Comprehensive Comparisons between Grafted Kynam Agarwood and Normal Agarwood on Traits, Composition, and In Vitro Activation of AMPK
Abstract
1. Introduction
2. Results and Discussion
2.1. Sample Characteristics and Microscopic Inspection
2.2. Analysis of Physicochemical Indicators and Content of Key Component Groups
2.3. Mass Spectrometric Analysis of Chemical Components in Agarwood and Analysis of Common Components
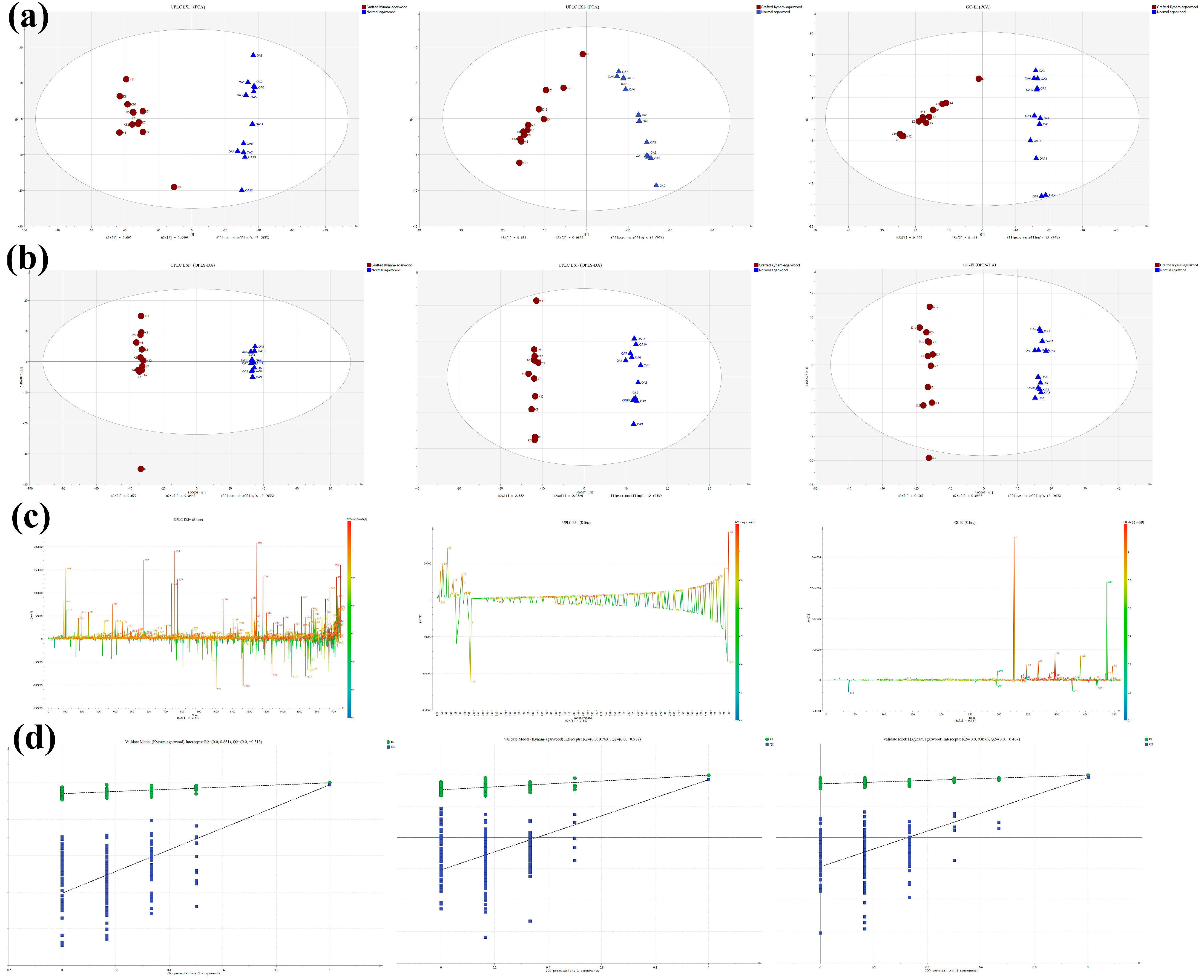
2.4. Mass Spectrometry-Based High-Throughput Techniques for Processing Raw Data of Two Types of Agarwood and Obtaining Global Difference Components
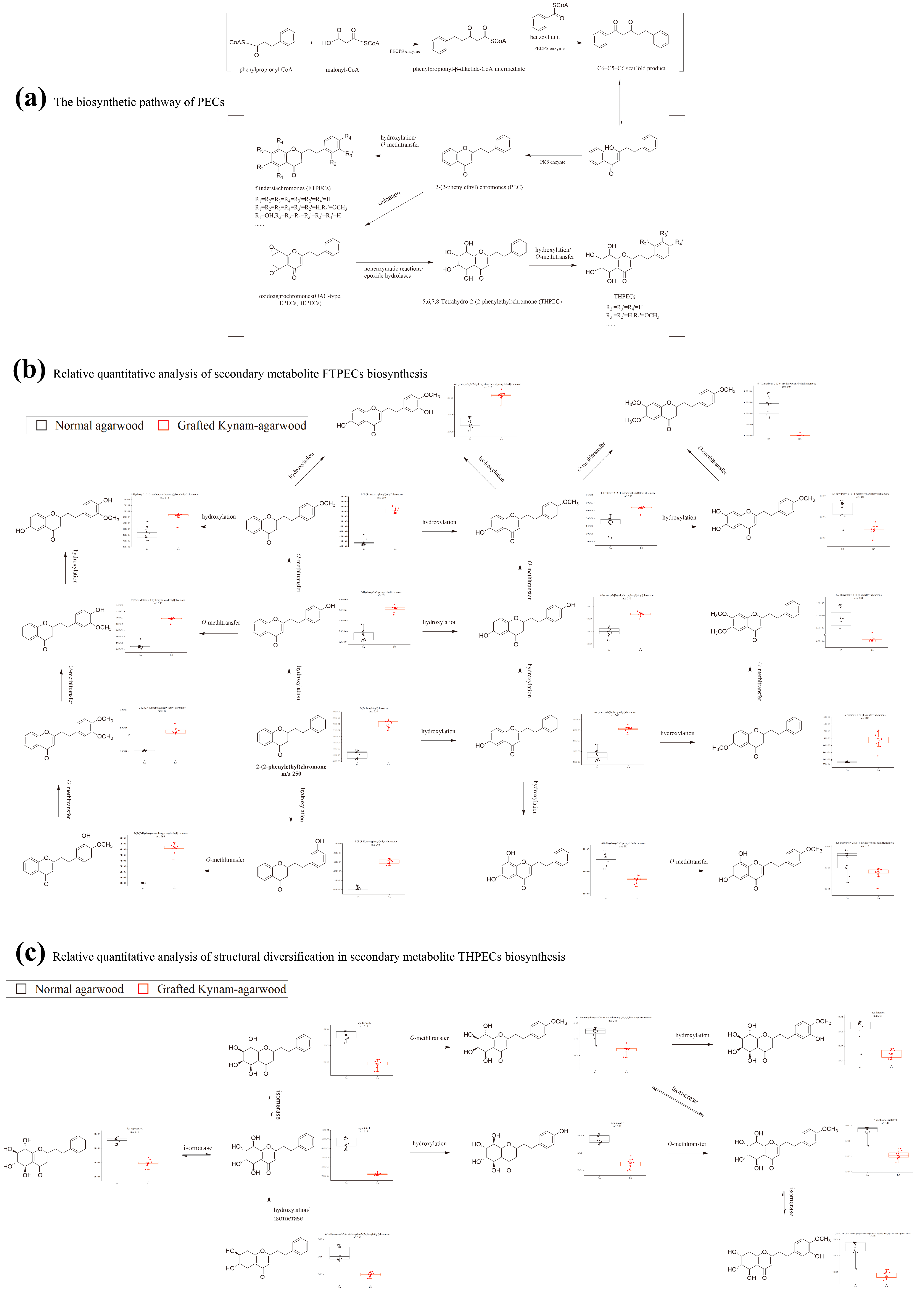
2.5. Identification of Differential Makers between GKA and Normal Agarwood and Differential Analysis of PECs Biosynthesis Pathway
2.6. Identification of Potential Volatile Fragrance Markers in GKA
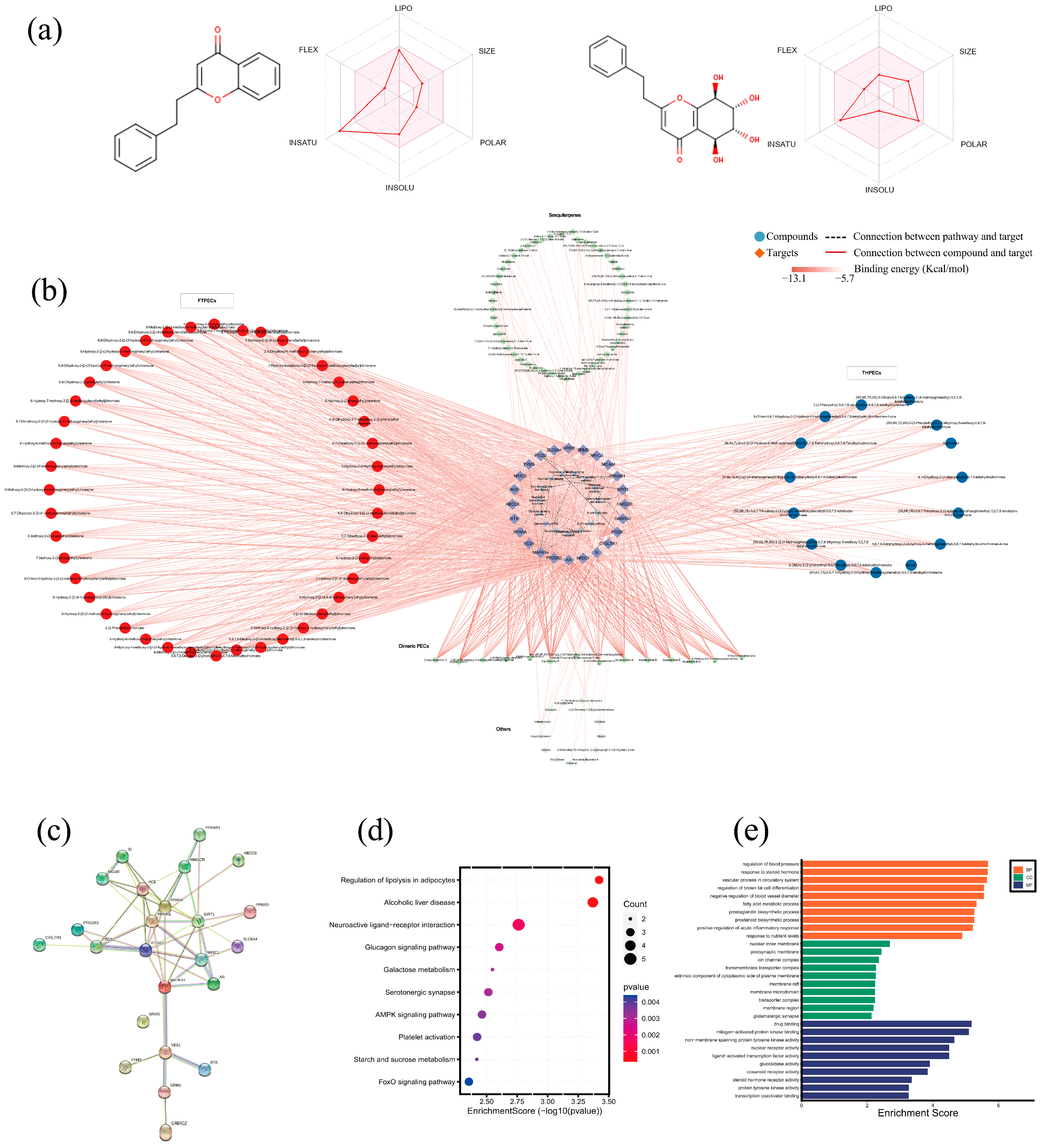
2.7. Molecular Docking Reveals the Major Active Ingredients Group of Agarwood and In Vitro Validation of the Activated AMPK
3. Materials and Methods
3.1. Agarwood Materials and Sample Preparation
3.2. Microscopic Analysis and Content Determination of the Total Composition Groups
3.3. GC-MS Analysis Conditions
3.4. UPLC/Q-TOF-MS Analysis Conditions
3.5. Data Preprocessing and Analysis
3.6. Multivariate Analysis and the Identification of Optimized Features
3.7. Computer-Aided Screening
3.7.1. Target Protein and Ligand Preparation
3.7.2. Molecular Docking Simulation
3.7.3. Network Analysis
3.8. Cell Culture and Treatment
3.9. Western Blot Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
References
- Hashim, Y.Z.; Kerr, P.G.; Abbas, P.; Salleh, H.M. Aquilaria spp. (agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 189, 331–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Yang, Y.; Feng, J.; Liu, P.; Sui, C.; Wei, J. Trunk surface agarwood-inducing technique with Rigidoporus vinctus: An efficient novel method for agarwood production. PLoS ONE 2018, 13, e0198111. [Google Scholar] [CrossRef] [PubMed]
- Shivanand, P.; Arbie, N.F.; Krishnamoorthy, S.; Ahmad, N. Agarwood-The Fragrant Molecules of a Wounded Tree. Molecules 2022, 27, 3386. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Han, X.; Sun, Y.; Chen, H.; Yang, Y.; Liu, Y.; Meng, H.; Gao, Z.; Xu, Y.; Zhang, Z. Overview of sesquiterpenes and chromones of agarwood originating from four main species of the genus Aquilaria. RSC Adv. 2019, 9, 4113–4130. [Google Scholar] [CrossRef]
- Xie, Y.; Li, L.; Chen, Y.; Yang, Y.; Xu, H.; Wang, Z.; Yang, L. Rapid authentication of agarwood by using liquid extraction surface analysis mass spectrometry (LESA-MS). Phytochem. Anal. 2020, 31, 801–808. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1, pp. 192–193. [Google Scholar]
- Yang, D.-L.; Wang, H.; Guo, Z.-K.; Li, W.; Mei, W.-L.; Dai, H.-F. Fragrant agarofuran and eremophilane sesquiterpenes in agarwood ‘Qi-Nan’ from Aquilaria sinensis. Phytochem. Lett. 2014, 8, 121–125. [Google Scholar] [CrossRef]
- Huang, S.; Mei, W.; Zeng, J.; Dai, H. The textual research of Chinese herbal medicine qinan and its historical origin. China Trop. Agric. 2021, 1, 41–48. [Google Scholar]
- Kang, Y. Molecular identification of Aquilaria species with distribution records in China using DNA barcode technology. Mitochondrial DNA Part B 2021, 6, 1525–1535. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, T.; Zhang, Y.; Wang, Q.; Li, G. Characterization of the incense ingredients of cultivated grafting Kynam by TG-FTIR and HS-GC-MS. Fitoterapia 2020, 142, 104493. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Feng, J.; Chen, D.; Yang, Y.; Liu, P.; Yu, Z.; Wei, J. Remarkable phytochemical characteristics of Chi-Nan Agarwood induced from new-found Chi-Nan germplasm of Aquilaria sinensis compared with ordinary Agarwood. Int. J. Anal. Chem. 2021, 2021, 5593730. [Google Scholar] [CrossRef]
- Feng, J.; Hou, W.C.; Chen, L.L.; Chen, X.Q.; Yang, Y.; Liu, Y.Y.; Wei, J.H. Quality analysis and evaluation of agarwood produced by “Chi-nan” germplasm based on standard of Chinese Pharmacopoeia. Mod. Chin. Med. 2022, 24, 432–437. [Google Scholar]
- Kao, W.Y.; Hsiang, C.Y.; Ho, S.C.; Ho, T.Y.; Lee, K.T. Chemical Profiles of Incense Smoke Ingredients from Agarwood by Headspace Gas Chromatography-Tandem Mass Spectrometry. Molecules 2018, 23, 2969. [Google Scholar] [CrossRef]
- Chen, X.; Wang, C.; Feng, J.; Chen, D.; Wei, J.; Liu, Y. Comparative analysis of chemical constituents and anti-oxidant and anti-inflammatory activities of six representative agarwood essential oils. Chin. Tradit. Herb. Drugs 2022, 18, 5720–5730. [Google Scholar]
- Dong, M.; Du, H.; Li, X.; Zhang, L.; Wang, X.; Wang, Z.; Jiang, H. Discovery of Biomarkers and Potential Mechanisms of Agarwood Incense Smoke Intervention by Untargeted Metabolomics and Network Pharmacology. Drug Des. Dev. Ther. 2022, 16, 265–278. [Google Scholar] [CrossRef]
- Yao, C.; Qi, L.; Zhong, F.; Li, N.; Ma, Y. An integrated chemical characterization based on FT-NIR, GC-MS and LC-MS for the comparative metabolite profiling of wild and cultivated agarwood. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2022, 1188, 123056. [Google Scholar] [CrossRef]
- Liao, G.; Dong, W.H.; Yang, J.L.; Li, W.; Wang, J.; Mei, W.L.; Dai, H.F. Monitoring the Chemical Profile in Agarwood Formation within One Year and Speculating on the Biosynthesis of 2-(2-Phenylethyl)Chromones. Molecules 2018, 23, 1261. [Google Scholar] [CrossRef]
- Fan, M.; Yang, W.; He, M.; Li, Y.; Peng, Z.; Wang, G. Occurrence, synthesis and biological activity of 2-(2-phenyethyl)chromones. Eur. J. Med. Chem. 2022, 237, 114397. [Google Scholar] [CrossRef]
- Huo, H.X.; Gu, Y.F.; Sun, H.; Zhang, Y.F.; Liu, W.J.; Zhu, Z.X.; Shi, S.P.; Song, Y.L.; Jin, H.W.; Zhao, Y.F.; et al. Anti-inflammatory 2-(2-phenylethyl)chromone derivatives from Chinese agarwood. Fitoterapia 2017, 118, 49–55. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, D.L.; Wei, J.H.; Feng, J.; Zhang, Z.; Yang, Y.; Zheng, W. Four New 2-(2-Phenylethyl)chromone Derivatives from Chinese Agarwood Produced via the Whole-Tree Agarwood-Inducing Technique. Molecules 2016, 21, 1433. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Song, L.; Wang, Y.; Qiu, H.; Yang, Y.; Li, C.; Wang, Z.; Han, Z.; Yang, L. Seven new 2-(2-phenylethyl) chromone derivatives from agarwood of Aquilaria agallocha with inhibitory effects on nitric oxide production. Fitoterapia 2022, 159, 105177. [Google Scholar] [CrossRef]
- Wang, S.L.; Hwang, T.L.; Chung, M.I.; Sung, P.J.; Shu, C.W.; Cheng, M.J.; Chen, J.J. New Flavones, a 2-(2-Phenylethyl)-4H-chromen-4-one Derivative, and Anti-Inflammatory Constituents from the Stem Barks of Aquilaria sinensis. Molecules 2015, 20, 20912–20925. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Mei, W.L.; Zeng, Y.B.; Guo, Z.K.; Zhao, Y.X.; Wang, H.; Zuo, W.J.; Dong, W.H.; Wang, Q.H.; Dai, H.F. 2-(2-phenylethyl)chromone derivatives in Chinese agarwood “Qi-Nan” from Aquilaria sinensis. Planta Med. 2013, 79, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.N.; Mei, W.L.; Dong, W.H.; Kong, F.D.; Li, W.; Yuan, J.Z.; Dai, H.F. Two new 2-(2-Hydroxy-2-phenylethyl)chromens from agarwood originating from Aquilaria crassna. J. Asian Nat. Prod. Res. 2018, 20, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Kong, F.D.; Wang, H.; Mei, W.L.; Dai, H.F. Qinanmer, a new compound from Chinese agarwood ‘Qi-Nan’ originating from Aquilaria sinensis. J. Asian Nat. Prod. Res. 2017, 19, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R. New chromone and triglyceride from Cucumis melo seeds. Nat. Prod. Commun. 2014, 9, 205–208. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Yang, L.; Kong, F.D.; Dong, W.H.; Li, W.; Chen, H.Q.; Wang, H.; Cai, C.H.; Gai, C.J.; Mei, W.L.; et al. Three new 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones and one new dimeric 2-(2-phenylethyl)chromone from agarwood of Aquilaria crassna Pierre ex Lecomte in Laos. Nat. Prod. Res. 2021, 35, 2295–2302. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, D.L.; Yu, Z.X.; Wang, C.H.; Feng, J.; Meng, Y.; Wei, J.H. New 2-(2-phenylethyl)chromone derivatives from agarwood and their inhibitory effects on tumor cells. Nat. Prod. Res. 2020, 34, 1721–1727. [Google Scholar] [CrossRef]
- Suzuki, A.; Miyake, K.; Saito, Y.; Rasyid, F.A.; Tokuda, H.; Takeuchi, M.; Suzuki, N.; Ichiishi, E.; Fujie, T.; Goto, M.; et al. Phenylethylchromones with In Vitro Antitumor Promoting Activity from Aquilaria filaria. Planta Med. 2017, 83, 300–305. [Google Scholar] [CrossRef]
- Ahn, S.; Ma, C.T.; Choi, J.M.; An, S.; Lee, M.; Le, T.H.V.; Pyo, J.J.; Lee, J.; Choi, M.S.; Kwon, S.W. Adiponectin-secretion-promoting phenylethylchromones from the agarwood of Aquilaria malaccensis. J. Nat. Prod. 2019, 82, 259–264. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.L.; Dong, W.H.; Li, W.; Wang, P.; Cao, X.; Yuan, J.Z.; Chen, H.Q.; Mei, W.L.; Dai, H.F. Sesquiterpenoids and 2-(2-phenylethyl)chromones respectively acting as α-glucosidase and tyrosinase inhibitors from agarwood of an Aquilaria plant. J. Enzym. Inhib. Med. Chem. 2019, 34, 853–862. [Google Scholar] [CrossRef]
- Yoon, J.S.; Lee, M.K.; Sung, S.H.; Kim, Y.C. Neuroprotective 2-(2-phenylethyl)chromones of Imperata cylindrica. J. Nat. Prod. 2006, 69, 290–291. [Google Scholar] [CrossRef]
- Williams, D.A.; Smith, C.; Zhang, Y. An efficient procedure for the preparation of natural products bearing the 2-(2-phenylethyl)chromone skeleton. Tetrahedron Lett. 2013, 54, 4292–4295. [Google Scholar] [CrossRef]
- Mi, C.N.; Yuan, J.Z.; Zhu, M.M.; Yang, L.; Wei, Y.M.; Wang, H.; Long, W.X.; Mei, W.L.; Dai, H.F. 2-(2-Phenylethyl)chromone derivatives: Promising α-glucosidase inhibitors in agarwood from Aquilaria filaria. Phytochemistry 2021, 181, 112578. [Google Scholar] [CrossRef]
- Shibata, S.; Sugiyama, T.; Uekusa, Y.; Masui, R.; Narukawa, Y.; Kiuchi, F. Five new 2-(2-phenylethyl)chromone derivatives from agarwood. J. Nat. Med. 2020, 74, 561–570. [Google Scholar] [CrossRef]
- Sugiyama, T.; Narukawa, Y.; Shibata, S.; Masui, R.; Kiuchia, F. New 2-(2-Phenylethyl)chromone Derivatives and Inhibitors of Phosphodiesterase (PDE) 3A from Agarwood. Nat. Prod. Commun. 2016, 11, 795–797. [Google Scholar] [CrossRef]
- Yu, M.; He, Q.Q.; Chen, X.Q.; Feng, J.; Wie, J.H.; Liu, Y.Y. Chemical and Bioactivity Diversity of 2-(2-Phenylethyl)chromones in Agarwood: A Review. Chem. Biodivers 2022, 19, e202200490. [Google Scholar] [CrossRef]
- Ma, T.; Tian, X.; Zhang, B.; Li, M.; Wang, Y.; Yang, C.; Wu, J.; Wei, X.; Qu, Q.; Yu, Y.; et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022, 603, 159–165. [Google Scholar] [CrossRef]
- Shu, H.; Hang, W.; Peng, Y.; Nie, J.; Wu, L.; Zhang, W.; Wang, D.W.; Zhou, N. Trimetazidine Attenuates Heart Failure by Improving Myocardial Metabolism via AMPK. Front. Pharmacol. 2021, 12, 707399. [Google Scholar] [CrossRef]
- Khaliq, H.A.; Alhouayek, M.; Quetin-Leclercq, J.; Muccioli, G.G. 5’AMP-activated protein kinase: An emerging target of phytochemicals to treat chronic inflammatory diseases. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef]
- Xia, C.; Wang, G.; Chen, L.; Geng, H.; Yao, J.; Bai, Z.; Deng, L. Trans-gnetin H isolated from the seeds of Paeonia species induces autophagy via inhibiting mTORC1 signalling through AMPK activation. Cell Prolif. 2022, e13360. [Google Scholar] [CrossRef]
- Miao, Z.F.; Adkins-Threats, M.; Burclaff, J.R.; Osaki, L.H.; Sun, J.X.; Kefalov, Y.; He, Z.; Wang, Z.N.; Mills, J.C. A Metformin-Responsive Metabolic Pathway Controls Distinct Steps in Gastric Progenitor Fate Decisions and Maturation. Cell Stem Cell 2020, 26, 910–925.e6. [Google Scholar] [CrossRef] [PubMed]
- Śledź, P.; Caflisch, A. Protein structure-based drug design: From docking to molecular dynamics. Curr. Opin. Struct. Biol. 2018, 48, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Jiang, S.; Zhang, J.; Weng, Q.; Yu, Y.; Li, H.; Tian, S.; Ding, X.; Hu, S.; Yang, Y.; et al. Breviscapine alleviates NASH by inhibiting TGF-β-activated kinase 1-dependent signaling. Hepatology 2021, 76, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Divekar, S.D.; Storchan, G.B.; Sperle, K.; Veselik, D.J.; Johnson, E.; Dakshanamurthy, S.; Lajiminmuhip, Y.N.; Nakles, R.E.; Huang, L.; Martin, M.B. The role of calcium in the activation of estrogen receptor-alpha. Cancer Res. 2011, 71, 1658–1668. [Google Scholar] [CrossRef] [PubMed]
- Cyrus, K.; Wang, Q.; Sharawi, Z.; Noguchi, G.; Kaushal, M.; Chang, T.; Rydzewski, W.; Yeguech, W.; Gibrel, F.; Psaltis, J.B.; et al. Role of calcium in hormone-independent and -resistant breast cancer. Int. J. Cancer 2021, 149, 1817–1827. [Google Scholar] [CrossRef]
- Powers, C.N.; Setzer, W.N. A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements. Silico Pharmacol. 2015, 3, 4. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, F.; Liang, G.; Han, Y.; Xu, N.; Pan, J.; Luo, M.; Yang, W.; Zeng, L. Exploration of the Molecular Mechanism of Polygonati Rhizoma in the Treatment of Osteoporosis Based on Network Pharmacology and Molecular Docking. Front. Endocrinol. 2021, 12, 815891. [Google Scholar] [CrossRef]
- Yang, J.; Mei, W.; Xu, H.; Zuo, W.; Dai, H. Determination of Chromones Content in Agarwood by Using UV Spectrophotometry. J. Trop. Biol. 2014, 4, 400–404. [Google Scholar]
- Wang, X.H.; Gao, B.W.; Nakashima, Y.; Mori, T.; Zhang, Z.X.; Kodama, T.; Lee, Y.E.; Zhang, Z.K.; Wong, C.P.; Liu, Q.Q.; et al. Identification of a diarylpentanoid-producing polyketide synthase revealing an unusual biosynthetic pathway of 2-(2-phenylethyl)chromones in agarwood. Nat. Commun. 2022, 13, 348. [Google Scholar] [CrossRef]
- Yagura, T.; Shibayama, N.; Ito, M.; Kiuchi, F.; Honda, G. Three novel diepoxy tetrahydrochromones from agarwood artificially produced by intentional wounding. Tetrahedron Lett. 2005, 46, 4395–4398. [Google Scholar] [CrossRef]
- Naef, R. The volatile and semi-volatile constituents of agarwood, the infected heartwood of Aquilaria species: A review. Flavour Fragr. J. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.Q.; Wang, H.; Mei, W.L.; Dai, H.F. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef]
- Ishihara, M.; Masatsugu, Y.; Uneyama, K. Preparation of (−)-guaia-1 (10), 11-dien-15, 2-olide and (−)-2α-hydroxyguaia-1 (10), 11-dien-15-oic acid, fragrant sesquiterpenes in agarwood (Aquilaria agallocha Roxb.). Tetrahedron 1992, 48, 10265–10276. [Google Scholar] [CrossRef]
- Hassan, J.U.; Kaleem, I.; Rasool, A.; Xu, K.; Tahir, R.A.; Lv, B.; Li, C. Engineered Saccharomyces cerevisiae for the de novo synthesis of the aroma compound longifolene. Chem. Eng. Sci. 2020, 226, 115799. [Google Scholar] [CrossRef]
- Ning, Z.W.; Zhai, L.X.; Peng, J.; Zhao, L.; Huang, T.; Lin, C.Y.; Chen, W.H.; Luo, Z.; Xiao, H.T.; Bian, Z.X. Simultaneous UPLC-TQ-MS/MS determination of six active components in rat plasma: Application in the pharmacokinetic study of Cyclocarya paliurus leaves. Chin. Med. 2019, 14, 28. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Guo, M.; Li, C.; Lei, Y.; Xu, S.; Zhao, D.; Lu, X.-Y. Role of the adipose PPARγ-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol. Psychiatry 2017, 22, 1056–1068. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Yi, J.; Yang, Z.; Zhang, Z.; Li, Z. Promotion of adiponectin multimerization by emodin: A novel AMPK activator with PPARγ-agonist activity. J. Cell Biochem. 2012, 113, 3547–3558. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, Y.; Peng, H.; Rui, J.; Zhang, Z.; Wang, S.; Li, Z. WSF-P-1, a novel AMPK activator, promotes adiponectin multimerization in 3T3-L1 adipocytes. Biosci. Biotechnol. Biochem. 2017, 81, 1529–1535. [Google Scholar] [CrossRef]
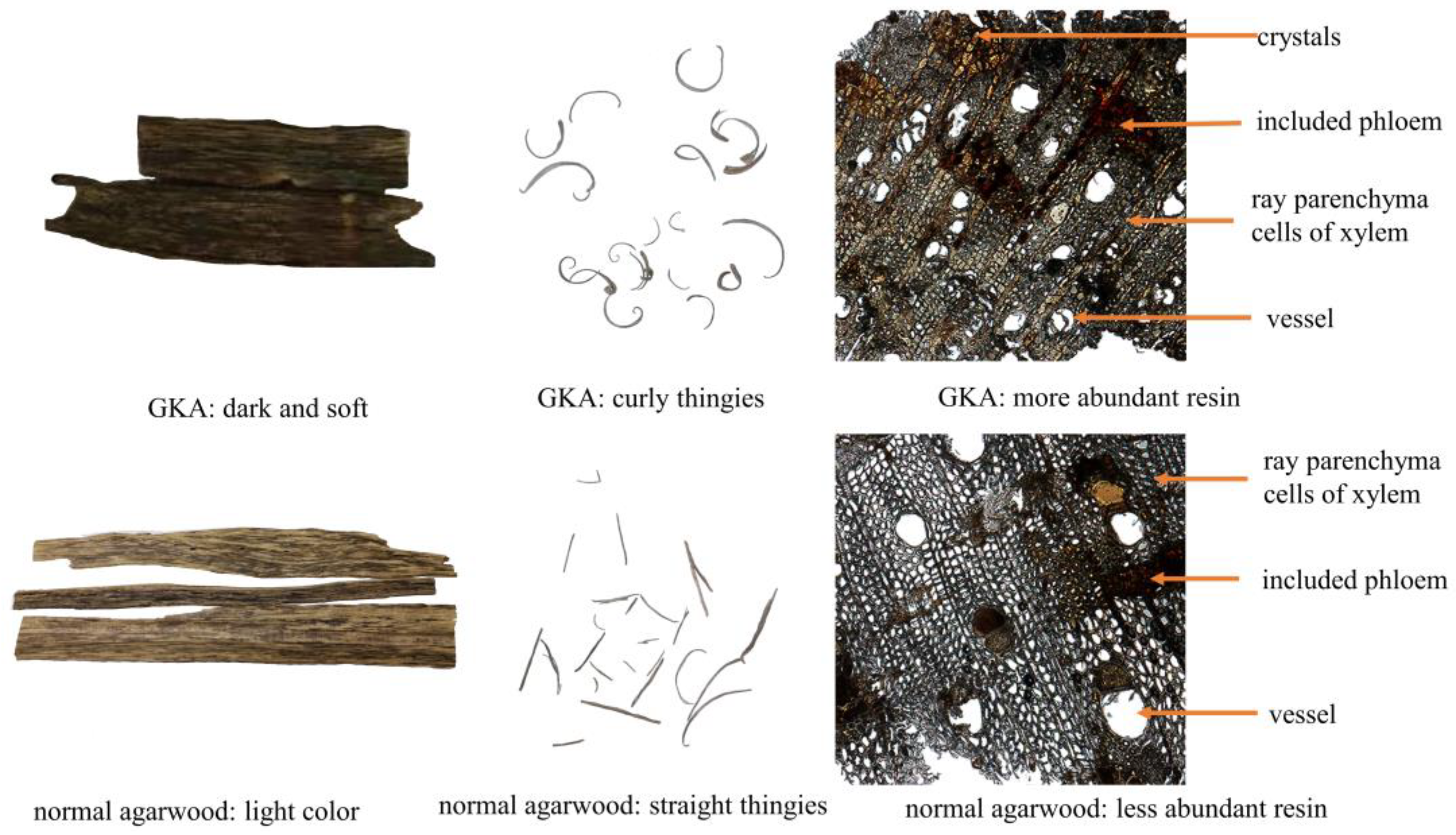
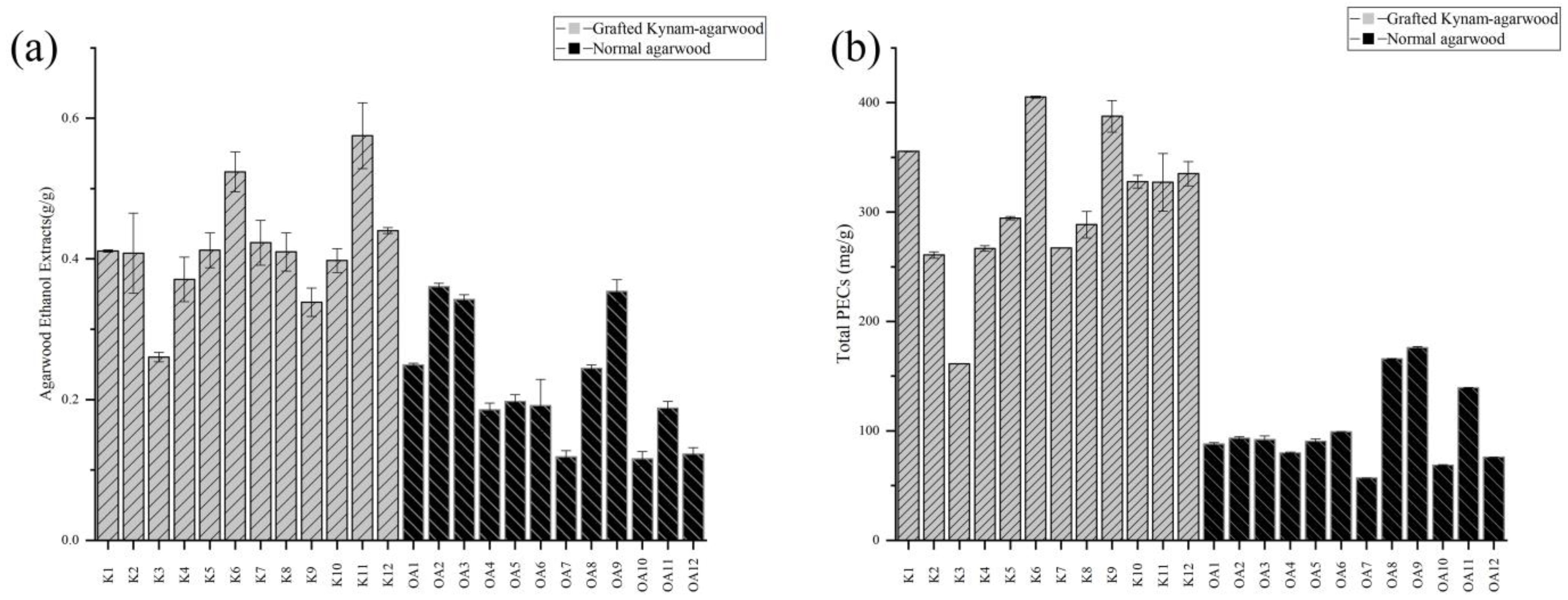
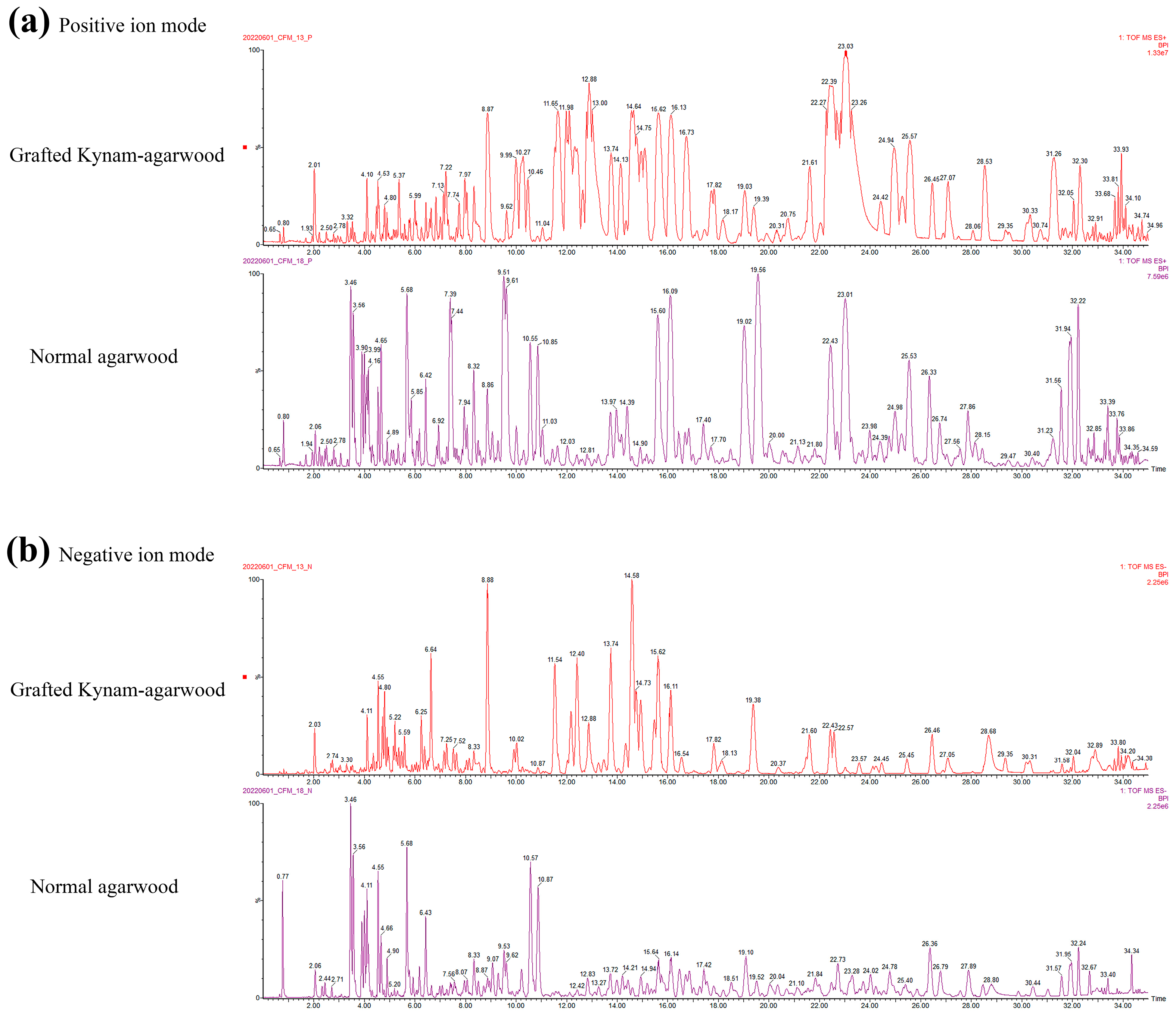
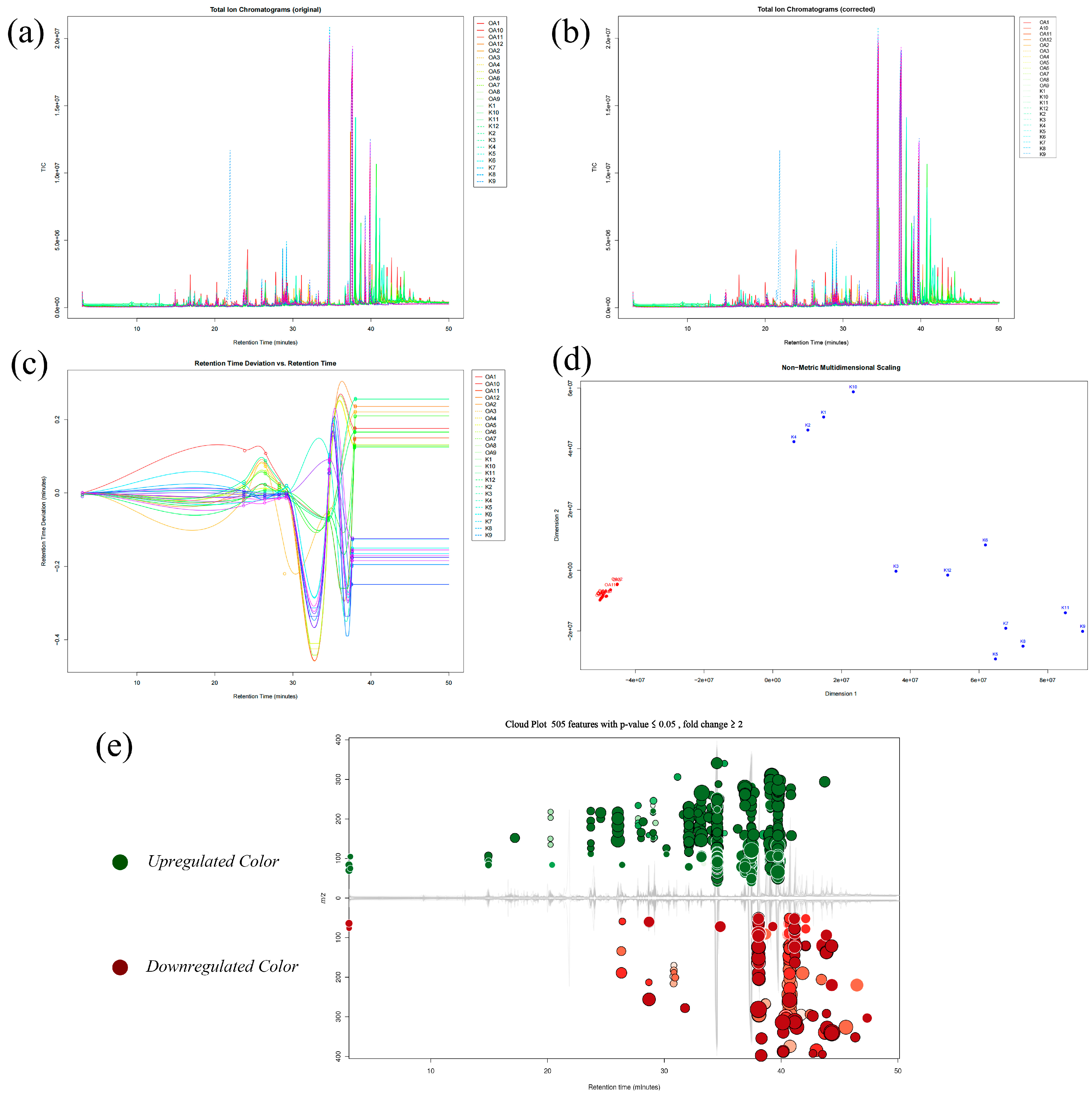

| Contents | Normal Agarwood | Grafted Kynam Agarwood | p-Value |
|---|---|---|---|
| Ethanol extracts (g/g) | 0.223 ± 0.089 | 0.414 ± 0.080 | 0.000015235 |
| Total PECs (mg/g) | 102.197 ± 37.902 | 306.402 ± 65.450 | 2.96989E−08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Huang, Y.; Luo, L.; Wang, Q.; Huang, N.; Zhang, Z.; Li, Z. Comprehensive Comparisons between Grafted Kynam Agarwood and Normal Agarwood on Traits, Composition, and In Vitro Activation of AMPK. Molecules 2023, 28, 1667. https://doi.org/10.3390/molecules28041667
Chen F, Huang Y, Luo L, Wang Q, Huang N, Zhang Z, Li Z. Comprehensive Comparisons between Grafted Kynam Agarwood and Normal Agarwood on Traits, Composition, and In Vitro Activation of AMPK. Molecules. 2023; 28(4):1667. https://doi.org/10.3390/molecules28041667
Chicago/Turabian StyleChen, Fengming, Yu Huang, Lu Luo, Qiaochu Wang, Nanxi Huang, Zhijie Zhang, and Zhen Li. 2023. "Comprehensive Comparisons between Grafted Kynam Agarwood and Normal Agarwood on Traits, Composition, and In Vitro Activation of AMPK" Molecules 28, no. 4: 1667. https://doi.org/10.3390/molecules28041667
APA StyleChen, F., Huang, Y., Luo, L., Wang, Q., Huang, N., Zhang, Z., & Li, Z. (2023). Comprehensive Comparisons between Grafted Kynam Agarwood and Normal Agarwood on Traits, Composition, and In Vitro Activation of AMPK. Molecules, 28(4), 1667. https://doi.org/10.3390/molecules28041667






