Anxiolytic-like Effect of Inhaled Cinnamon Essential Oil and Its Main Component Cinnamaldehyde in Animal Models
Abstract
1. Introduction
2. Results
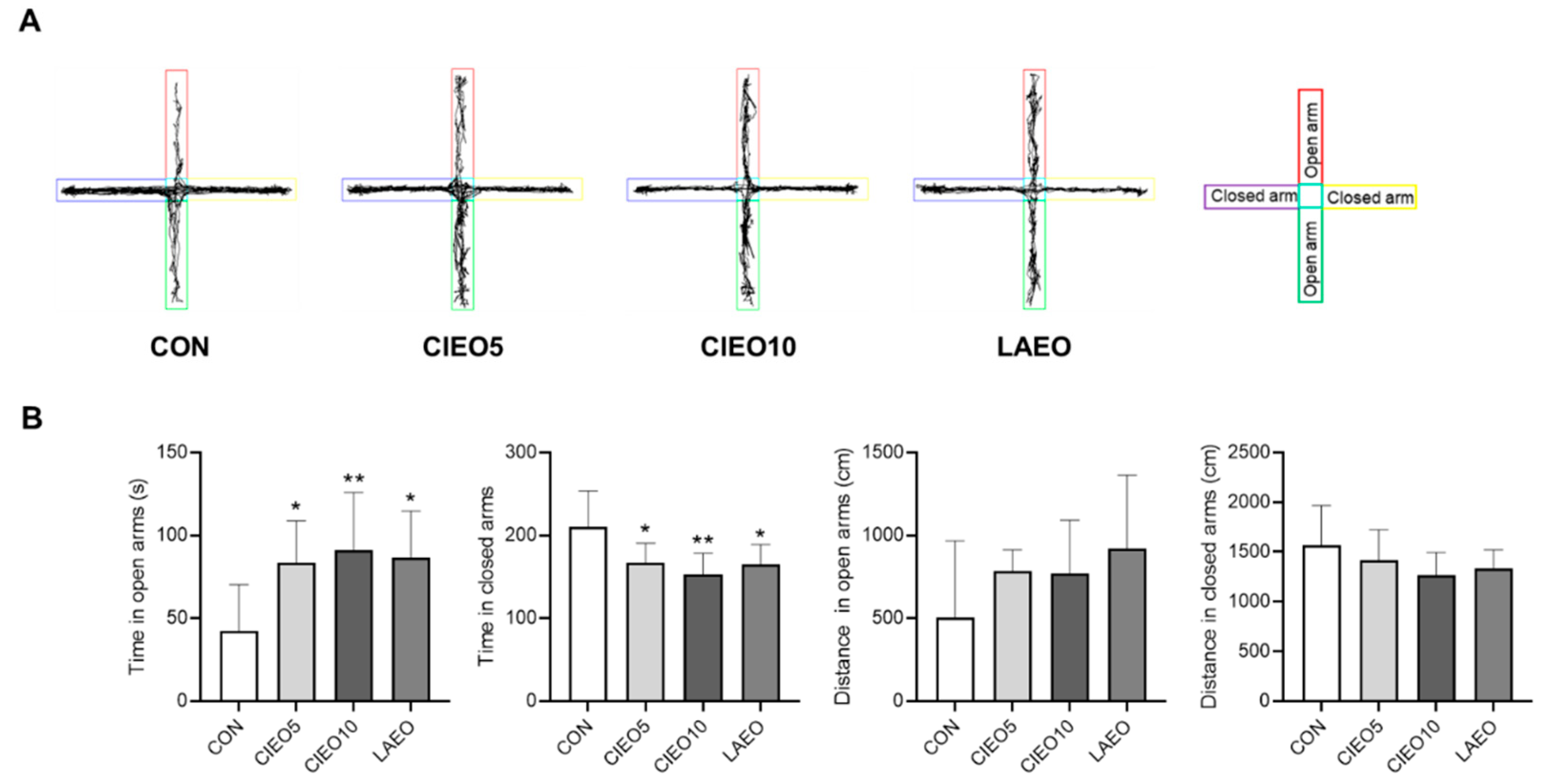
2.1. Effects of CIEO on Anxiety-like Behavior in Mice
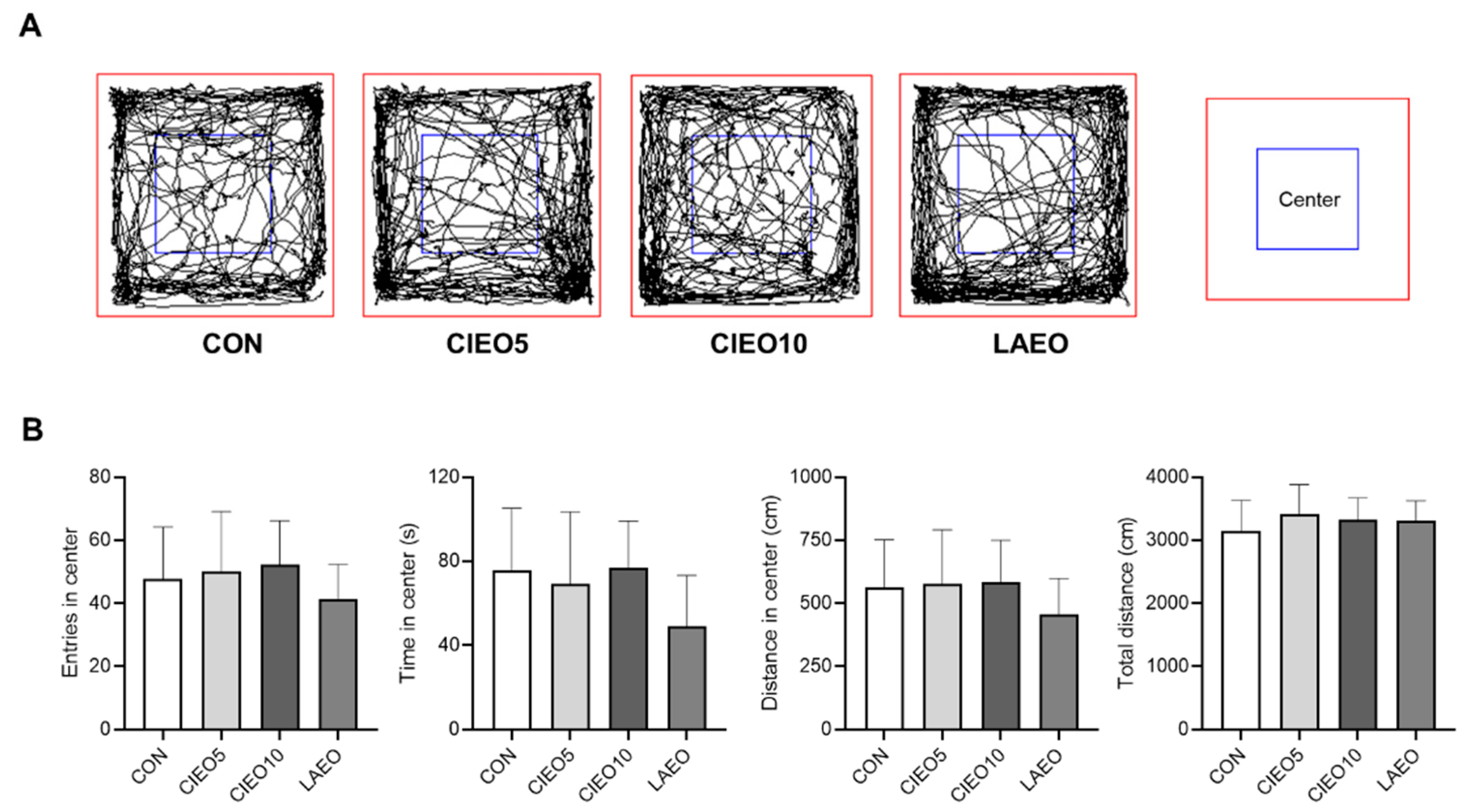
2.2. Effects of CIEO on Locomotor Activity in Mice
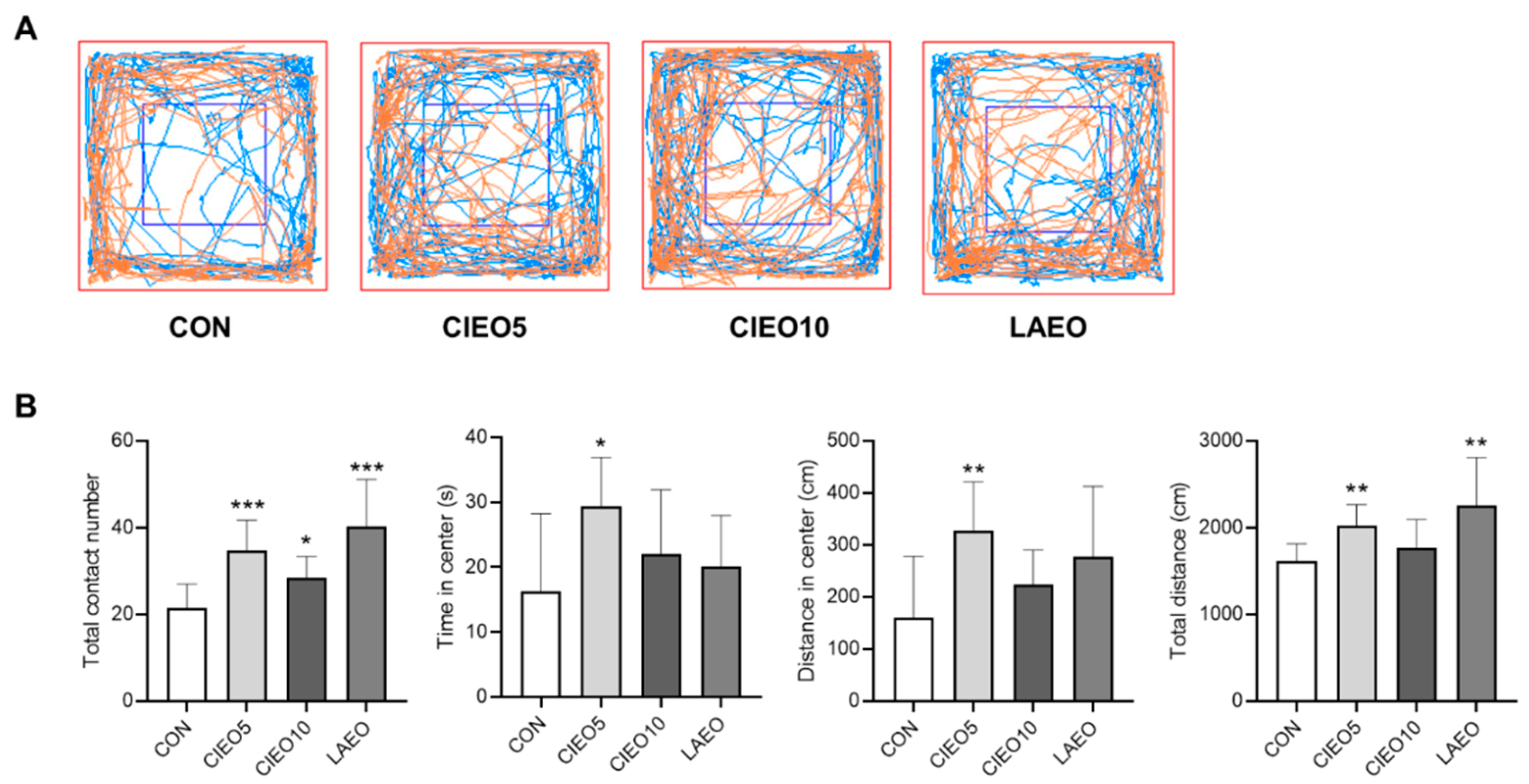
2.3. Effects of CIEO on Social Behavior in Mice
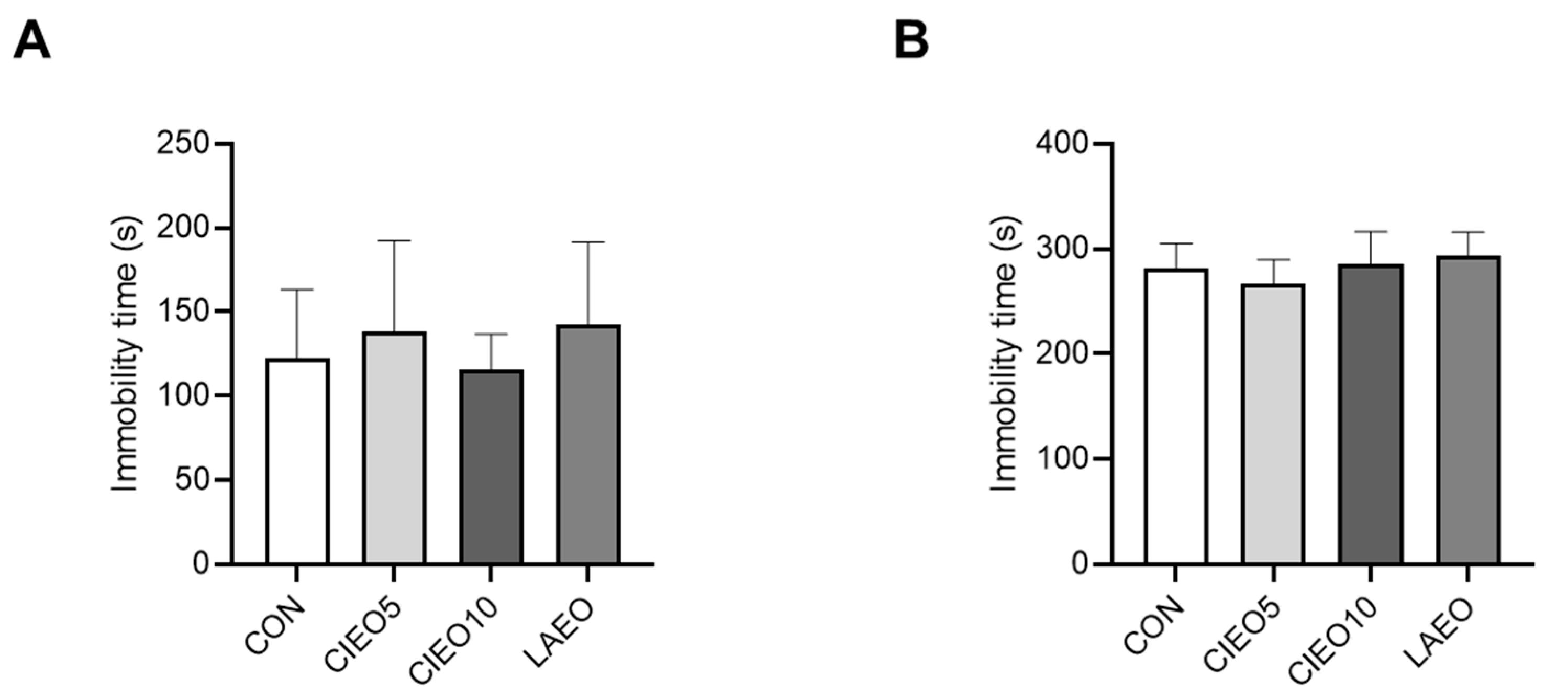
2.4. Effects of CIEO on Depression-like Behavior in Mice
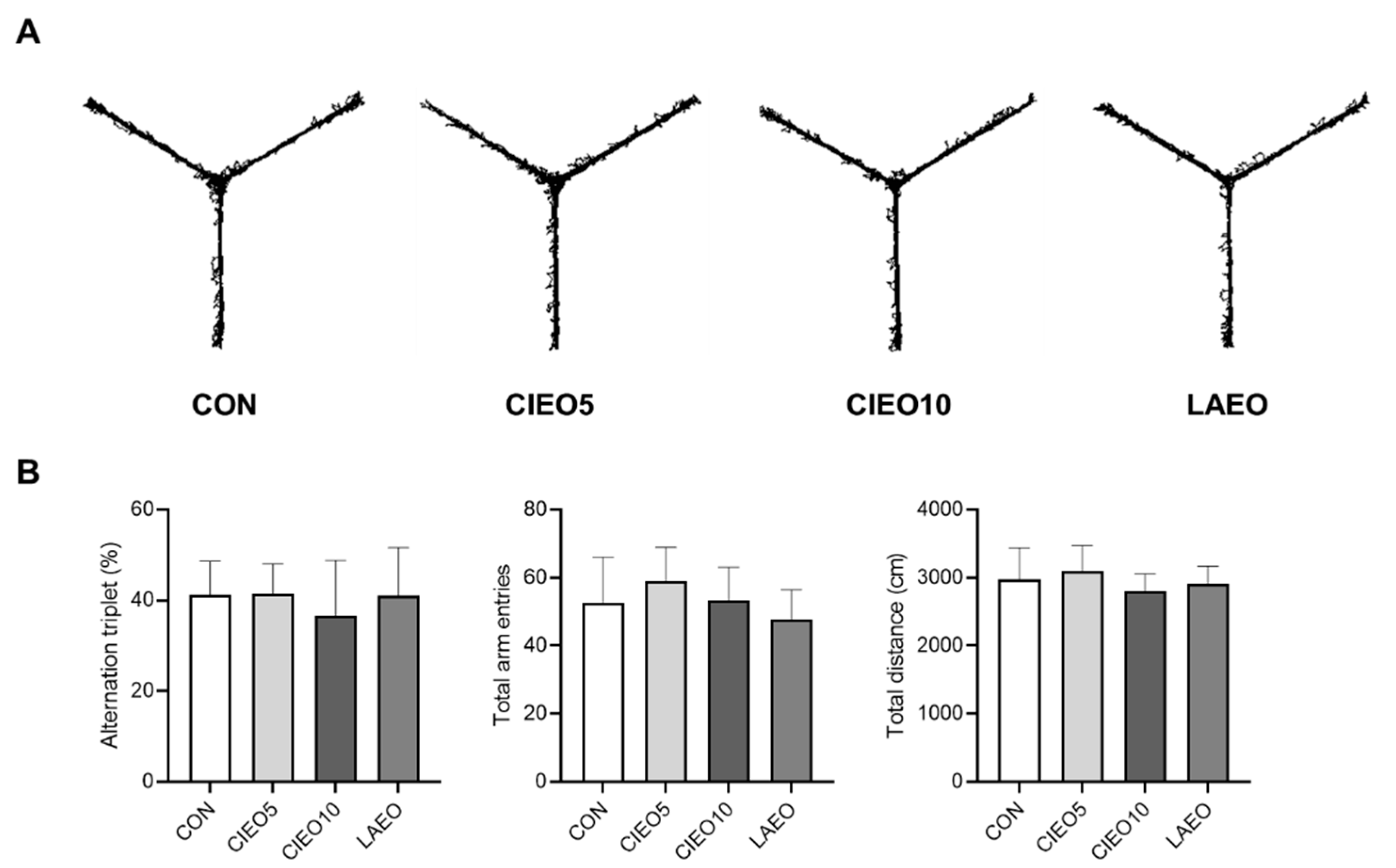
2.5. Effects of CIEO on Working Memory in Mice
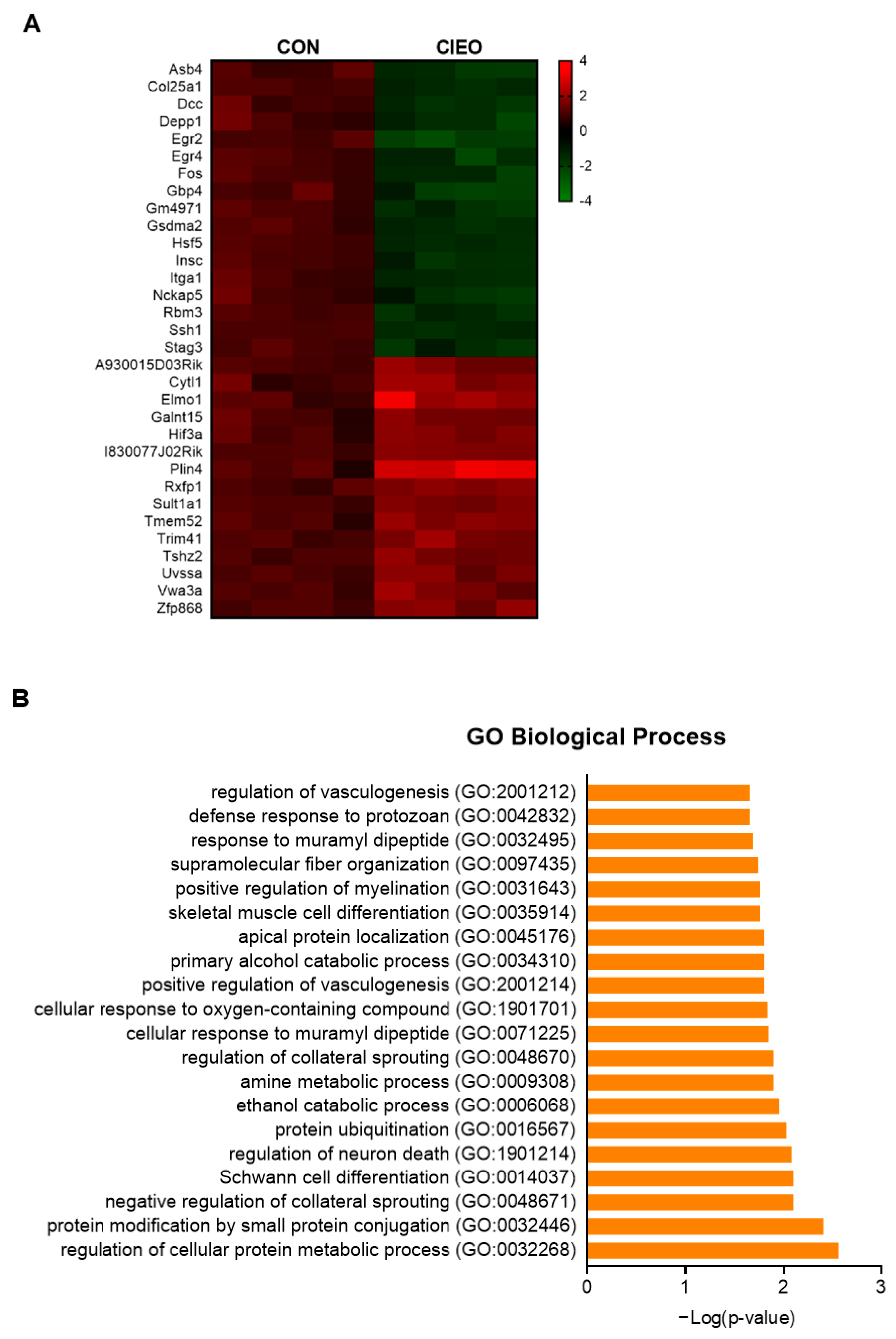
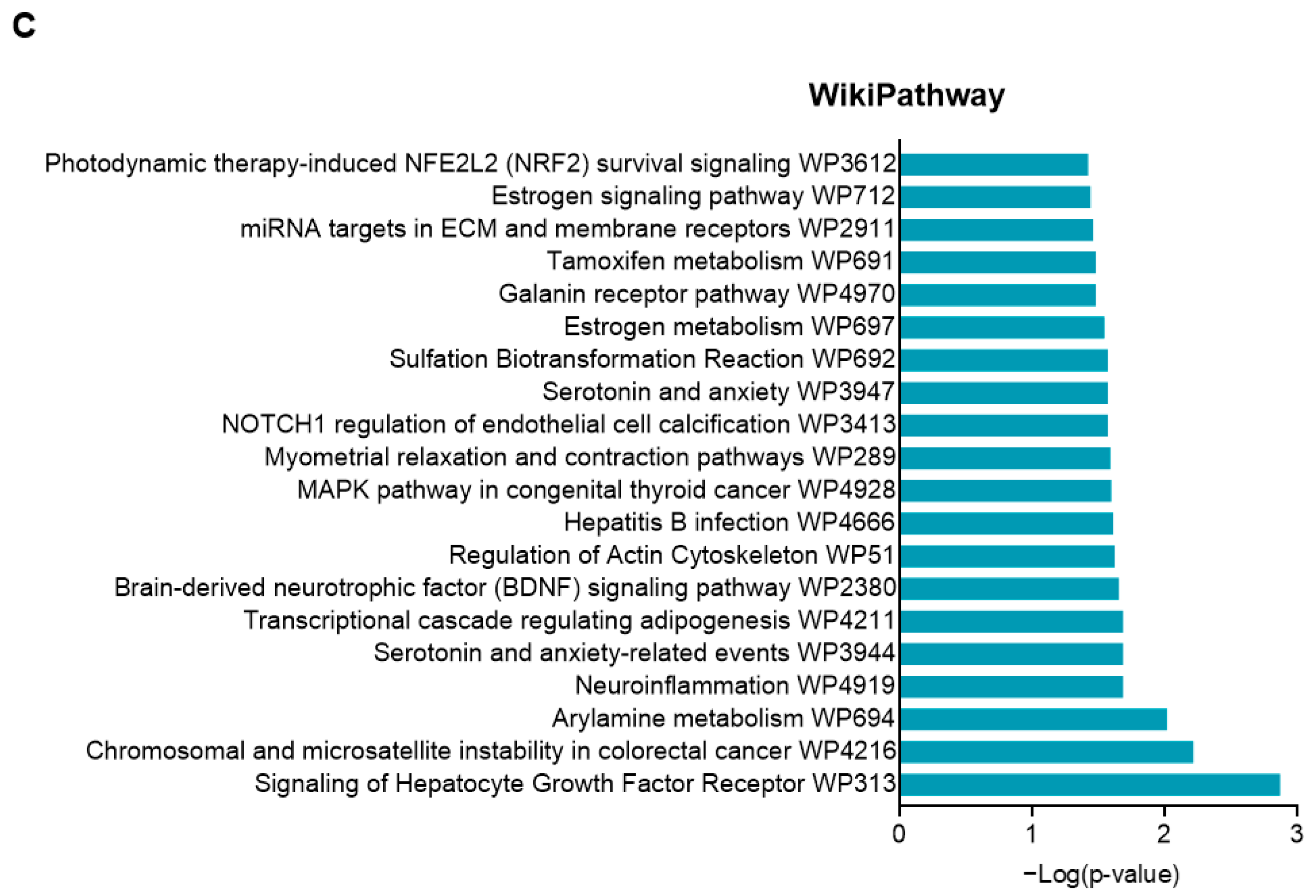
2.6. Effects of CIEO on Gene Expression in the Hippocampus
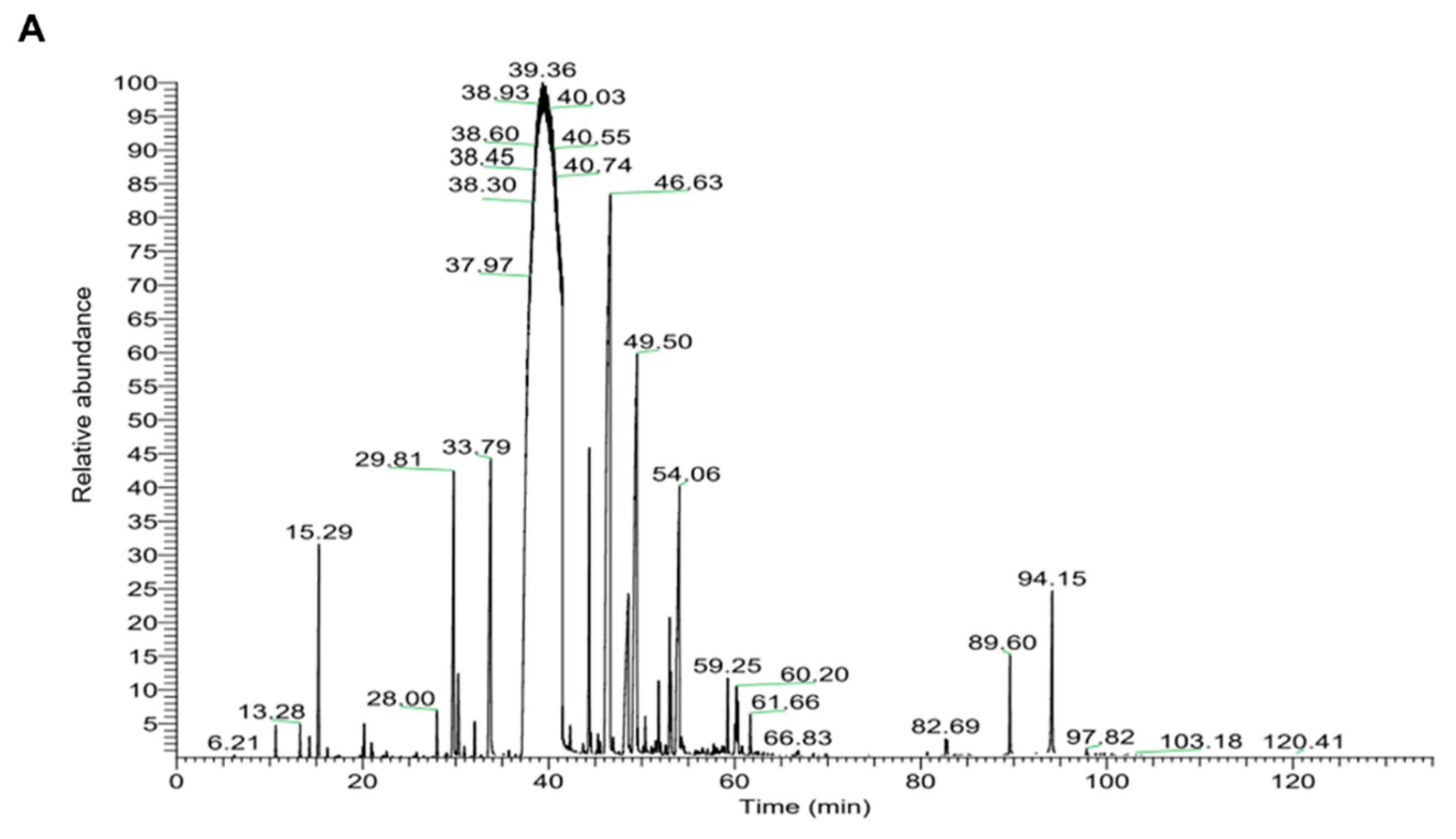
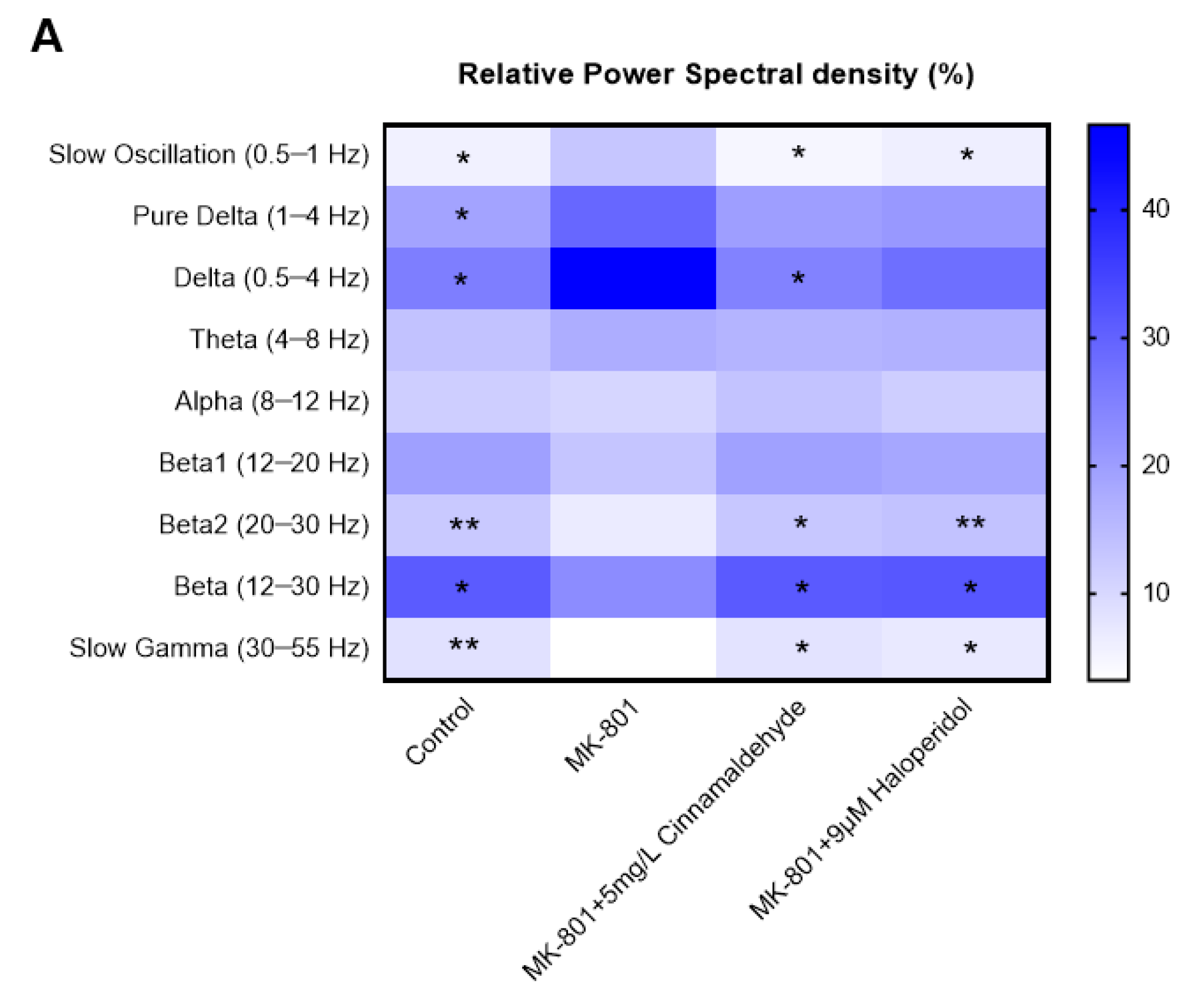
2.7. Chemical Composition of CIEO
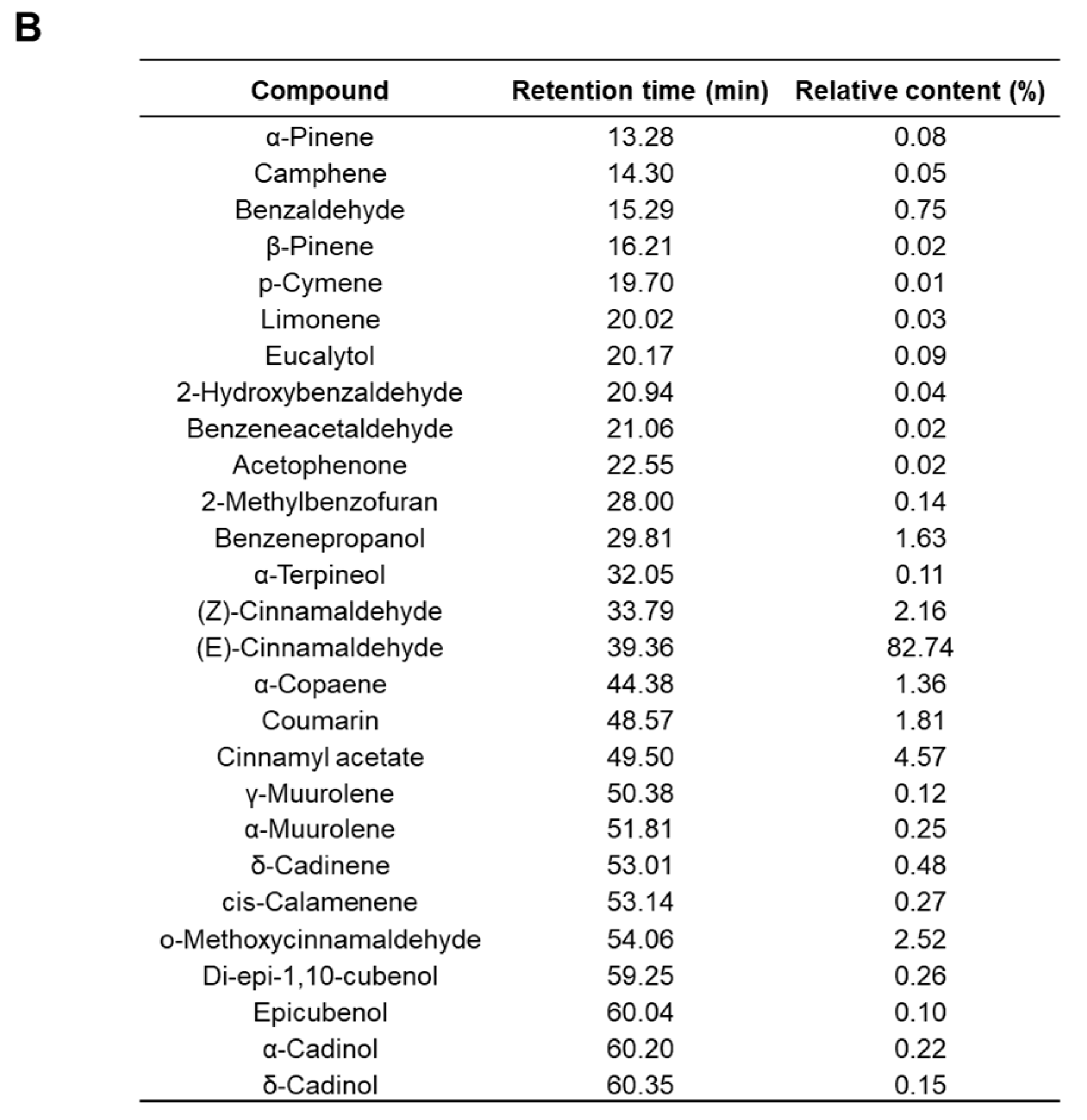
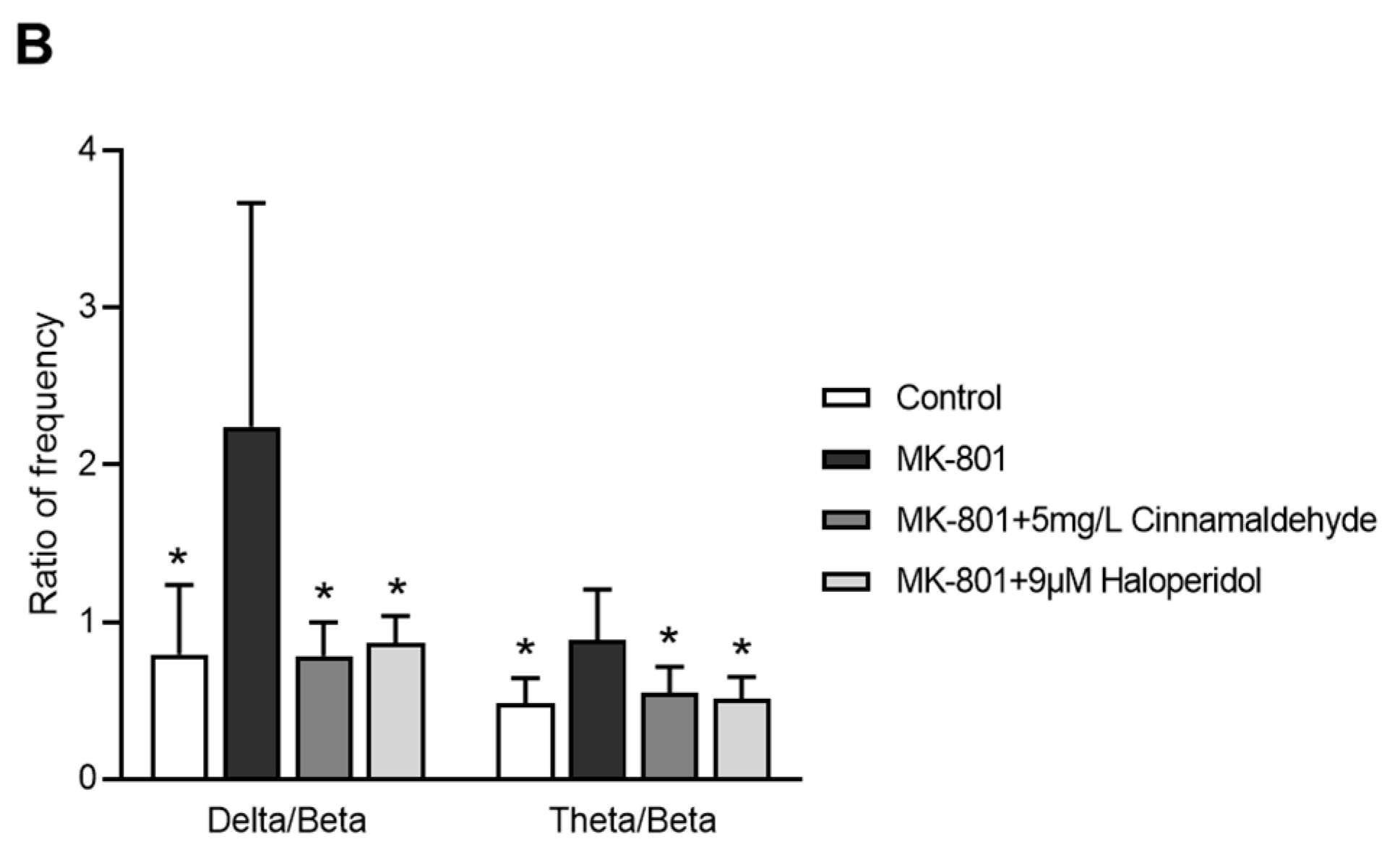
2.8. Effects of Cinnamaldehyde on EEG in an MK-801-Induced Anxiety-like Model in Zebrafish
3. Discussion
4. Materials and Methods
4.1. Preparation of Cinnamon Essential Oil
4.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
4.3. Animal and Treatment with Cinnamon Essential Oil
4.4. Elevated plus Maze (EPM) Test
4.5. Open Field Test (OFT)
4.6. Social Interaction Test
4.7. Tail Suspension Test (TST)
4.8. Forced Swimming Test (FST)
4.9. Y Maze Test
4.10. Sample Collection and Microarray Analysis
4.11. Electroencephalogram (EEG) Signal in Zebrafish
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, N.; Yao, L. Anxiolytic effect of essential oils and their constituents: A review. J. Agric. Food Chem. 2019, 67, 13790–13808. [Google Scholar] [CrossRef]
- Cryan, J.F.; Sweeney, F.F. The age of anxiety: Role of animal models of anxiolytic action in drug discovery. Br. J. Pharmacol. 2011, 164, 1129–1161. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Hosseinian-Far, A.; Jalali, R.; Vaisi-Raygani, A.; Rasoulpoor, S.; Mohammadi, M.; Rasoulpoor, S.; Khaledi-Paveh, B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: A systematic review and meta-analysis. Glob. Health 2020, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.B.; Singewald, N. Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol. Ther. 2019, 204, 107402. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-M.; Han, C.; Bahk, W.-M.; Lee, S.-J.; Patkar, A.A.; Masand, P.S.; Pae, C.-U. Addressing the side effects of contemporary antidepressant drugs: A comprehensive review. Chonnam Med. J. 2018, 54, 101–112. [Google Scholar] [CrossRef]
- Marazziti, D.; Mucci, F.; Tripodi, B.; Carbone, M.G.; Muscarella, A.; Falaschi, V.; Baroni, S. Emotional blunting, cognitive impairment, bone fractures, and bleeding as possible side effects of long-term use of SSRIs. Clin. Neuropsychiatry 2019, 16, 75. [Google Scholar]
- Katz, G.M.D. Tachyphylaxis/Tolerance to Antidepressive Medications: A Review. Isr. J. Psychiatry Relat. Sci. 2011, 48, 129–135. [Google Scholar]
- Bratsos, S.; Saleh, S.N. Clinical efficacy of ketamine for treatment-resistant depression. Cureus 2019, 11, e5189. [Google Scholar] [CrossRef]
- Zaidi, S.F.; Aziz, M.; Muhammad, J.S.; Kadowaki, M. Review: Diverse pharmacological properties of Cinnamomum cassia: A review. Pak. J. Pharm. Sci. 2015, 28, 1433–1438. [Google Scholar]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef]
- Aponso, M.; Patti, A.; Bennett, L.E. Dose-related effects of inhaled essential oils on behavioural measures of anxiety and depression and biomarkers of oxidative stress. J. Ethnopharmacol. 2020, 250, 112469. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, R.; Pazgoohan, N.; Seresht, H.R.; Amin, B. Repeated systemic administration of the cinnamon essential oil possesses anti-anxiety and anti-depressant activities in mice. Iran J. Basic Med. Sci. 2017, 20, 708–714. [Google Scholar] [PubMed]
- Perry, N.; Perry, E. Aromatherapy in the management of psychiatric disorders. CNS Drugs 2006, 20, 257–280. [Google Scholar] [CrossRef]
- de Sousa, D.P.; de Almeida Soares Hocayen, P.; Andrade, L.N.; Andreatini, R. A Systematic Review of the Anxiolytic-Like Effects of Essential Oils in Animal Models. Molecules 2015, 20, 18620–18660. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation Aromatherapy via Brain-Targeted Nasal Delivery: Natural Volatiles or Essential Oils on Mood Disorders. Front. Pharmacol. 2022, 13, 860043. [Google Scholar] [CrossRef]
- Lukas, G.; Brindle, S.D.; Greengard, P. The route of absorption of intraperitoneally administered compounds. J. Pharmacol. Exp. Ther. 1971, 178, 562–564. [Google Scholar]
- Koyama, S.; Heinbockel, T. The effects of essential oils and terpenes in relation to their routes of intake and application. Int. J. Mol. Sci. 2020, 21, 1558. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Chan, C.K.Y.; Gegechkori, V.; Morton, D.W. Models for skin and brain penetration of major components from essential oils used in aromatherapy for dementia patients. J. Biomol. Struct. Dyn. 2020, 38, 2402–2411. [Google Scholar] [CrossRef]
- Fung, T.K.H.; Lau, B.W.M.; Ngai, S.P.C.; Tsang, H.W.H. Therapeutic Effect and Mechanisms of Essential Oils in Mood Disorders: Interaction between the Nervous and Respiratory Systems. Int. J. Mol. Sci. 2021, 22, 4844. [Google Scholar] [CrossRef]
- Tucker, L.B.; McCabe, J.T. Measuring Anxiety-Like Behaviors in Rodent Models of Traumatic Brain Injury. Front. Behav. Neurosci. 2021, 15, 682935. [Google Scholar] [CrossRef]
- Leite, M.P.; Fassin Jr, J.; Baziloni, E.M.; Almeida, R.N.; Mattei, R.; Leite, J.R. Behavioral effects of essential oil of Citrus aurantium L. inhalation in rats. Rev. Bras. Farmacogn. 2008, 18, 661–666. [Google Scholar] [CrossRef]
- Carvalho-Freitas, M.I.; Costa, M. Anxiolytic and sedative effects of extracts and essential oil from Citrus aurantium L. Biol. Pharm. Bull. 2002, 25, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.I.; de Aquino Neto, M.R.; Teixeira Neto, P.F.; Moura, B.A.; do Amaral, J.F.; de Sousa, D.P.; Vasconcelos, S.M.; de Sousa, F.C. Central nervous system activity of acute administration of isopulegol in mice. Pharmacol. Biochem. Behav. 2007, 88, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Okuda, M.; Fujita, Y.; Takada-Takatori, Y.; Sugimoto, H.; Urakami, K. Aromatherapy improves cognitive dysfunction in senescence-accelerated mouse prone 8 by reducing the level of amyloid beta and tau phosphorylation. PLoS ONE 2020, 15, e0240378. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.X.; Bai, X.; Wang, M.J.; Yan, J.Z.; Cao, Y.J. The Effects of Aromatherapy on Anxiety and Depression in People with Cancer: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 1335. [Google Scholar] [CrossRef]
- Chioca, L.R.; Ferro, M.M.; Baretta, I.P.; Oliveira, S.M.; Silva, C.R.; Ferreira, J.; Losso, E.M.; Andreatini, R. Anxiolytic-like effect of lavender essential oil inhalation in mice: Participation of serotonergic but not GABAA/benzodiazepine neurotransmission. J. Ethnopharmacol. 2013, 147, 412–418. [Google Scholar] [CrossRef]
- Mehlen, P.; Mazelin, L. The dependence receptors DCC and UNC5H as a link between neuronal guidance and survival. Biol. Cell 2003, 95, 425–436. [Google Scholar] [CrossRef]
- Popa, N.; Bachar, D.; Roberts, A.C.; Santangelo, A.M.; Gascon, E. Region-specific microRNA alterations in marmosets carrying SLC6A4 polymorphisms are associated with anxiety-like behavior. eBioMedicine 2022, 82, 104159. [Google Scholar] [CrossRef]
- Poirier, R.; Cheval, H.; Mailhes, C.; Garel, S.; Charnay, P.; Davis, S.; Laroche, S. Distinct functions of egr gene family members in cognitive processes. Front. Neurosci. 2008, 2, 47–55. [Google Scholar] [CrossRef]
- Yan, Y.; Tan, X.; Wu, X.; Shao, B.; Wu, X.; Cao, J.; Xu, J.; Jin, W.; Li, L.; Xu, W. Involvement of early growth response-2 (Egr-2) in lipopolysaccharide-induced neuroinflammation. J. Mol. Histol. 2013, 44, 249–257. [Google Scholar] [CrossRef]
- Stankiewicz, A.M.; Goscik, J.; Majewska, A.; Swiergiel, A.H.; Juszczak, G.R. The Effect of Acute and Chronic Social Stress on the Hippocampal Transcriptome in Mice. PLoS ONE 2015, 10, e0142195. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Song, J.Y.; Joo, E.J.; Lee, K.Y.; Shin, S.Y.; Lee, Y.H.; Ahn, Y.M.; Kim, Y.S. Genetic association of the EGR2 gene with bipolar disorder in Korea. Exp. Mol. Med. 2012, 44, 121–129. [Google Scholar] [CrossRef][Green Version]
- Joo, J.-Y.; Schaukowitch, K.; Farbiak, L.; Kilaru, G.; Kim, T.-K. Stimulus-specific combinatorial functionality of neuronal c-fos enhancers. Nat. Neurosci. 2016, 19, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Troakes, C.; Ingram, C.D. Anxiety behaviour of the male rat on the elevated plus maze: Associated regional increase in c-fos mRNA expression and modulation by early maternal separation. Stress 2009, 12, 362–369. [Google Scholar] [CrossRef]
- McQuade, J.M.S.; Tamashiro, K.L.K.; Wood, G.E.; Herman, J.P.; McEwen, B.S.; Sakai, R.R.; Zhang, J.; Xu, M. Deficient hippocampal c-fos expression results in reduced anxiety and altered response to chronic stress in female mice. Neurosci. Lett. 2006, 403, 125–130. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, X.; Xu, S.; Liu, W.; Gao, M.; Song, L. SOCS-1 suppresses neuroinflammation in intracranial hemorrhage by downregulating the c-Fos/KLF4/IL-17A axis. Eur. J. Neurosci. 2022. [Google Scholar] [CrossRef]
- Çomakli, S.; Köktürk, M.; Topal, A.; Özkaraca, M.; Ceyhun, S.B. Immunofluorescence/fluorescence assessment of brain-derived neurotrophic factor, c-Fos activation, and apoptosis in the brain of zebrafish (Danio rerio) larvae exposed to glufosinate. Neurotoxicology 2018, 69, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.; Vijayan, M.M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R711–R719. [Google Scholar] [CrossRef]
- Panula, P.; Chen, Y.C.; Priyadarshini, M.; Kudo, H.; Semenova, S.; Sundvik, M.; Sallinen, V. The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol. Dis. 2010, 40, 46–57. [Google Scholar] [CrossRef]
- Panula, P.; Sallinen, V.; Sundvik, M.; Kolehmainen, J.; Torkko, V.; Tiittula, A.; Moshnyakov, M.; Podlasz, P. Modulatory neurotransmitter systems and behavior: Towards zebrafish models of neurodegenerative diseases. Zebrafish 2006, 3, 235–247. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef] [PubMed]
- Maximino, C.; da Silva, A.W.; Araujo, J.; Lima, M.G.; Miranda, V.; Puty, B.; Benzecry, R.; Picanco-Diniz, D.L.; Gouveia, A., Jr.; Oliveira, K.R.; et al. Fingerprinting of psychoactive drugs in zebrafish anxiety-like behaviors. PLoS ONE 2014, 9, e103943. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.K.; Hua, K.-F.; Hsu, H.-Y.; Cheng, S.-S.; Lin, I.-F.; Chen, C.-J.; Chen, S.-T.; Chang, S.-T. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem. Toxicol. 2008, 46, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Ismail, I.A.; Kang, S.M.; Han, D.C.; Kwon, B.M. Cinnamaldehydes in cancer chemotherapy. Phytother. Res. 2016, 30, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Perdikaris, P.; Dermon, C.R. Behavioral and neurochemical profile of MK-801 adult zebrafish model: Forebrain β2-adrenoceptors contribute to social withdrawal and anxiety-like behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 115, 110494. [Google Scholar] [CrossRef]
- Gurudath, N.; Riley, H.B. Drowsy Driving Detection by EEG Analysis Using Wavelet Transform and K-means Clustering. Procedia Comput. Sci. 2014, 34, 400–409. [Google Scholar] [CrossRef]
- Newson, J.J.; Thiagarajan, T.C. EEG Frequency Bands in Psychiatric Disorders: A Review of Resting State Studies. Front. Hum. Neurosci. 2018, 12, 521. [Google Scholar] [CrossRef]
- Wei, H.; Chang, L.; Huang, Q.; Zhou, R. Relation between spontaneous electroencephalographic theta/beta power ratio and test anxiety. Neurosci. Lett. 2020, 737, 135323. [Google Scholar] [CrossRef]
- Poppelaars, E.S.; Harrewijn, A.; Westenberg, P.M.; van der Molen, M.J.W. Frontal delta-beta cross-frequency coupling in high and low social anxiety: An index of stress regulation? Cogn. Affect. Behav. Neurosci. 2018, 18, 764–777. [Google Scholar] [CrossRef]
- Emamghoreishi, M.; Farrokhi, M.R.; Amiri, A.; Keshavarz, M. The neuroprotective mechanism of cinnamaldehyde against amyloid-β in neuronal SHSY5Y cell line: The role of N-methyl-D-aspartate, ryanodine, and adenosine receptors and glycogen synthase kinase-3β. Avicenna J. Phytomed. 2019, 9, 271. [Google Scholar] [PubMed]
- Fu, Y.; Yang, P.; Zhao, Y.; Zhang, L.; Zhang, Z.; Dong, X.; Wu, Z.; Xu, Y.; Chen, Y. trans-Cinnamaldehyde inhibits microglial activation and improves neuronal survival against neuroinflammation in BV2 microglial cells with lipopolysaccharide stimulation. Evid. Based Complement. Altern. Med. 2017, 2017, 4730878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, X.; Dong, L.; Wen, Y.; Zheng, X.; Zhang, C.; Chen, R.; Zhang, Y.; Li, Y.; He, T. Cinnamaldehyde inhibits inflammation and brain damage in a mouse model of permanent cerebral ischaemia. Br. J. Pharmacol. 2015, 172, 5009–5023. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Berton, J.; Ferreira, T.N.; Santos, N.P.; Ferro, M.M.; Favero, G.M. Evaluation of depressive and anxious behavior with the use of propranolol in melanoma-bearing mice. Braz. Arch. Biol. Technol. 2021, 64. [Google Scholar] [CrossRef]
- Linck, V.M.; da Silva, A.L.; Figueiro, M.; Caramao, E.B.; Moreno, P.R.; Elisabetsky, E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Arad, M.; Terrillion, C.E.; Piantadosi, S.C.; Gould, T.D. The mouse forced swim test. J. Vis. Exp. 2012, 59, e3638. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Ugale, R.; Yilmazer-Hanke, D.; Stairs, D.; Dravid, S. GluN2C/GluN2D subunit-selective NMDA receptor potentiator CIQ reverses MK-801-induced impairment in prepulse inhibition and working memory in Y-maze test in mice. Br. J. Pharmacol. 2014, 171, 799–809. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.T.H.; Nguyen, N.P.K.; Tran, K.N.; Shin, H.-M.; Yang, I.-J. Anxiolytic-like Effect of Inhaled Cinnamon Essential Oil and Its Main Component Cinnamaldehyde in Animal Models. Molecules 2022, 27, 7997. https://doi.org/10.3390/molecules27227997
Nguyen LTH, Nguyen NPK, Tran KN, Shin H-M, Yang I-J. Anxiolytic-like Effect of Inhaled Cinnamon Essential Oil and Its Main Component Cinnamaldehyde in Animal Models. Molecules. 2022; 27(22):7997. https://doi.org/10.3390/molecules27227997
Chicago/Turabian StyleNguyen, Ly Thi Huong, Nhi Phuc Khanh Nguyen, Khoa Nguyen Tran, Heung-Mook Shin, and In-Jun Yang. 2022. "Anxiolytic-like Effect of Inhaled Cinnamon Essential Oil and Its Main Component Cinnamaldehyde in Animal Models" Molecules 27, no. 22: 7997. https://doi.org/10.3390/molecules27227997
APA StyleNguyen, L. T. H., Nguyen, N. P. K., Tran, K. N., Shin, H.-M., & Yang, I.-J. (2022). Anxiolytic-like Effect of Inhaled Cinnamon Essential Oil and Its Main Component Cinnamaldehyde in Animal Models. Molecules, 27(22), 7997. https://doi.org/10.3390/molecules27227997







