratantonio, D.; Hasan, M.M.; Sharifi, S.; Fathi, N.; Ullah, H.; Rastrelli, L. Apoptosis induced by luteolin in breast cancer: Mechanistic and therapeutic perspectives. Phytomedicine 2019, 59, 152883. [Google Scholar] [CrossRef] [PubMed]
- Castro e Silva, J.H.; Ferreira, R.S.; Pereira, E.P.; Braga-de-Souza, S.; Alves de Almeida, M.M.; Creusa dos Santos, C.; Butt, A.M.; Caiazzo, E.; Capasso, R.; Amaral da Silva, V.D.; et al. Amburana cearensis: Pharmacological and neuroprotective effects of its compounds. Molecules 2020, 25, 3394. [Google Scholar] [CrossRef]
- Xiong, L.; Lu, H.; Hu, Y.; Wang, W.; Liu, R.; Wan, X.; Fu, J. In vitro anti-motile effects of rhoifolin, a flavonoid extracted from Callicarpa nudiflora on breast cancer cells via downregulating podocalyxin-ezrin interaction during epithelial mesenchymal transition. Phytomedicine 2021, 93, 153486. [Google Scholar] [CrossRef]
- Rahman, S.A.S.N.; Abdul Wahab, N.; Abd Malek, S.N. In vitro morphological assessment of apoptosis induced by antiproliferative constituents from the rhizomes of Curcuma zedoaria. Evid. Based Complement. Altern. Med. 2013, 2013, 257108. [Google Scholar]
- Said, A.; Naeem, N.; Wahid, F.; Javed, A.; Rasheed, H.M.; Khan, T.; Sajjad, W.; Shah, K.; Siraj, S. Mechanisms underlying the wound healing and tissue regeneration properties of Chenopodium album. 3 Biotech 2020, 10, 452. [Google Scholar] [CrossRef]
- Khan, T.; Ali, S.; Qayyum, R.; Hussain, I.; Wahid, F.; Shah, A.J. Intestinal and vascular smooth muscle relaxant effect of Viscum album explains its medicinal use in hyperactive gut disorders and hypertension. BMC Complement. Altern. Med. 2016, 16, 251. [Google Scholar] [CrossRef]
- Khan, S.; Khan, T.; Shah, A.J. Total phenolic and flavonoid contents and antihypertensive effect of the crude extract and fractions of Calamintha vulgaris. Phytomedicine 2018, 47, 174–183. [Google Scholar] [CrossRef]
- John, B.; Sulaiman, C.T.; George, S.; Reddy, V.R.K. Total phenolics and flavonoids in selected medicinal plants from Kerala. Int. J. Pharm. Pharm. Sci. 2014, 6, 406–408. [Google Scholar]
- Vyas, S.; Kachhwaha, S.; Kothari, S.L. Comparative analysis of phenolic contents and total antioxidant capacity of Moringa oleifera Lam. Pharmacogn. J. 2015, 7, 44–51. [Google Scholar]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 2020, 332–349. [Google Scholar]
- Khan, Z.U.; Khan, T.; Mannan, A.; Ali, A.; Ni, J. In Vitro and Ex Vivo Evaluation of Mangifera indica L. Extract-Loaded Green Nanoparticles in Topical Emulsion against Oxidative Stress and Aging. Biomedicines 2022, 10, 2266. [Google Scholar] [CrossRef] [PubMed]
| Entry No. | Extract | Total Phenol (µg GAE/mg) |
|---|---|---|
| 1 | Crude | 588 ± 3.0 |
| 2 | n-Hexane | 54 ± 1.2 |
| 3 | Chloroform | 266 ± 1.0 |
| 4 | Ethyl acetate | 859 ± 1.0 |
| 5 | n-Butanol | 44 ± 2.1 |
| 6 | Aqueous | 77 ± 2.3 |
| Entry No. | Extract | Total Flavonoid (µg QE/mg) |
|---|---|---|
| 1 | Crude | 118 ± 2.0 |
| 2 | n-Hexane | 20 ± 1.1 |
| 3 | Chloroform | 79 ± 1.3 |
| 4 | Ethyl acetate | 315 ± 1.0 |
| 5 | n-Butanol | 24 ± 1.5 |
| 6 | Aqueous | 29 ± 1.4 |
| Crude Extract of G. velutinum | ||||||
|---|---|---|---|---|---|---|
| Entry # | Compound Name | RT | m/z | MS2 Fragmentation Pattern | Molecular Formula | Exact Mass |
| 1 | Quinic acid | 0.43 | 191.0568 | 111.1887 (100), 173.3876, 85.1423, 127.066, 146.063, 93.184 | C7H12O6 | 192.0633 |
| 2 | Trehalose | 0.55 | 341.1099 | 179.3955 (100), 161.3627, 143.3026, 119.2360, 113.2109 | C12H22O11 | 342.1162 |
| 3 | Gallocatechol | 1.14 | 305.0642 | 221.1006 (100), 179.1527, 273.0852, 261.1541 | C15H14O7 | 306.0739 |
| 4 | Citric acid | 1.71 | 191.0566 | 147.3730 (100), 111.1887, 85.1423 | C6H8O7 | 192.0270 |
| 5 | Isovitexin | 2.96 | 431.0991 | 311.1170 (100), 341.1700, 269.1172, 367.1852 | C21H20O10 | 432.1056 |
| 6 | Myricetin, 3-Galactopyranoside | 3.71 | 479.1910 | 316.6702 (100), 193.4596, 271.5861 | C21H20O13 | 480.0903 |
| 7 | Hyperoside | 4.02 | 463.5582 | 301.5882 (100) | C21H20O12 | 464.0954 |
| 8 | CGHTM, ME | 4.05 | 447.0950 | 285.5274 (100), 327.6831, 313.7282 | C17H26O11 | 406.1475 |
| 9 | Ellagic acid | 6.07 | 300.9967 | 257.0632 (100), 229.0459, 185.1000, 271.0589, 151.1032 | C14H6O8 | 302.0062 |
| 10 | Rutin | 6.38 | 609.0831 | 463.1980 (100), 301.1688 | C27H30O16 | 610.1533 |
| 11 | 9,12,13,TriHODE | 6.76 | 327.2881 | 229.2324 (100), 291.2702, 211.2155, 171.2333 | C18H32O5 | 328.2249 |
| 12 | FA 18:1+3O | 6.84 | 329.1445 | 229.2349 (100), 171.1871, 211.1918, 293.2625 | C18H34O5 | 330.2406 |
| 13 | Stearidonate (18:4n3) | 7.35 | 277.206 | 233.6909 (100), 205.7536, 179.5107 | C18H27O2- | 275.2011 |
| 14 | 8,8-DDPCM | 8.19 | 339.8762 | 183.4248 (100), 299.6149, 197.4348 | C19H18O6 | 342.1103 |
| 15 | 5,6,2′-Trimethoxyflavone | 8.77 | 311.166 | 183.1256 (100), 247.3156, 198.1505 | C18H16O5 | 312.0997 |
| 16 | MDTT | 9.06 | 311.1662 | 183.373 (100), 149.344, 271.601, 247.682 | C18H30O4 | 310.2144 |
| n-Hexane fraction | ||||||
| 17 | Azelaic acid | 6.24 | 187.2562 | 125.1266 (100), 143.2794, 97.4454, 159.1357 | C9H16O4 | 188.1048 |
| 12 | FA 18:1+3O | 6.84 | 329.1445 | 229.2349 (100), 171.1871, 211.1918, 293.2625 | C18H34O5 | 330.2406 |
| 18 | FA 18:4+2O | 7.15 | 307.1800 | 97.0354 (100), 267.0800 | C18H28O4 | 308.1987 |
| 19 | DGMG 18:3 | 7.41 | 721.3603 | 675.5287 (100), 397.2563, 415.2704 | C33H56O14 | 676.3670 |
| 20 | 9-HOA | 7.73 | 275.2000 | 231.2642 (100), 177.2366, 203.3373, 255.2677, 239.4132 | C18H30O3 | 294.2194 |
| 21 | 13-HODE | 7.92 | 295.3241 | 195.2709 (100), 171.2408, 179.2919, 251.3765, 181.2437 | C18H32O3 | 296.2351 |
| 22 | Docosanol | 8.97 | 325.2862 | 183.1292 (100) | C22H46O | 326.3548 |
| Chloroform fraction | ||||||
| 23 | p-Mentha-1-ene-6-one | 3.59 | 216.5700 | 171.0872 (100), 144.1471, 125.9763, 168.9540 | C10H16O | 152.1201 |
| 9 | Ellagic acid | 6.07 | 300.9967 | 257.0632 (100), 229.0459, 185.1000 271.0589, 151.1032 | C14H6O8 | 302.0062 |
| 24 | trans-piceid | 5.70 | 433.2105 | 387.2457 (100), 225.1868, 193.112, 313.1065 | C20H22O8 | 390.1314 |
| 25 | Vicenin-2 | 5.70 | 593.2812 | 473.1857 (100), 353.1384, 503.181, 383.1578, 413.2072 | C27H30O15 | 594.1584 |
| 26 | EGCG | 5.85 | 457.0732 | 331.1129 (100), 305.1384, 169.0689, 413.1637, 193.0952 | C22H18O11 | 458.0849 |
| 27 | Vitexin-2-O-rhamnoside | 6.01 | 577.1510 | 311.1990 (100), 413.2145, 341.1857, 293.2155, 395.136, 283.16 | C27H30O14 | 578.1635 |
| 28 | Isoquercetin | 6.01 | 463.3657 | 301.1005 (100), 316.0813, 343.0984 | C21H20O12 | 464.0954 |
| 10 | Rutin | 6.38 | 609.0831 | 463.1980 (100), 301.1688 | C27H30O16 | 610.1533 |
| 11 | 9,12,13,TriHODE | 6.76 | 327.2881 | 229.2324 (100), 291.2702, 211.2155, 171.2333 | C18H32O5 | 328.2249 |
| 29 | Maltotriose | 7.50 | 449.7303 | 502.4152 (100), 503.2143, 418.2113, 491.4700, 523.2600, 371.3013 | C18H32O16 | 504.1690 |
| 30 | 9,10-DiHOME | 7.52 | 313.2362 | 201.1504 (100), 183.1968, 293.0565, 277.2592, 171.1771, 195.1924 | C18H34O4 | 314.2457 |
| 15 | 5,6,2′-Trimethoxyflavone | 8.77 | 311.166 | 183.1256 (100), 247.3156, 198.1505 | C18H16O5 | 312.0997 |
| 31 | MGMG 18:3 | 7.72 | 559.308 | 331.4297 (100), 305.10051, 169.0092 | C27H46O9 | 514.3141 |
| 19 | DGMG 18:3 | 7.41 | 721.3603 | 675.5287 (100), 397.2563, 415.2704 | C33H56O14 | 676.3670 |
| 14 | 8,8-DDPCM | 8.19 | 339.8762 | 183.4248 (100), 299.6149, 197.4348 | C19H18O6 | 342.1103 |
| Ethyl acetate fraction | ||||||
| 32 | Gallic acid | 0.42 | 169.0149 | 125.2083 (100), 141.1750, 81.4432, 69.1584 | C7H6O5 | 170.0215 |
| 3 | Gallocatechol | 1.14 | 305.0642 | 221.1006 (100), 179.1527, 273.0852, 261.1541 | C15H14O7 | 306.0739 |
| 33 | Catechin | 2.20 | 289.1863 | 245.1277 (100), 205.1055, 179.1031 | C15H14O6 | 290.0790 |
| 5 | Isovitexin | 2.96 | 431.0991 | 311.1170 (100), 341.1700, 269.1172, 367.1852 | C21H20O10 | 432.1056 |
| 34 | 2-HCA | 3.50 | 163.0392 | 119.0751(100), 135.1361 | C9H8O3 | 164.0473 |
| 35 | Isokaempferide | 4.67 | 301.5982 | 257.5770 (100), 229.4959, 272.5718 | C16H12O6 | 300.0633 |
| 36 | Roseoside | 5.38 | 387.2834 | 207.0929 (100), 163.1177, 225.0903 | C19H30O8 | 386.1940 |
| 37 | 1,3,6-tri-O-galloylglucose | 5.80 | 635.0832 | 465.1552 (100), 483.1615, 313.1343, 423.1522, 591.1775, 221.1012 | C27H24O18 | 636.0962 |
| 26 | EGCG | 5.85 | 457.0732 | 331.1129 (100), 305.1384, 169.0689, 413.1637, 193.0952 | C22H18O11 | 458.0849 |
| 38 | Homoorientin | 5.89 | 447.0904 | 327.0962 (100), 357.0830, 285.0861, 313.0800 | C21H20O11 | 448.1005 |
| 28 | Isoquercetin | 6.01 | 463.3657 | 301.1005 (100), 316.0813, 343.0984 | C21H20O12 | 464.0954 |
| 39 | Kaempferol 3-O-glucoside | 6.13 | 447.0922 | 285.0800 (100), 301.0750, 307.1052 327.1223 | C21H20O11 | 448.1005 |
| 40 | Luteolin | 6.59 | 285.0380 | 241.1324 (100), 175.0822, 199.1310, 217.0913, 151.0380 | C15H10O6 | 286.0477 |
| 41 | Rhoifolin | 6.63 | 577.1302 | 269.1225 (100), 431.1875, 413.1603, 307.1234, 327.1476 | C27H30O14 | 578.1635 |
| 11 | 9,12,13-TriHODE | 6.76 | 327.2881 | 229.2324 (100), 291.2702, 211.2155, 171.2333 | C18H32O5 | 328.2249 |
| 12 | FA 18:1+3O | 6.84 | 329.1445 | 229.2349 (100), 171.1871, 211.1918, 293.2625 | C18H34O5 | 330.2406 |
| 22 | Docosanol | 8.97 | 325.2862 | 183.1292 (100) | C22H46O | 326.3548 |
| 14 | 8,8-DDPCM | 8.19 | 339.8762 | 183.4248 (100), 299.6149, 197.4348 | C19H18O6 | 342.1103 |
| n-Butanol fraction | ||||||
| 1 | Quinic acid | 0.43 | 191.057 | 111.1887 (100), 85.1423, 127.2440, 146.063, 93.184 | C7H12O6 | 192.0633 |
| 2 | Trehalose | 0.50 | 341.1099 | 179.3955 (100), 161.3627, 143.3026, 119.2360, 113.2109 | C12H22O11 | 342.1162 |
| 5 | Isovitexin | 2.96 | 431.0991 | 311.1170 (100), 341.1700, 269.1172, 367.1852 | C21H20O10 | 432.1056 |
| 7 | Hyperoside | 4.02 | 463.0932 | 301.5882 (100) | C21H20O12 | 464.0954 |
| 42 | Luteolin 4’-O-glucoside | 4.16 | 447.0980 | 284.582 (100), 327.7294, 315.7570, 255.572 | C21H20O11 | 448.1005 |
| Aqueous fraction | ||||||
| 2 | Trehalose | 0.55 | 341.1099 | 179.3955 (100), 161.3627, 143.3026, 119.2360, 113.2109 | C12H22O11 | 342.1162 |
| 43 | 6,7-Dimethyl-4-hydroxycoumarin | 0.67 | 188.3989 | 131.3810 (100), 147.4211, 85.2340 | C11H10O3 | 190.0629 |
| 4 | Citric acid | 1.71 | 191.0566 | 147.3730 (100), 111.1887, 85.1423 | C6H8O7 | 192.0270 |
| 44 | Arabinose | 3.36 | 174.9568 | 131.2210 (100), 147.2940, 155.4096 | C5H10O5 | 150.0528 |
| 35 | Isokaempferide | 4.67 | 301.5982 | 257.5770 (100), 229.4959, 272.5718 | C16H12O6 | 300.0633 |
| 14 | 8,8-DDPCM | 8.19 | 339.8762 | 183.4248 (100), 299.6149, 197.4348 | C19H18O6 | 342.1103 |
| 45 | 1-THONONE | 8.44 | 311.175 | 183.5197 (100), 149.3188 | C15H20O7 | 312.1209 |
| 46 | 7-Methoxyflavonol | 8.49 | 266.6690 | 97.0762 (100), 245.4699 | C16H12O4 | 268.0735 |
| Serial No. | Abbreviation | Compound Name |
|---|---|---|
| 1 | CGHTM, ME | Cyclopenta(c)pyran-4-carboxylic acid, 1-(beta-D-glucopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-4a,7-dihydroxy-7-methyl-, methyl ester |
| 2 | 8,8-DDPCM | (8,8-dimethyl-2,10-dioxo-9H-pyrano[2,3-f]chromen-9-yl) (Z)-2-methylbut-2-enoate |
| 3 | MDTT | Methyl (2E,4E,8E)-7,13-dihydroxy-4,8,12-trimethyltetradeca-2,4,8-trienoate |
| 4 | 9,12,13,TriHODE | (10E,15E)-9,12,13-trihydroxyoctadeca-10,15-dienoic acid |
| 5 | EGCG | Epigallocatechin gallate |
| 6 | FA 18:1+3O | 9,12,13-Trihydroxy-10-octadecenoic acid |
| 7 | FA 18:4+2O | (10E,12E,14E)-16-hydroxy-9-oxooctadeca-10,12,14-trienoic acid |
| 8 | 9-HOA | 9-Hydroxy-10E,12Z,15Z-octadecatrienoic acid |
| 9 | 13-HODE | 13-hydroxy-9,11-octadecadienoic acid |
| 10 | 9,10-DiHOME | (12Z)-9,10-Dihydroxyoctadec-12-enoic acid |
| 11 | 2-HCA | 2-Hydroxycinnamic acid |
| 12 | 1-THONONE | 1-[2-methyl-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]ethanone |
| Entry No. | Sample Name | MCF-7 Cell Line IC50 (µg/mL) | PC-3 Cell Line IC50 (µg/mL) |
|---|---|---|---|
| 1 | Crude | 431.78 | 89.02 |
| 2 | n-Hexane fraction | 523.63 | 325.87 |
| 3 | Chloroform fraction | 222.27 | 27.63 |
| 4 | Ethyl acetate fraction | 226.35 | 196.83 |
| 5 | n-Butanol fraction | ≤50% (Not applicable) | 123.36 |
| 6 | Aqueous fraction | 522.41 | 36.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.L.; Bashir, K.; Rasheed, H.M.; Rahman, J.U.; Ikram, M.; Shah, A.J.; Majrashi, K.A.; Alnasser, S.M.; Menaa, F.; Khan, T. LC-MS/MS-Based Metabolomic Profiling of Constituents from Glochidion velutinum and Its Activity against Cancer Cell Lines. Molecules 2022, 27, 9012. https://doi.org/10.3390/molecules27249012
Shah SL, Bashir K, Rasheed HM, Rahman JU, Ikram M, Shah AJ, Majrashi KA, Alnasser SM, Menaa F, Khan T. LC-MS/MS-Based Metabolomic Profiling of Constituents from Glochidion velutinum and Its Activity against Cancer Cell Lines. Molecules. 2022; 27(24):9012. https://doi.org/10.3390/molecules27249012
Chicago/Turabian StyleShah, Syed Luqman, Kashif Bashir, Hafiz Majid Rasheed, Jamil Ur Rahman, Muhammad Ikram, Abdul Jabbar Shah, Kamlah Ali Majrashi, Sulaiman Mohammed Alnasser, Farid Menaa, and Taous Khan. 2022. "LC-MS/MS-Based Metabolomic Profiling of Constituents from Glochidion velutinum and Its Activity against Cancer Cell Lines" Molecules 27, no. 24: 9012. https://doi.org/10.3390/molecules27249012
APA StyleShah, S. L., Bashir, K., Rasheed, H. M., Rahman, J. U., Ikram, M., Shah, A. J., Majrashi, K. A., Alnasser, S. M., Menaa, F., & Khan, T. (2022). LC-MS/MS-Based Metabolomic Profiling of Constituents from Glochidion velutinum and Its Activity against Cancer Cell Lines. Molecules, 27(24), 9012. https://doi.org/10.3390/molecules27249012






