Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves
Abstract
1. Introduction
2. Results and Discussion
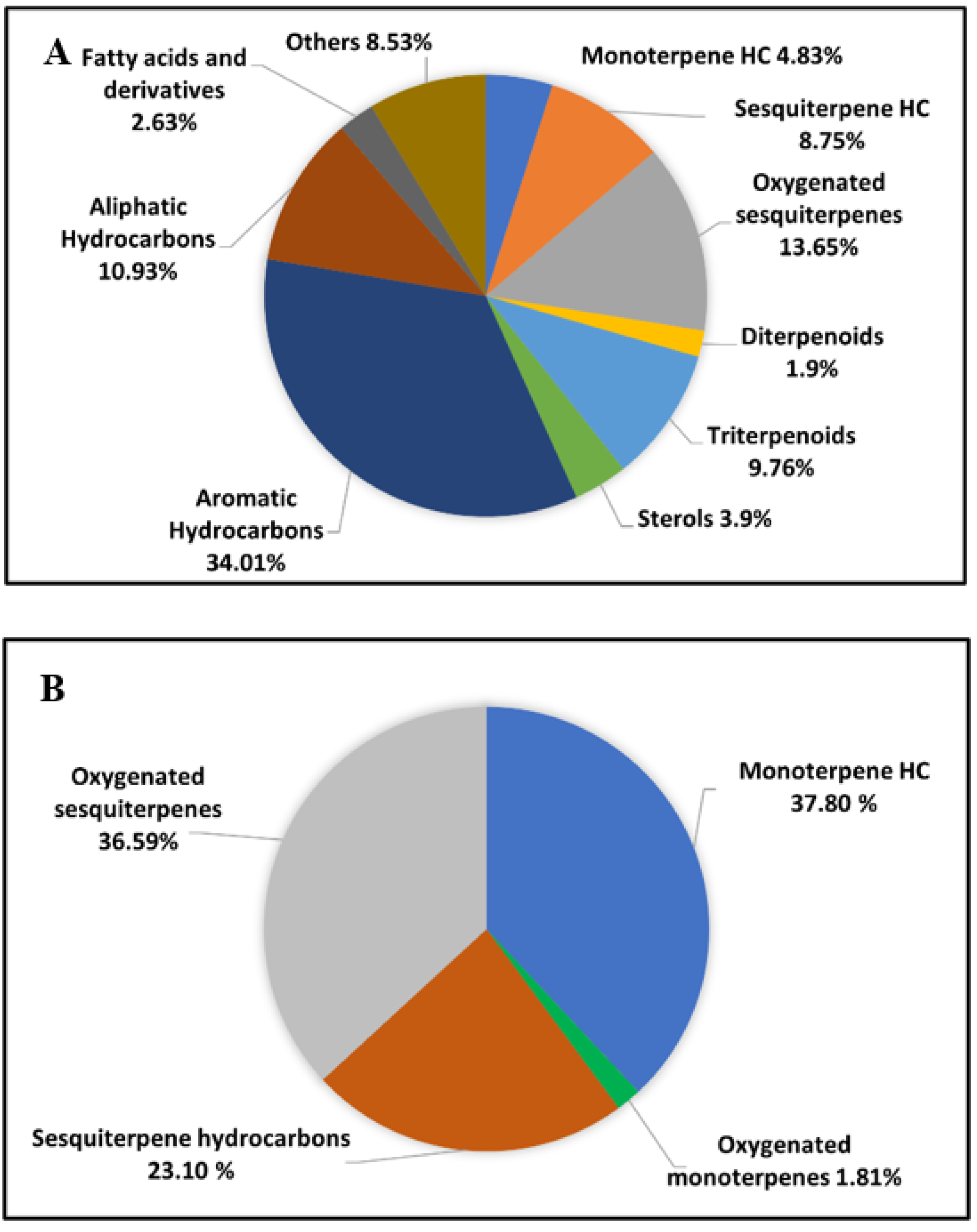
2.1. GC/MS Analysis of the n-Hexane Extract and Essential Oil of Psidium guajava
2.2. Total Phenolic and Flavonoid Content of the n-Hexane Extract of P. guajava Leaves
2.3. Antioxidant Potential of the n-Hexane Extract and Essential Oil Isolated from P. guajava Leaves
2.4. Enzyme Inhibitory Activity of the n-Hexane Extract and the Essential Oil Isolated from P. guajava Leaves
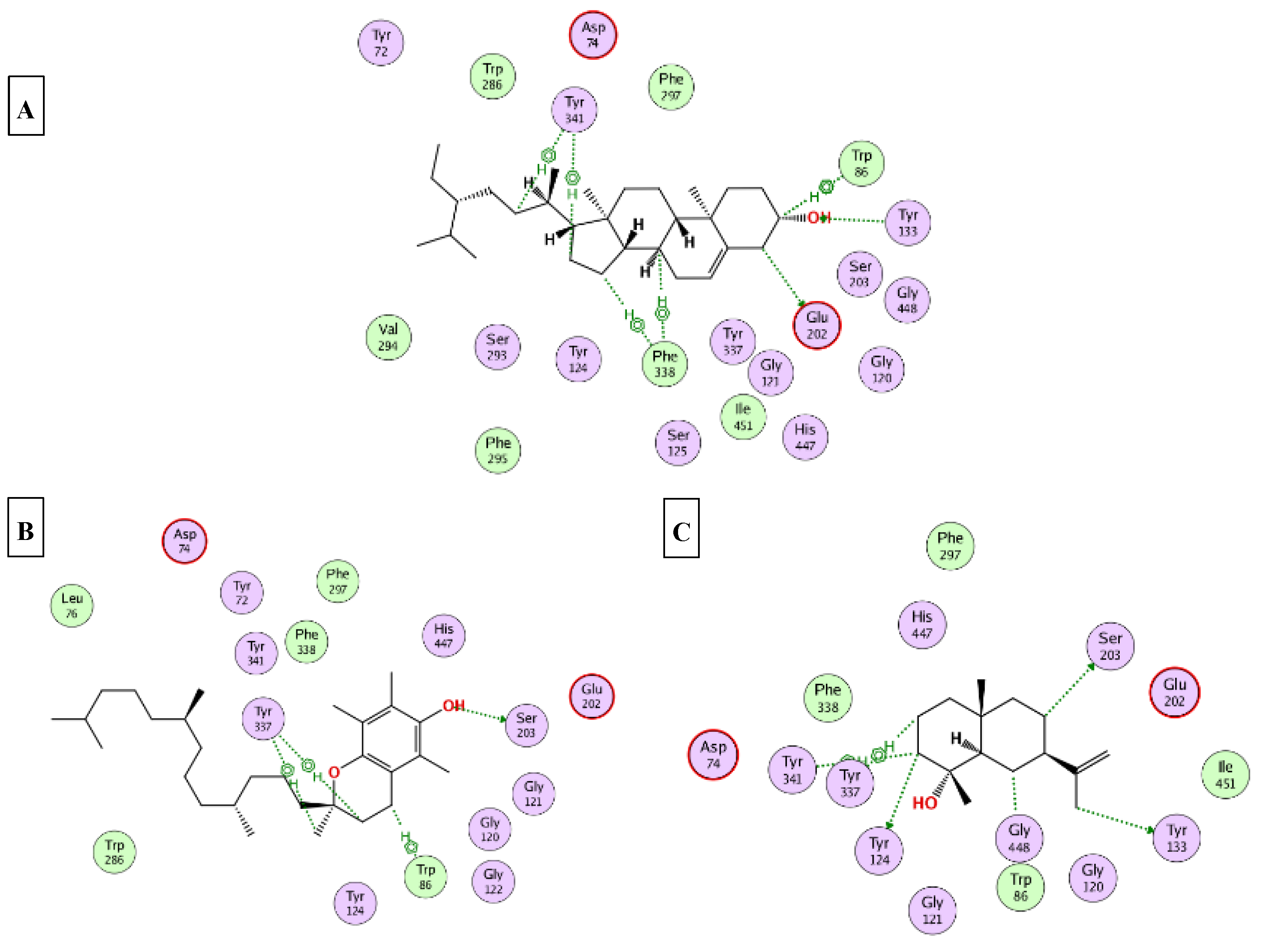
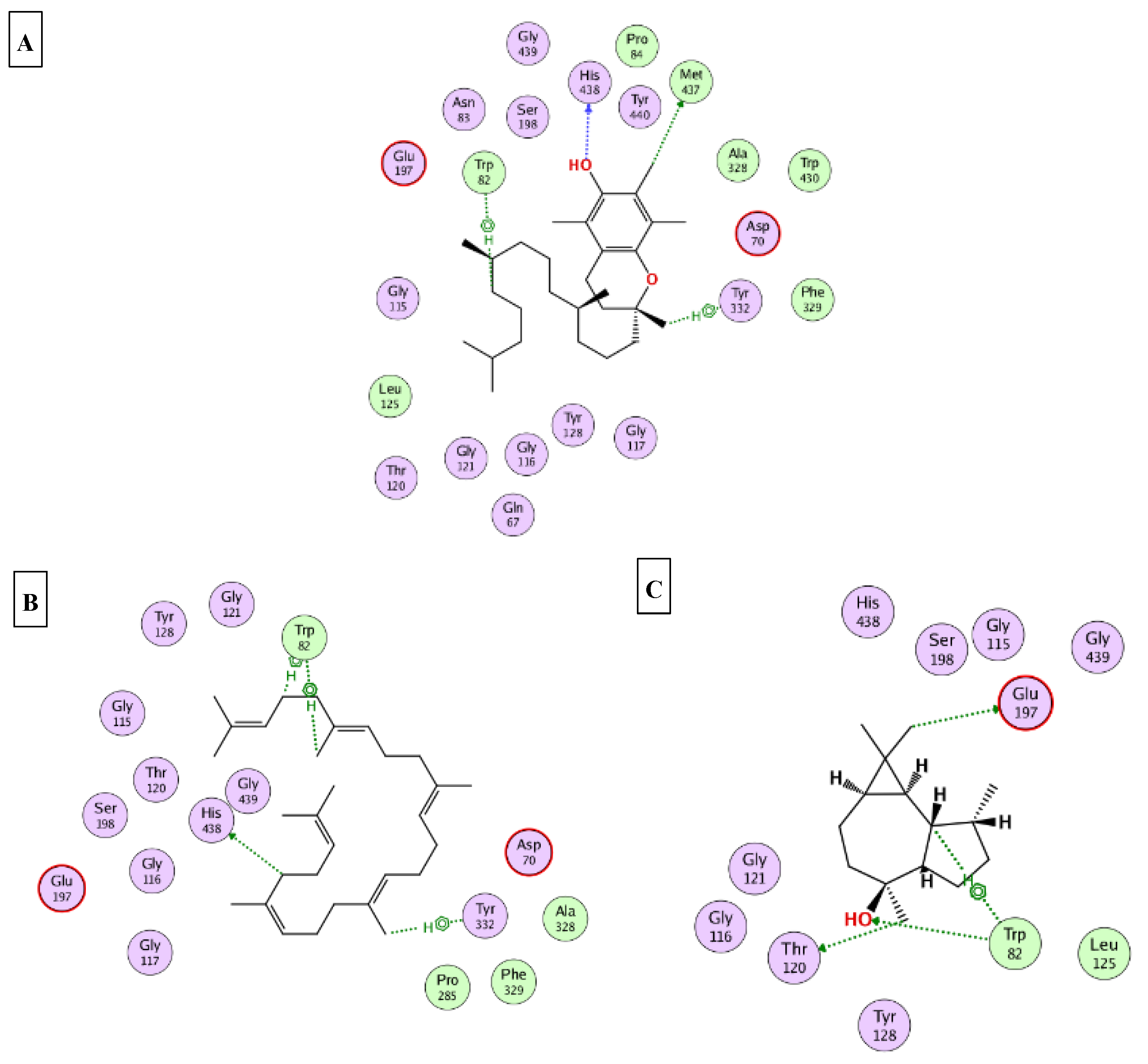
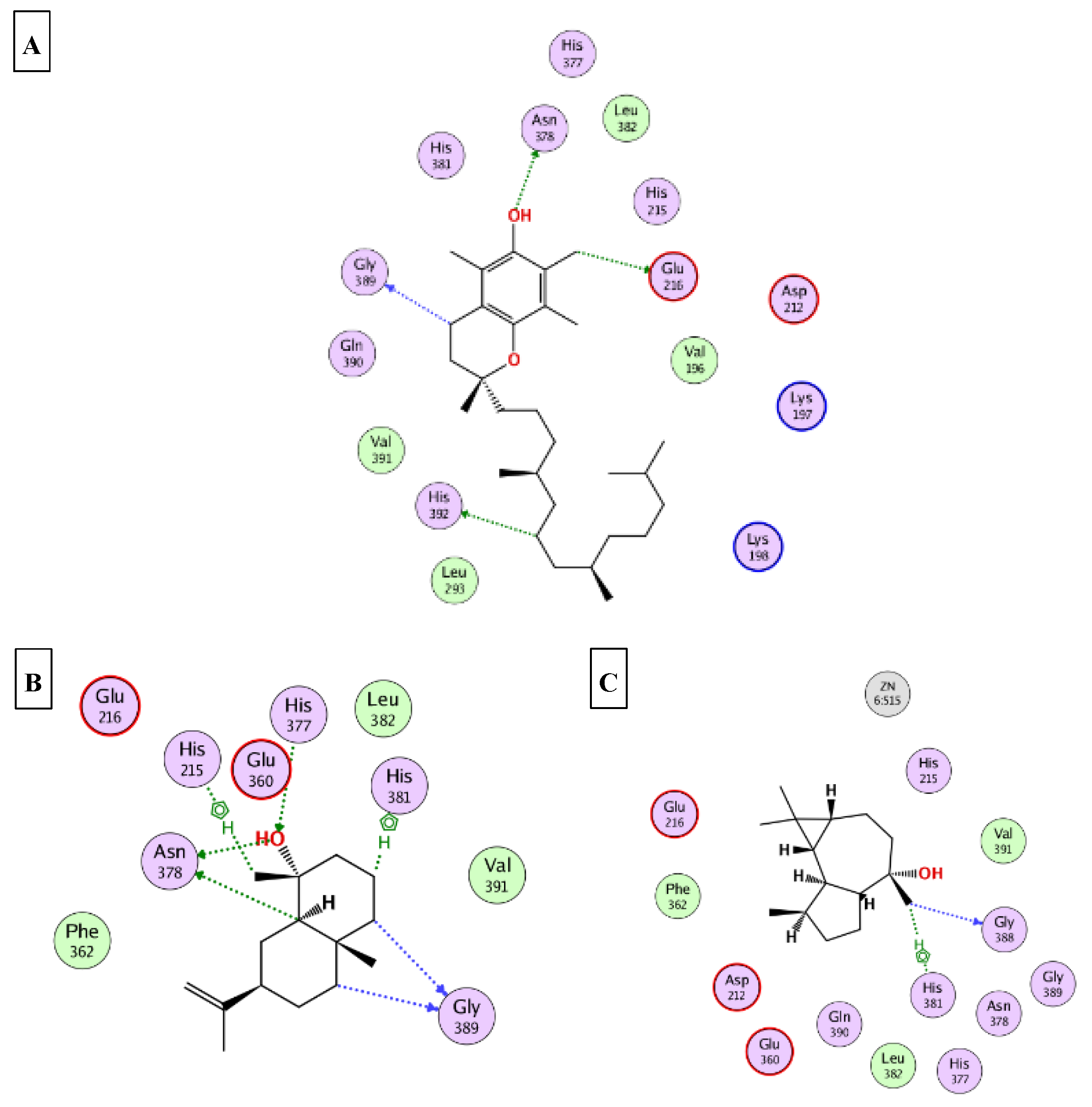
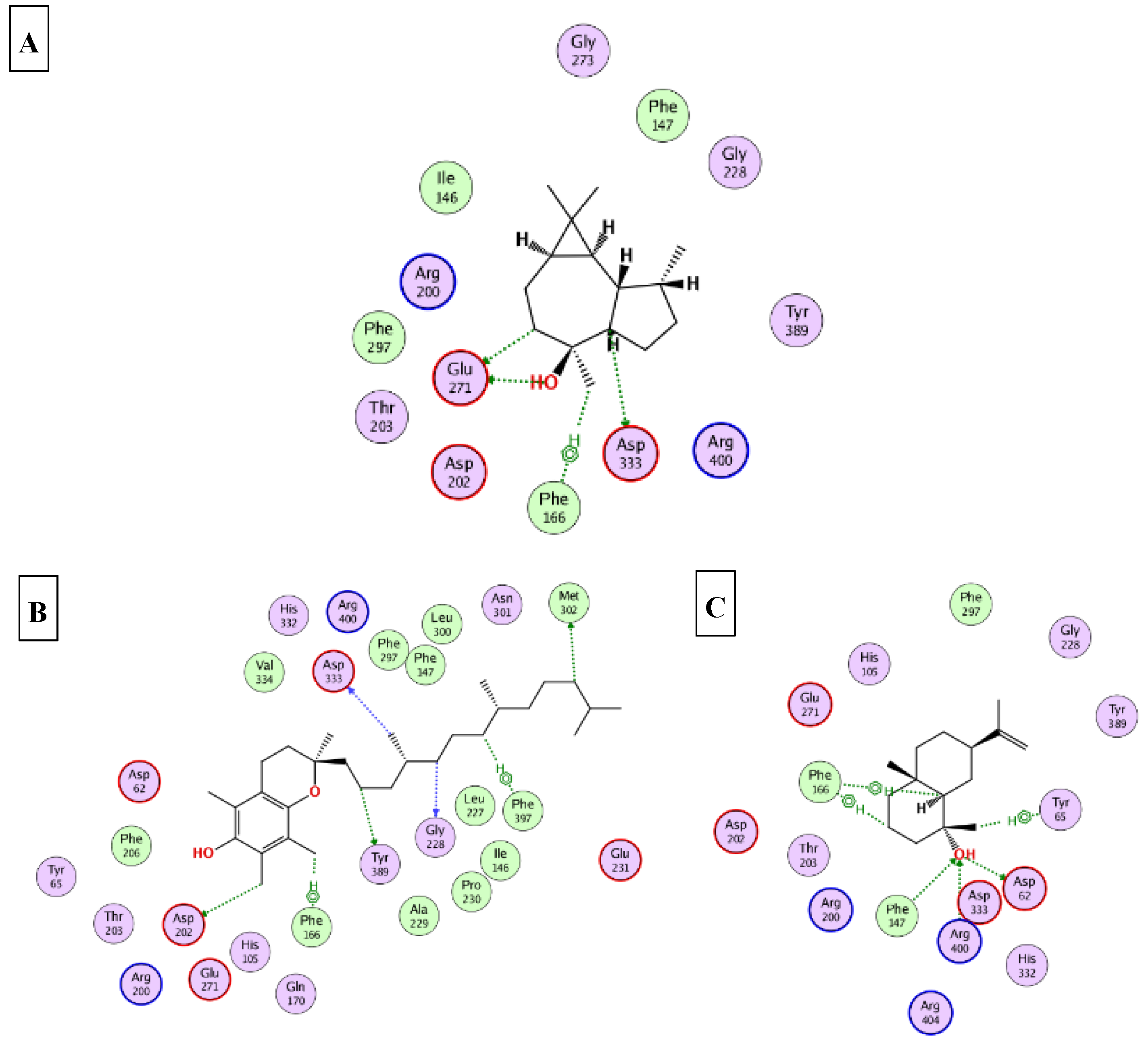
2.5. Molecular Docking
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of the Essential Oil
3.3. Preparation of the n-Hexane Extract
3.4. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
3.5. Compounds Identification
3.6. Total Phenolic and Flavonoid Content
3.7. Antioxidant and Enzyme Inhibitory Assays
3.8. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elgindi, M.R.; El-Nassar Singab, A.B.; Aly, S.H.; Mahmoud, I.I.; Mohamed Elgindi, C.R. Phytochemical Investigation and Antioxidant Activity of Hyophorbe verschaffeltii (Arecaceae). J. Pharmacogn. Phytochem. 2016, 5, 39–46. [Google Scholar]
- Aly, S.H.; Elissawy, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Efferth, T.; Singab, A.N.B. The Pharmacology of the Genus Sophora (Fabaceae): An Updated Review. Phytomedicine 2019, 64, 153070. [Google Scholar] [CrossRef] [PubMed]
- Ads, E.N.; Hassan, S.I.; Rajendrasozhan, S.; Hetta, M.H.; Aly, S.H.; Ali, M.A. Isolation, Structure Elucidation and Antimicrobial Evaluation of Natural Pentacyclic Triterpenoids and Phytochemical Investigation of Different Fractions of Ziziphus spina-christi (L.) Stem Bark Using LCHRMS Analysis. Molecules 2022, 27, 1805. [Google Scholar] [CrossRef] [PubMed]
- Chaves-López, C.; Usai, D.; Donadu, M.G.; Serio, A.; González-Mina, R.T.; Simeoni, M.C.; Molicotti, P.; Zanetti, S.; Pinna, A.; Paparella, A. Potential of: Borojoa patinoi Cuatrecasas Water Extract to Inhibit Nosocomial Antibiotic Resistant Bacteria and Cancer Cell Proliferation in Vitro. Food Funct. 2018, 9, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Saber, F.R.; Aly, S.H.; Khallaf, M.A.; El-Nashar, H.A.S.; Fahmy, N.M.; El-Shazly, M.; Radha, R.; Prakash, S.; Kumar, M.; Taha, D.; et al. Hyphaene thebaica (Areceaeae) as a Promising Functional Food: Extraction, Analytical Techniques, Bioactivity, Food, and Industrial Applications. Food Anal. Methods 2022. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activity of Pinus Species Essential Oils and Their Constituents. J. Enzyme Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Caputo, L.; De Martino, L.; Sakr, S.H.; De Feo, V.; Camele, I. Study of Bio-Pharmaceutical and Antimicrobial Properties of Pomegranate (Punica granatum L.) Leathery Exocarp Extract. Plants 2021, 10, 153. [Google Scholar] [CrossRef]
- Xiao, J.; Tundis, R. Natural Products for Alzheimer’s Disease Therapy: Basic and Application. J. Pharm. Pharmacol. 2013, 65, 1679–1680. [Google Scholar] [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campin, J.; Martnez, J.A.; Milagro, F.I. Antidiabetic Effects of Natural Plant Extracts via Inhibition of Carbohydrate Hydrolysis Enzymes with Emphasis on Pancreatic Alpha Amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of Inhibition of α-Amylase and α-Glucosidase by Aqueous Extract of Morinda lucida Benth Leaf. Biomed Res. Int. 2013, 2013, 527570. [Google Scholar] [CrossRef]
- Fang, L.; Kraus, B.; Lehmann, J.; Heilmann, J.; Zhang, Y.; Decker, M. Design and Synthesis of Tacrine-Ferulic Acid Hybrids as Multi-Potent Anti-Alzheimer Drug Candidates. Bioorg. Med. Chem. Lett. 2008, 18, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.; Yaqub, A.G.; Sanda, K.A.; Nicholas, A.O.; Arastus, W.; Muhammad, M.; Abdullahi, S. Review on Diabetes, Synthetic Drugs and Glycemic Effects of Medicinal Plants. Sect. Title Pharmacol. 2013, 7, 2628–2637. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; Neri-Numa, I.A.; de Araújo, F.F.; Pastore, G.M. A Critical Review of Some Fruit Trees from the Myrtaceae Family as Promising Sources for Food Applications with Functional Claims. Food Chem. 2020, 306, 125630. [Google Scholar] [CrossRef] [PubMed]
- Apel, M.A.; Sobral, M.; Schapoval, E.E.S.; Henriques, A.T.; Menut, C.; Bessiere, J.-M. Volatile Constituents of Eugenia Mattosii Legr (Myrtaceae). J. Essent. Oil Res. 2005, 17, 284–285. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Chemical and Biological Characterization of Melaleuca Alternifolia Essential Oil. Plants 2022, 11, 558. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Eldehna, W.M.; Al-Rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Aly, S.H. GC/MS Analysis of Essential Oil and Enzyme Inhibitory Activities of Syzygium cumini (Pamposia) Grown in Docking Studies. Molecules 2021, 26, 6984. [Google Scholar] [CrossRef]
- Sobeh, M.; El-Raey, M.; Rezq, S.; Abdelfattah, M.A.O.; Petruk, G.; Osman, S.; El-Shazly, A.M.; El-Beshbishy, H.A.; Mahmoud, M.F.; Wink, M. Chemical Profiling of Secondary Metabolites of Eugenia Uniflora and Their Antioxidant, Anti-Inflammatory, Pain Killing and Anti-Diabetic Activities: A Comprehensive Approach. J. Ethnopharmacol. 2019, 240, 111939. [Google Scholar] [CrossRef]
- de Moraes, Â.A.B.; de Jesus Pereira Franco, C.; Ferreira, O.O.; Varela, E.L.P.; do Nascimento, L.D.; Cascaes, M.M.; da Silva, D.R.P.; Percário, S.; de Oliveira, M.S.; de Aguiar Andrade, E.H. Myrcia Paivae O.Berg (Myrtaceae) Essential Oil, First Study of the ChemicalComposition and Antioxidant Potential. Molecules 2022, 27, 5460. [Google Scholar] [CrossRef]
- Ferreira Macedo, J.G.; Linhares Rangel, J.M.; de Oliveira Santos, M.; Camilo, C.J.; Martins da Costa, J.G.; Maria de Almeida Souza, M. Therapeutic Indications, Chemical Composition and Biological Activity of Native Brazilian Species from Psidium Genus (Myrtaceae): A Review. J. Ethnopharmacol. 2021, 278, 114248. [Google Scholar] [CrossRef]
- Arima, H.; Danno, G. Isolation of Antimicrobial Compounds from Guava (Psidium guajava L.) and TheirStructural Elucidation. Biosci. Biotechnol. Biochem. 2002, 66, 1727–1730. [Google Scholar] [CrossRef]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Yang, M.; Chen, W.; Li, X.; Zhu, X. Melatonin Treatment Improves Postharvest Preservation and Resistance of Guava Fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Rouseff, R.L.; Onagbola, E.O.; Smoot, J.M.; Stelinski, L.L. Sulfur Volatiles in Guava (Psidium guajava L.) Leaves: Possible Defense Mechanism. J. Agric. Food Chem. 2008, 56, 8905–8910. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Feng, C.; Lin, X.; Liu, S.; Li, Y.; Kang, M. A Chromosome-Level Genome Assembly Provides Insights into Ascorbic Acid Accumulation and Fruit Softening in Guava (Psidium guajava). Plant Biotechnol. J. 2021, 19, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.A.J.; Estevam, E.B.B.; Silva, T.S.; Nicolella, H.D.; Furtado, R.A.; Alves, C.C.F.; Souchie, E.L.; Martins, C.H.G.; Tavares, D.C.; Barbosa, L.C.A.; et al. Antibacterial and Antiproliferative Activities of the Fresh Leaf Essential Oil of Psidium guajava L. (Myrtaceae). Brazilian J. Biol. 2019, 79, 697–702. [Google Scholar] [CrossRef]
- Ashraf, A.; Sarfraz, R.A.; Rashid, M.A.; Mahmood, A.; Shahid, M.; Noor, N. Chemical Composition, Antioxidant, Antitumor, Anticancer and Cytotoxic Effects of Psidium guajava Leaf Extracts. Pharm. Biol. 2016, 54, 1971–1981. [Google Scholar] [CrossRef]
- Metwally, A.M.; Omar, A.A.; Harraz, F.M.E.S.S. Phytochemical Investigation and Antimicrobial Activity of Psidium guajava L. Leaves. Pharmacogn. Mag. 2010, 6, 212. [Google Scholar]
- da Silva, I.; JR, C.; G, P.; Maranho LT, P.M. Leaf Extract of Eugenia Uniflora L. Prevents Episodic Memory Impairment Induced by Streptozotocin in Rats. Pharmacognosy Res. 2019, 11, 24–30. [Google Scholar] [CrossRef]
- Hassan, E.M.; El Gendy, A.E.N.G.; Abd-ElGawad, A.M.; Elshamy, A.I.; Farag, M.A.; Alamery, S.F.; Omer, E.A. Comparative Chemical Profiles of the Essential Oils from Different Varieties of Psidium guajava L. Molecules 2020, 26, 119. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Amarowicz, R.; Saurabh, V.; Nair, M.S.; Maheshwari, C.; Sasi, M.; Prajapati, U.; Hasan, M.; Singh, S.; et al. Guava (Psidium guajava L.) Leaves: Nutritional Composition. Foods 2021, 10, 752. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhu, H.; Wang, J.; Zhang, H. Chemical Composition, Antibacterial, Antioxidant and Enzyme Inhibitory Activities of the Essential Oil from Leaves of Psidium guajava L. Chem. Biodivers. 2022, 19. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Bai, X.; Feng, J.; Xia, X.; Li, F. Inhibitory Effect of Guava Leaf Polyphenols on Advanced Glycation End Products of Frozen Chicken Meatballs (-18C) and Its Mechanism Analysis. Foods 2022, 11, 2509. [Google Scholar] [CrossRef] [PubMed]
- Naseer, S.; Hussain, S.; Naeem, N.; Pervaiz, M.; Rahman, M. The Phytochemistry and Medicinal Value of Psidium guajava (Guava). Clin. Phytoscience 2018, 4. [Google Scholar] [CrossRef]
- Arain, A.; Hussain Sherazi, S.T.; Mahesar, S.A. Essential Oil From Psidium guajava Leaves: An Excellent Source of β-Caryophyllene. Nat. Prod. Commun. 2019, 14, 1934578X19843007. [Google Scholar] [CrossRef]
- E Silva, R.C.; Da Costa, J.S.; De Figueiredo, R.O.; Setzer, W.N.; Da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Monoterpenes and Sesquiterpenes of Essential Oils from Psidium Species and Their Biological Properties. Molecules 2021, 26, 965. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Priya, R.M. Phytochemical and Biopharmaceutical Aspects of Psidium guajava (L.) Essential Oil: A Review. Res. J. Med. Plant 2011, 5, 432–442. [Google Scholar] [CrossRef]
- Beltrame, B.M.; Klein-Junior, L.C.; Schwanz, M.; Henriques, A.T. Psidium L. Genus: A Review on Its Chemical Characterization, Preclinical and Clinical Studies. Phyther. Res. 2021, 35, 4795–4803. [Google Scholar] [CrossRef] [PubMed]
- de Souza Cardoso, J.; Oliveira, P.S.; Bona, N.P.; Vasconcellos, F.A.; Baldissarelli, J.; Vizzotto, M.; Soares, M.S.P.; Ramos, V.P.; Spanevello, R.M.; Lencina, C.L.; et al. Antioxidant, Antihyperglycemic, and Antidyslipidemic Effects of Brazilian-NativeFruit Extracts in an Animal Model of Insulin Resistance. Redox Rep. 2018, 23, 41–46. [Google Scholar] [CrossRef]
- Alvarenga, F.Q.; Mota, B.C.F.; Leite, M.N.; Fonseca, J.M.S.; Oliveira, D.A.; de Andrade Royo, V.; e Silva, M.L.A.; Esperandim, V.; Borges, A.; Laurentiz, R.S. In Vivo Analgesic Activity, Toxicity and Phytochemical Screening of theHydroalcoholic Extract from the Leaves of Psidium Cattleianum Sabine. J. Ethnopharmacol. 2013, 150, 280–284. [Google Scholar] [CrossRef]
- Camacho-Hernández, I.L.; Cisneros-Rodríguez, C.; Uribe-Beltrán, M.J.; Ríos-Morgan, A.; Delgado-Vargas, F. Antifungal Activity of Fruit Pulp Extract from Psidium Sartorianum. Fitoterapia 2004, 75, 401–404. [Google Scholar] [CrossRef]
- Bouchoukh, I.; Hazmoune, T.; Boudelaa, M.; Bensouici, C.; Zellagui, A. Anticholinesterase and Antioxidant Activities of Foliar Extract from a Tropical Species: Psidium guajava L. (Myrtaceae) Grown in Algeria. Curr. Issues Pharm. Med. Sci. 2019, 32, 160–167. [Google Scholar] [CrossRef]
- Al-Madhagy, S.A.; Mostafa, N.M.; Youssef, F.S.; Awad, G.E.A.; Eldahshan, O.A.; Singab, A.N.B. Metabolic Profiling of a Polyphenolic-Rich Fraction of: Coccinia Grandis Leaves Using LC-ESI-MS/MS and in Vivo Validation of Its Antimicrobial and Wound Healing Activities. Food Funct. 2019, 10, 6267–6275. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.M.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. Cytotoxic Activity and Molecular Docking of a Novel Biflavonoid Isolated from Jacaranda Acutifolia (Bignoniaceae). Nat. Prod. Res. 2016, 30, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Singab, A.N.B. A New Antidiabetic and Anti-Inflammatory Biflavonoid from Schinus Polygama (Cav.) Cabrera Leaves. Nat. Prod. Res. 2022, 36, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.H.; Elissawy, A.M.; Fayez, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Neuroprotective Effects of Sophora Secundiflora, Sophora Tomentosa Leaves and Formononetin on Scopolamine-Induced Dementia. Nat. Prod. Res. 2020, 35, 1–5. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A. Investigation of Antioxidant Potentials of Solvent Extracts from DifferentAnatomical Parts of Asphodeline Anatolica E. Tuzlaci: An Endemic Plant to Turkey. African J. Tradit. Complement. Altern. Med. AJTCAM 2014, 11, 481–488. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-rashood, S.T.; Eldahshan, O.A. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus Sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Mostafa, N.M.; El-Shazly, M.; Eldahshan, O.A. The Role of Plant-Derived Compounds in Managing Diabetes Mellitus: A Review of Literature from 2014 To 2019. Curr. Med. Chem. 2020, 28, 4694–4730. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Aly, S.H.; Ahmadi, A.; El-Shazly, M. The Impact of Polyphenolics in the Management of Breast Cancer: Mechanistic Aspects and Recent Patents. Recent Pat. Anticancer. Drug Discov. 2021, 17, 358–379. [Google Scholar] [CrossRef]
- Gul, M.Z.; Bhakshu, L.M.; Ahmad, F.; Kondapi, A.K.; Qureshi, I.A.; Ghazi, I.A. Evaluation of Abelmoschus Moschatus Extracts for Antioxidant, Free Radical Scavenging, Antimicrobial and Antiproliferative Activities Using in Vitro Assays. BMC Complement. Altern. Med. 2011, 11, 64. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Kis, B.; Pavel, I.Z.; Avram, S.; Moaca, E.A.; Herrero San Juan, M.; Schwiebs, A.; Radeke, H.H.; Muntean, D.; Diaconeasa, Z.; Minda, D.; et al. Antimicrobial Activity, in Vitro Anticancer Effect (MCF-7 Breast Cancer Cell Line), Antiangiogenic and Immunomodulatory Potentials of Populus Nigra L. Buds Extract. BMC Complement. Med. Ther. 2022, 22, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, L.D.; de Moraes, A.A.B.; da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; de Aguiar Andrade, E.H.; de Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Tepe, A.S.; Ozaslan, M. Anti-Alzheimer, Anti-Diabetic, Skin-Whitening, and Antioxidant Activities of the Essential Oil of Cinnamomum zeylanicum. Ind. Crops Prod. 2020, 145, 112069. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical Composition of Mentha Pulegium and Rosmarinus officinalis Essential Oilsand Their Antileishmanial, Antibacterial and Antioxidant Activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- KO, T.-F.; Weng, Y.-M.; Chiou, R.Y.Y. Squalene Content and Antioxidant Activity of Terminalia Catappa Leaves and Seeds. J. Agric. Food Chem. 2002, 50, 5343–5348. [Google Scholar] [CrossRef]
- Shahat, E.A.; Bakr, R.O.; Eldahshan, O.A.; Ayoub, N.A. Chemical Composition and Biological Activities of the Essential Oil from Leaves and Flowers of Pulicaria Incisa Sub. Candolleana (Family Asteraceae). Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef]
- Sodhi, S.; Sharma, A.; Brar, A.P.S.; Brar, R.S. Effect of α Tocopherol and Selenium on Antioxidant Status, Lipid Peroxidation and Hepatopathy Induced by Malathion in Chicks. Pestic. Biochem. Physiol. 2008, 90, 82–86. [Google Scholar] [CrossRef]
- Weng, X.C.; Wang, W. Antioxidant Activity of Compounds Isolated from Salvia Plebeia. Food Chem. 2000, 71, 489–493. [Google Scholar] [CrossRef]
- Wei, A.; Shibamoto, T. Antioxidant Activities and Volatile Constituents of Various Essential Oils. J. Agric. Food Chem. 2007, 55, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Zhang, W.; He, Y.; Li, G.; Shen, T. Chemical Composition, Antioxidant and Antimicrobial Activities of Essential Oil from Leontopodium Longifolium Ling. J. Essent. Oil-Bearing Plants 2018, 21, 175–180. [Google Scholar] [CrossRef]
- Kraujalis, P.; Venskutonis, P.R. Supercritical Carbon Dioxide Extraction of Squalene and Tocopherols from Amaranth and Assessment of Extracts Antioxidant Activity. J. Supercrit. Fluids 2013, 80, 78–85. [Google Scholar] [CrossRef]
- Ahmad, S.; Beg, Z.H. Hypolipidemic and Antioxidant Activities of Thymoquinone and Limonene in Atherogenic Suspension Fed Rats. Food Chem. 2013, 138, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Bacanli, M.; Başaran, A.A.; Başaran, N. The Antioxidant and Antigenotoxic Properties of Citrus Phenolics Limonene and Naringin. Food Chem. Toxicol. 2015, 81, 160–170. [Google Scholar] [CrossRef]
- Calleja, M.A.; Vieites, J.M.; Montero-Meterdez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The Antioxidant Effect of β-Caryophyllene Protects Rat Liver from Carbon Tetrachloride-Induced Fibrosis by Inhibiting Hepatic Stellate Cell Activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria Crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of Inhibition of Acetylcholinesterase by Monoterpenoids and Implications for Pest Control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Zarrad, K.; Hamouda, A.B.; Chaieb, I.; Laarif, A.; Jemâa, J.M. Ben Chemical Composition, Fumigant and Anti-Acetylcholinesterase Activity of the Tunisian Citrus Aurantium L. Essential Oils. Ind. Crops Prod. 2015, 76, 121–127. [Google Scholar] [CrossRef]
- Gad, H.A.; Mamadalieva, N.Z.; Böhmdorfer, S.; Rosenau, T.; Zengin, G.; Mamadalieva, R.Z.; Al Musayeib, N.M.; Ashour, M.L. GC-MS Based Identification of the Volatile Components of Six Astragalus Species from Uzbekistan and Their Biological Activity. Plants 2021, 10, 124. [Google Scholar] [CrossRef]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Amjad Kamal, M.; Ojha, S. Polypharmacological Properties and Therapeutic Potential of β-Caryophyllene: A Dietary Phytocannabinoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, B.; Yang, F.; Sun, Q.; Yang, Z.; Zhu, L. Chemical Composition and Anti-Acetylcholinesterase Activity of Flower Essential Oils of Artemisia annua at Different Flowering Stage. Iran. J. Pharm. Res. 2011, 10, 265–271. [Google Scholar]
- Chear, N.J.Y.; Khaw, K.Y.; Murugaiyah, V.; Lai, C.S. Cholinesterase Inhibitory Activity and Chemical Constituents of Stenochlaena Palustris Fronds at Two Different Stages of Maturity. J. Food Drug Anal. 2016, 24, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. α-Glucosidase and α-Amylase Inhibitors from Seed Oil: A Review of Liposoluble Substance to Treat Diabetes. Crit. Rev. Food Sci. Nutr. 2017, 57, 3438–3448. [Google Scholar] [CrossRef] [PubMed]
- Javadi, N.; Abas, F.; Hamid, A.A.; Simoh, S.; Shaari, K.; Ismail, I.S.; Mediani, A.; Khatib, A. GC-MS-Based Metabolite Profiling of Cosmos caudatus Leaves Possessing Alpha-Glucosidase Inhibitory Activity. J. Food Sci. 2014, 79, C1130–C1136. [Google Scholar] [CrossRef] [PubMed]
- You, D.H.; Park, J.W.; Yuk, H.G.; Lee, S.C. Antioxidant and Tyrosinase Inhibitory Activities of Different Parts of Guava (Psidium guajava L.). Food Sci. Biotechnol. 2011, 20, 1095–1100. [Google Scholar] [CrossRef]
- Obaid, R.J.; Mughal, E.U.; Naeem, N.; Sadiq, A.; Alsantali, R.I.; Jassas, R.S.; Moussa, Z.; Ahmed, S.A. Natural and Synthetic Flavonoid Derivatives as New Potential Tyrosinase Inhibitors: A Systematic Review. RSC Adv. 2021, 11, 22159–22198. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural Skin-Whitening Compounds for the Treatment of Melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin Whitening Agents: Medicinal Chemistry Perspective of Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 9781932633214. [Google Scholar]
- NIST. The National Institute of Standards and Technology (NIST) Chemistry WebBook. NIST Standard Reference Database Number 69. Available online: Http://Webbook.Nist.Gov/Chemistry/ (accessed on 5 July 2022).
- Aly, S.H.; Elissawy, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Variability of the Chemical Composition of the Essential Oils of Flowers and the Alkaloid Contents of Leaves of Sophora Secundiflora and Sophora Tomentosa. J. Essent. Oil-Bearing Plants 2020, 442–452. [Google Scholar] [CrossRef]
- Aly, S.H.; Elissawy, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Phytochemical Investigation Using GC/MS Analysis and Evaluation of Antimicrobial and Cytotoxic Activities of the Lipoidal Matter of Leaves of Sophora Secundiflora and Sophora Tomentosa. Arch. Pharm. Sci. Ain Shams Univ. 2020, 4, 207–214. [Google Scholar] [CrossRef]
- Ashmawy, A.; Mostafa, N.; Eldahshan, O. GC/MS Analysis and Molecular Profiling of Lemon Volatile Oil against Breast Cancer. J. Essent. Oil-Bearing Plants 2019, 22, 903–916. [Google Scholar] [CrossRef]
- Singab, A.N.B.; Mostafa, N.M.; Eldahshan, O.A.; Ashour, M.L.; Wink, M. Profile of Volatile Components of Hydrodistilled and Extracted Leaves of Jacaranda Acutifolia and Their Antimicrobial Activity against Foodborne Pathogens. Nat. Prod. Commun. 2014, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In Vitro Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Profile of Potentilla Thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and Enzyme Inhibitory Potential of Two Potentilla Species (P. Speciosa L. and P. Reptans Willd.) and Their Chemical Composition. Front. Pharmacol. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure of Human Tyrosinase Related Protein 1 Reveals a Binuclear Zinc ActiveSite Important for Melanogenesis. Angew. Chem. Int. Ed. Engl. 2017, 56, 9812–9815. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.L.; Brazzolotto, X.; MacDonald, I.R.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef]
- Shen, X.; Saburi, W.; Gai, Z.; Kato, K.; Ojima-Kato, T.; Yu, J.; Komoda, K.; Kido, Y.; Matsui, H.; Mori, H.; et al. Structural Analysis of the α-Glucosidase HaG Provides New Insights into SubstrateSpecificity and Catalytic Mechanism. Acta Crystallogr. D. Biol. Crystallogr. 2015, 71, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.K.; Li, C.; Withers, S.G.; Brayer, G.D. Order and Disorder: Differential Structural Impacts of Myricetin and EthylCaffeate on Human Amylase, an Antidiabetic Target. J. Med. Chem. 2012, 55, 10177–10186. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, Y.; Yin, W.; Li, J.; Wang, W.; Bai, F.; Xu, S.; Gong, Q.; Peng, T.; Hong, Y.; et al. Kinetics-Driven Drug Design Strategy for Next-Generation Acetylcholinesterase Inhibitors to Clinical Candidate. J. Med. Chem. 2021, 64, 1844–1855. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal Chemistry and the Molecular Operating Environment (MOE): Application of QSAR and Molecular Docking to Drug Discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]

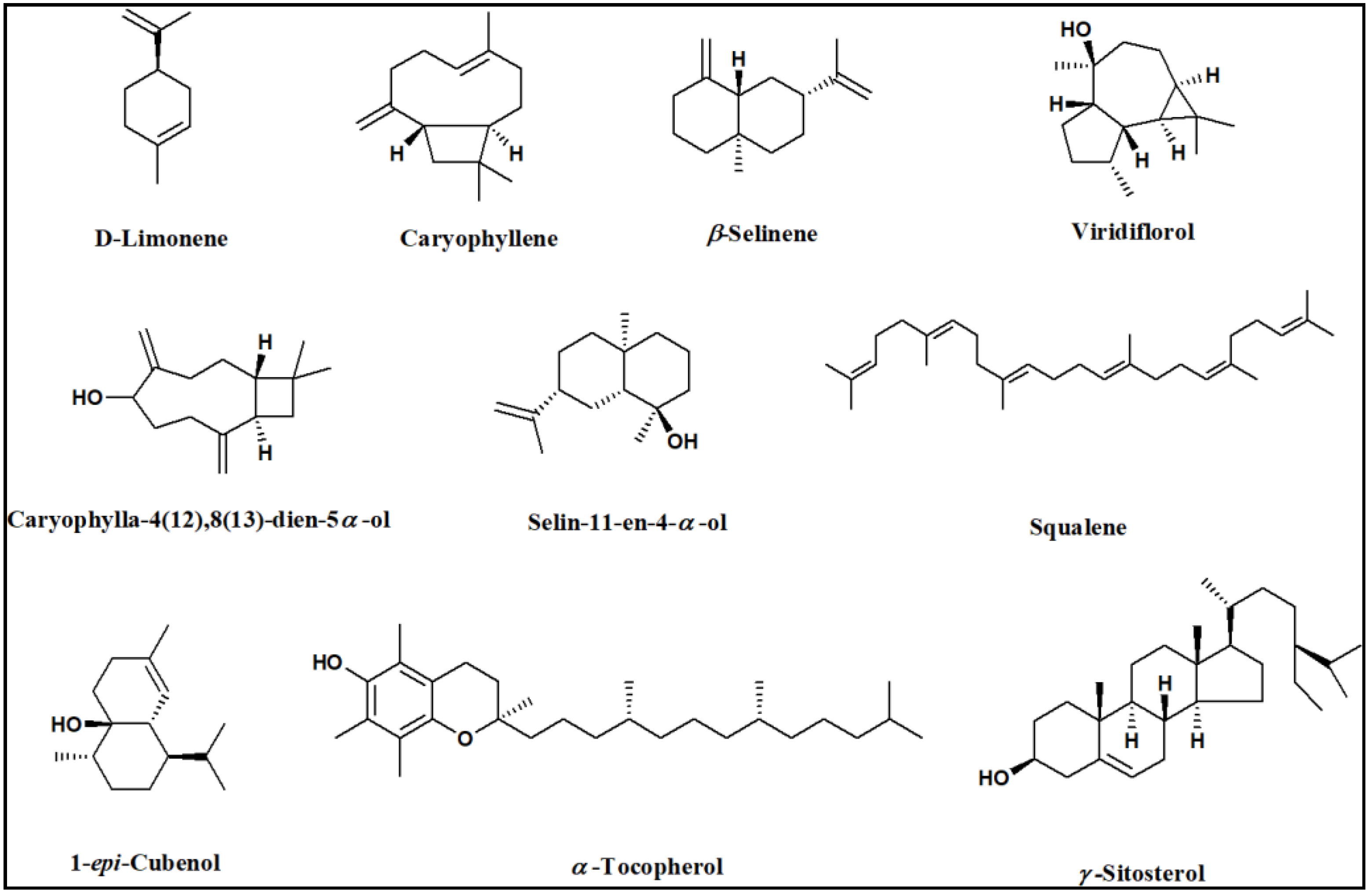

| No. | Rt (min) | Compound | RIExp. a | RILit b | Molecular Formula | Content (%) | |
|---|---|---|---|---|---|---|---|
| PGH | PGO | ||||||
| 1 | 7.16 | α-Pinene | 931 | 931 | C10H16 | - | 0.99 |
| 2 | 8.99 | n-Decane | 999 | 1000 | C10H22 | 1.44 | - |
| 3 | 9.94 | p-Cymene | 1024 | 1024 | C10H14 | - | 0.13 |
| 4 | 10.09 | D-Limonene | 1029 | 1029 | C10H16 | 4.83 | 36.68 |
| 5 | 12.15 | n-Undecane | 1099 | 1100 | C11H24 | 3.61 | - |
| 6 | 14.15 | 2-Methylundecane | 1164 | 1165 | C12H26 | 0.70 | - |
| 7 | 14.98 | trans-p-Mentha-1(7),8-dien-2-ol | 1188 | 1185 | C10H16O | - | 0.50 |
| 8 | 15.07 | α-Terpineol | 1191 | 1189 | C10H18O | - | 0.64 |
| 9 | 15.25 | n-Dodecane | 1199 | 1200 | C12H26 | 4.15 | - |
| 10 | 15.67 | 3,6-Dimethylundecane | 1213 | 1210 | C13H28 | 0.58 | - |
| 11 | 15.95 | trans-Carveol | 1221 | 1220 | C10H16O | - | 0.15 |
| 12 | 16.20 | cis-p-Mentha-1(7),8-dien-2-ol | 1229 | 1235 | C10H16O | - | 0.52 |
| 13 | 18.21 | n-Tridecane | 1299 | 1300 | C13H28 | 0.45 | - |
| 14 | 20.43 | α-Copaene | 1378 | 1376 | C15H24 | 0.51 | 0.59 |
| 15 | 21.65 | β-Caryophyllene | 1424 | 1424 | C15H24 | 3.80 | 8.41 |
| 16 | 22.16 | Alloaromadendrene | 1443 | 1442 | C15H24 | 0.42 | 1.60 |
| 17 | 22.54 | Humulene (α-Caryophyllene) | 1458 | 1455 | C15H24 | - | 1.00 |
| 18 | 22.74 | epi-β-Caryophyllene | 1465 | 1466 | C15H24 | - | 0.37 |
| 19 | 23.13 | γ-Muurolene | 1480 | 1479 | C15H24 | - | 0.40 |
| 20 | 23.41 | β-Selinene | 1491 | 1486 | C15H24 | 1.22 | 4.10 |
| 21 | 23.64 | β-Guaiene | 1500 | 1500 | C15H24 | 1.05 | 2.94 |
| 22 | 23.75 | α-Bisabolene | 1504 | 1506 | C15H24 | - | 1.12 |
| 23 | 23.92 | β -Bisabolene | 1511 | 1512 | C15H24 | - | 1.33 |
| 24 | 24.11 | γ-Cadinene | 1519 | 1513 | C15H24 | - | 0.23 |
| 25 | 24.33 | cis-Calamenene | 1528 | 1529 | C15H22 | - | 0.67 |
| 26 | 24.56 | Cubenene | 1537 | 1533 | C15H24 | 1.75 | 0.34 |
| 27 | 24.79 | Ledol | 1547 | 1549 | C15H26O | 1.27 | - |
| 28 | 25.28 | Dodecanoic acid | 1566 | 1566 | C12H24O | 1.20 | 0.88 |
| 29 | 25.54 | Caryophyllene alcohol | 1577 | 1572 | C15H26O | - | 0.35 |
| 30 | 25.68 | Caryophyllene oxide | 1582 | 1583 | C15H24O | - | 0.20 |
| 31 | 25.89 | Viridiflorol | 1591 | 1592 | C15H26O | 0.95 | 9.68 |
| 32 | 26.09 | Globulol | 1598 | 1590 | C15H26O | - | 0.49 |
| 33 | 26.15 | Benzene, (1-methylnonyl)- | 1602 | 1616 | C16H26 | 1.26 | - |
| 34 | 26.42 | β-Atlantol | 1613 | 1608 | C15H24O | - | 1.29 |
| 35 | 26.51 | β-Himachalene oxide | 1616 | 1616 | C15H24O | - | 0.81 |
| 36 | 26.60 | Humulene epoxide II | 1620 | 1620 | C15H24O | - | 0.61 |
| 37 | 26.70 | Alloaromadendrene oxide-(1) | 1624 | 1625 | C15H24O | - | 0.26 |
| 38 | 26.82 | γ-Eudesmol | 1630 | 1632 | C15H26O | 2.08 | 0.39 |
| 39 | 26.94 | 1-epi-Cubenol | 1635 | 1630 | C15H26O | 4.51 | 1.38 |
| 40 | 27.08 | Caryophylla-4(12),8(13)-dien-5ꞵ-ol | 1640 | 1640 | C15H24O | - | 1.87 |
| 41 | 27.17 | Caryophylla-4(12),8(13)-dien-5α-ol | 1645 | 1641 | C15H24O | 3.64 | 6.48 |
| 42 | 27.37 | α-Cadinol | 1653 | 1654 | C15H26O | - | 0.97 |
| 43 | 27.61 | Selin-11-en-4-α-ol | 1663 | 1659 | C15H26O | - | 6.35 |
| 44 | 27.69 | Benzene, (1-ethylnonyl)- | 1668 | 1670 | C17H28 | 2.93 | - |
| 45 | 27.83 | epi-β-Bisabolol | 1672 | 1672 | C15H26O | - | 0.69 |
| 46 | 27.96 | Khusilol | 1678 | 1676 | C14H20O | - | 1.99 |
| 47 | 28.19 | α-Bisabolone oxide A | 1688 | 1686 | C14H22O2 | - | 0.55 |
| 48 | 28.29 | 11αH-Himachal-4-en-1β-ol | 1692 | 1699 | C15H26O | - | 1.35 |
| 49 | 28.55 | Benzene, (1-methyldecyl)- | 1704 | 1715 | C17H28 | 3.46 | - |
| 50 | 29.14 | Benzene, (1-pentylheptyl)- | 1728 | 1718 | C18H30 | 3.60 | - |
| 51 | 29.25 | Benzene, (1-butyloctyl)- | 1733 | 1725 | C18H30 | 3.71 | - |
| 52 | 29.52 | Benzene, (1-propylnonyl)- | 1744 | 1741 | C18H30 | 2.70 | - |
| 53 | 30.01 | Benzene, (1-ethyldecyl)- | 1764 | 1767 | C18H30 | 2.55 | - |
| 54 | 30.85 | Benzene, (1-methylundecyl)- | 1799 | 1797 | C18H30 | 2.94 | - |
| 55 | 31.31 | Benzene, (1-pentyloctyl)- | 1822 | 1819 | C19H32 | 3.19 | - |
| 56 | 31.46 | Benzene, (1-butylnonyl)- | 1830 | 1825 | C19H32 | 2.65 | - |
| 57 | 31.73 | Benzene, (1-propyldecyl)- | 1844 | 1838 | C19H32 | 1.76 | - |
| 58 | 32.23 | Benzene, (1-ethylundecyl)- | 1870 | 1866 | C19H32 | 1.50 | - |
| 59 | 33.05 | Benzene, (1-methyldodecyl)- | 1912 | 1911 | C19H32 | 1.76 | - |
| 60 | 37.04 | Phytol | 2115 | 2114 | C20H40O | 1.90 | - |
| 61 | 38.37 | Palmitic acid, butyl ester | 2187 | 2188 | C20H40O2 | 0.75 | - |
| 62 | 41.38 | Eicosanoic acid, methyl ester | 2358 | 2339 | C21H42O2 | 0.64 | - |
| 63 | 41.52 | Linolenic acid, ethyl ester | 2366 | - | C20H34O2 | 0.83 | - |
| 64 | 48.77 | Squalene | 2834 | 2835 | C30H50 | 9.76 | - |
| 65 | 50.27 | Hexacosanoic acid, methyl ester | 2942 | 2940 | C27H54O2 | 0.41 | - |
| 66 | 53.07 | α-Tocopherol | 3152 | 3149 | C29H50O2 | 8.53 | - |
| 67 | 55.98 | γ-Sitosterol | 3352 | 3351 | C29H50O | 3.90 | - |
| Monoterpene hydrocarbons | 4.83 | 37.80 | |||||
| Oxygenated monoterpenes | - | 1.81 | |||||
| Sesquiterpene hydrocarbons | 8.75 | 23.10 | |||||
| Oxygenated sesquiterpenes | 13.65 | 36.59 | |||||
| Diterpenoids | 1.90 | - | |||||
| Triterpenoids | 9.76 | - | |||||
| Sterols | 3.90 | - | |||||
| Aromatic Hydrocarbons | 34.01 | - | |||||
| Aliphatic Hydrocarbons | 10.93 | - | |||||
| Fatty acids and fatty acids derivatives | 2.63 | - | |||||
| Others | 8.53 | - | |||||
| Total identified compounds | 98.89 | 99.30 | |||||
| Samples | DPPH | ABTS | CUPRAC | FRAP | MCA | PM |
|---|---|---|---|---|---|---|
| (mg TE/g) | (mg TE/g) | (mg TE/g) | (mg TE/g) | (mg EDTAE/g) | (mmol TE/g) | |
| n-Hexane extract | n.a. | n.a. | 70.80 ± 1.46 | 26.01 ± 0.97 | 24.83 ± 0.35 | 2.0 ± 0.07 |
| Essential oil | n.a. | n.a. | 18.17 ± 0.08 | 12.08 ± 0.17 | 9.02 ± 1.2 | 2.58 ± 0.14 |
| Samples | AChE Inhibition | BChE Inhibition | Tyrosinase Inhibition | α-Amylase Inhibition | α-Glucosidase Inhibition |
|---|---|---|---|---|---|
| (mg GALAE/g) | (mg GALAE/g) | (mg KAE/g) | (mmol ACAE/g) | (mmol ACAE/g) | |
| n-Hexane extract | n.a. | n.a. | 33.91 ± 2.25 | 0.52 ± 0.01 | 0.67 ± 0.03 |
| Essential oil | n.a. | 6.85 ± 0.03 | 61.70 ± 3.21 | 0.13 ± 0.01 | 1.49 ± 0.01 |
| Compound | Docking Scores Kcal/mol | ||||
|---|---|---|---|---|---|
| AChE 7D9O | BChE 6ESJ | Tyrosinase 5M8Q | α-Amylase 4GQQ | α-Glucosidase 3WY2 | |
| D-Limonene | −9.2 | −7.2 | −7.3 | −6.1 | −7.9 |
| β-Caryophyllene | −9.3 | −8.7 | −7.2 | −6.3 | −7.9 |
| β-Selinene | −9.5 | −7.7 | −6.8 | −7.7 | −7.6 |
| Viridiflorol | −11.4 | −10.7 | −9.3 | −6.3 | −13.6 |
| 1-epi-Cubenol | −10.2 | −8.8 | −6.9 | −6.4 | −8.3 |
| Caryophylla-4(12),8(13)-dien-5α-ol | −11.4 | −9.1 | −7.6 | −6.8 | −8.9 |
| Selin-11-en-4-α-ol | −13.4 | −9.3 | −9.5 | −7.8 | −12.5 |
| Squalene | −12.3 | −11.1 | −7.9 | −6.4 | −9.1 |
| α-Tocopherol | −14.2 | −13.9 | −9.5 | −8.9 | −12.5 |
| γ-Sitosterol | −15.4 | −9.4 | −8.2 | −7.1 | −9.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aly, S.H.; Eldahshan, O.A.; Al-Rashood, S.T.; Binjubair, F.A.; El Hassab, M.A.; Eldehna, W.M.; Dall’Acqua, S.; Zengin, G. Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves. Molecules 2022, 27, 8979. https://doi.org/10.3390/molecules27248979
Aly SH, Eldahshan OA, Al-Rashood ST, Binjubair FA, El Hassab MA, Eldehna WM, Dall’Acqua S, Zengin G. Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves. Molecules. 2022; 27(24):8979. https://doi.org/10.3390/molecules27248979
Chicago/Turabian StyleAly, Shaza H., Omayma A. Eldahshan, Sara T. Al-Rashood, Faizah A. Binjubair, Mahmoud A. El Hassab, Wagdy M. Eldehna, Stefano Dall’Acqua, and Gokhan Zengin. 2022. "Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves" Molecules 27, no. 24: 8979. https://doi.org/10.3390/molecules27248979
APA StyleAly, S. H., Eldahshan, O. A., Al-Rashood, S. T., Binjubair, F. A., El Hassab, M. A., Eldehna, W. M., Dall’Acqua, S., & Zengin, G. (2022). Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves. Molecules, 27(24), 8979. https://doi.org/10.3390/molecules27248979










