Synchronous Extraction, Antioxidant Activity Evaluation, and Composition Analysis of Carbohydrates and Polyphenols Present in Artichoke Bud
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of the Investigated Factors on the Carbohydrate and Polyphenol Contents
2.1.1. Effects of Ultrasonic Powers on the Carbohydrate and Polyphenol Contents in Artichoke Bud
2.1.2. Effects of Different Powder Mass on the Carbohydrate and Polyphenol Contents in Artichoke Bud
2.1.3. Effects of Different Extraction Temperatures on Carbohydrate and Polyphenol Contents in Artichoke Bud
2.1.4. Effects of Different Ultrasonic Times on the Carbohydrate and Polyphenol Contents in Artichoke Bud
2.1.5. Effects of Different Concentrations of Ammonium Sulfate on the Carbohydrate and Polyphenol Contents in Artichoke Bud
2.1.6. Effects of Different Alcohol–Water Ratios on the Polysaccharide and Polyphenol Contents in Artichoke Bud
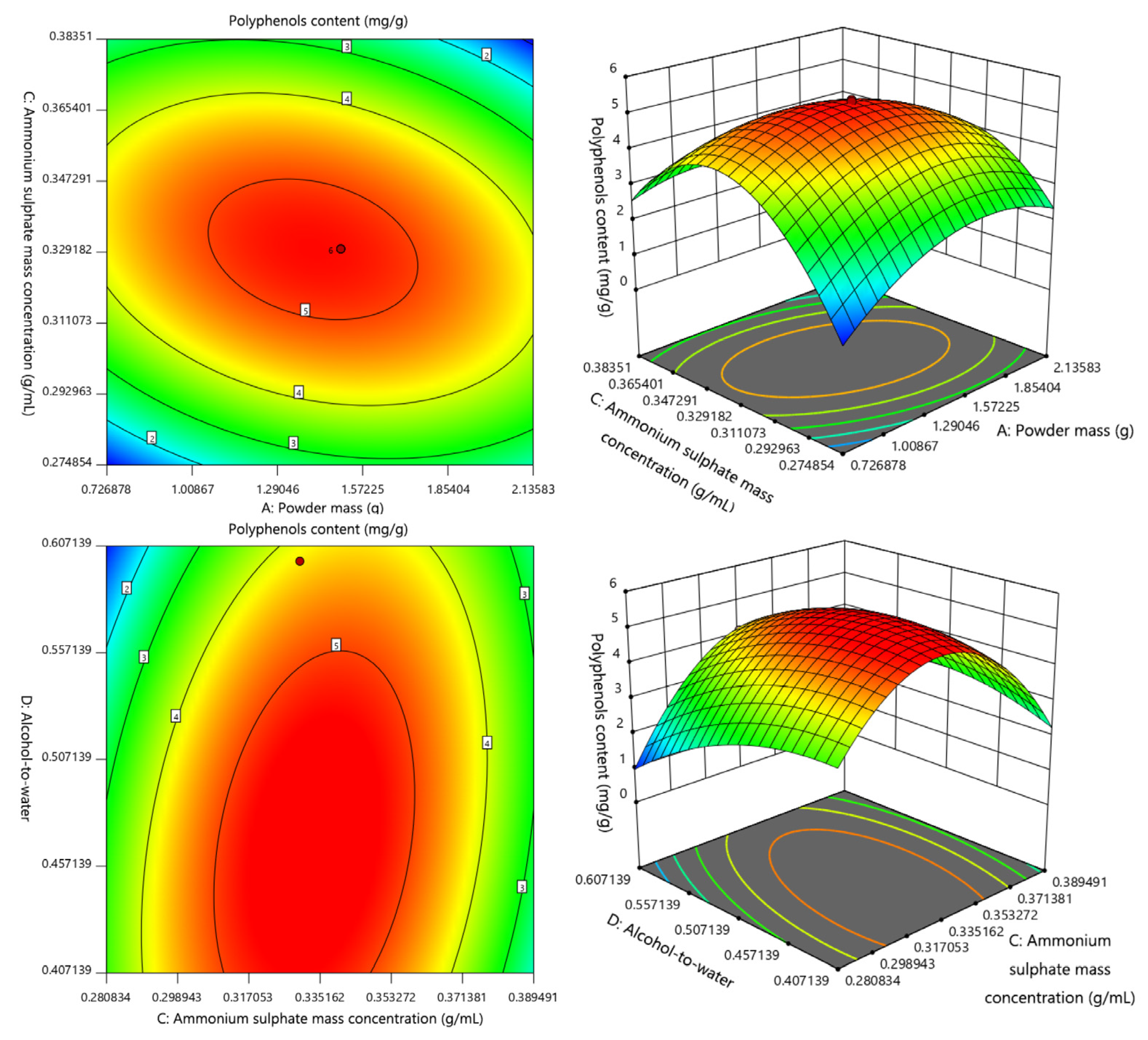
2.2. Box–Behnken Response Surface Experimental Results
2.3. Optimal Process Validation
2.4. Evaluation of Antioxidant Activity In Vitro
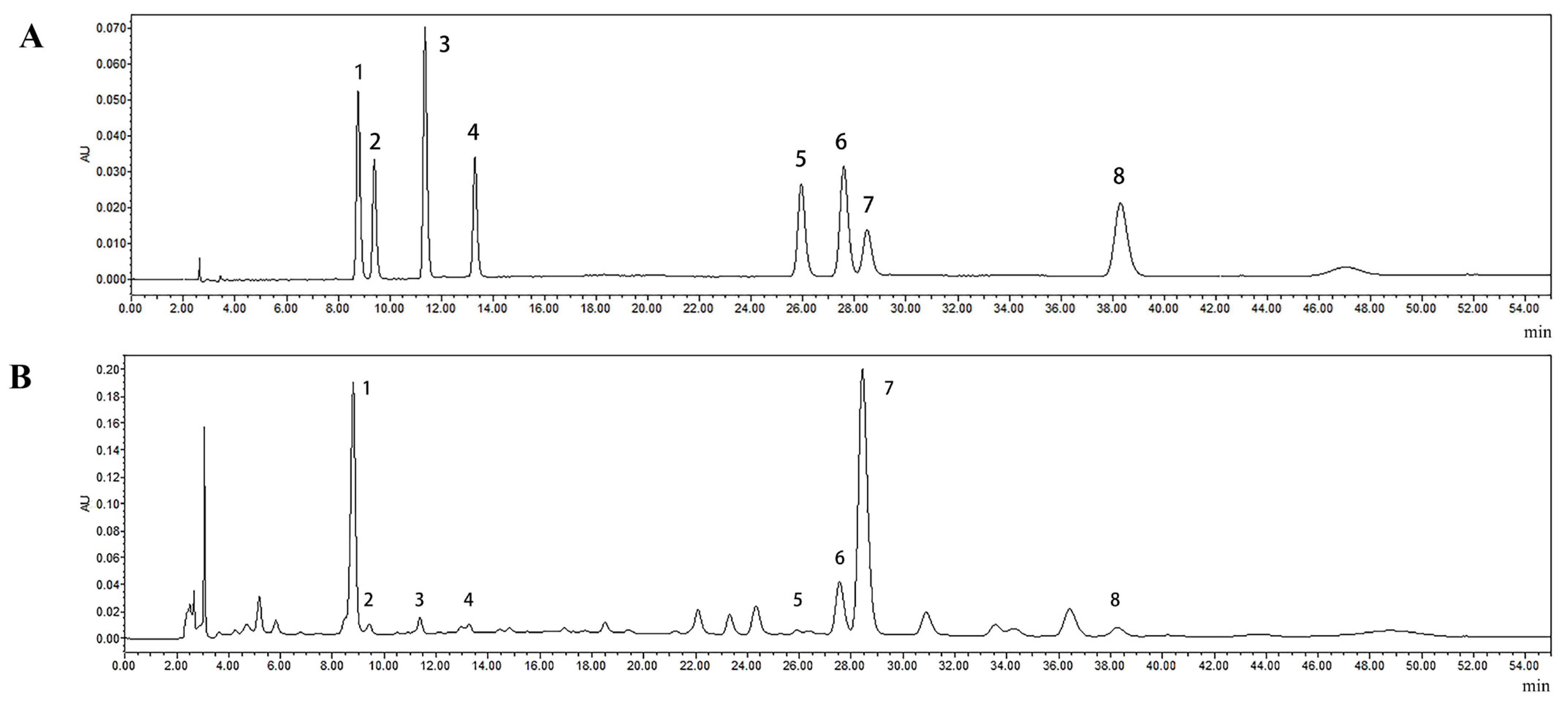
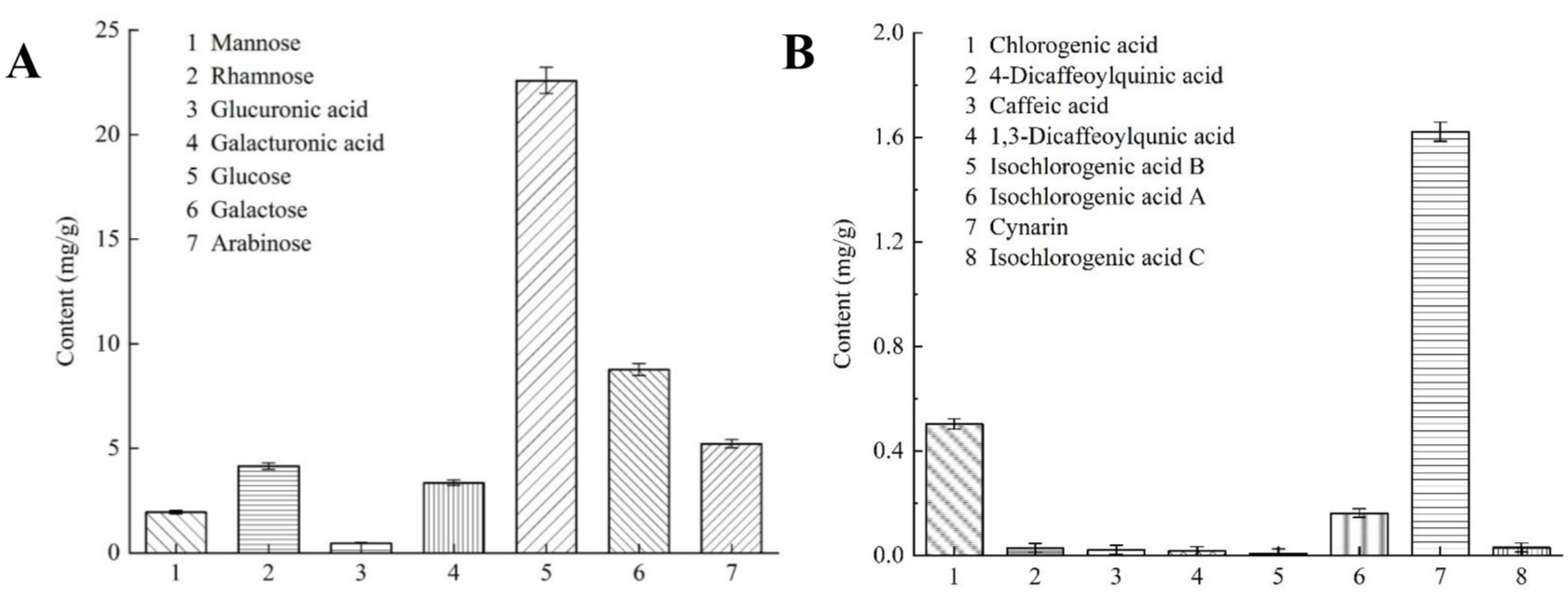
2.5. Results of HPLC Analysis of Polysaccharide and Polyphenol Compositions
3. Materials and Methods
3.1. Materials
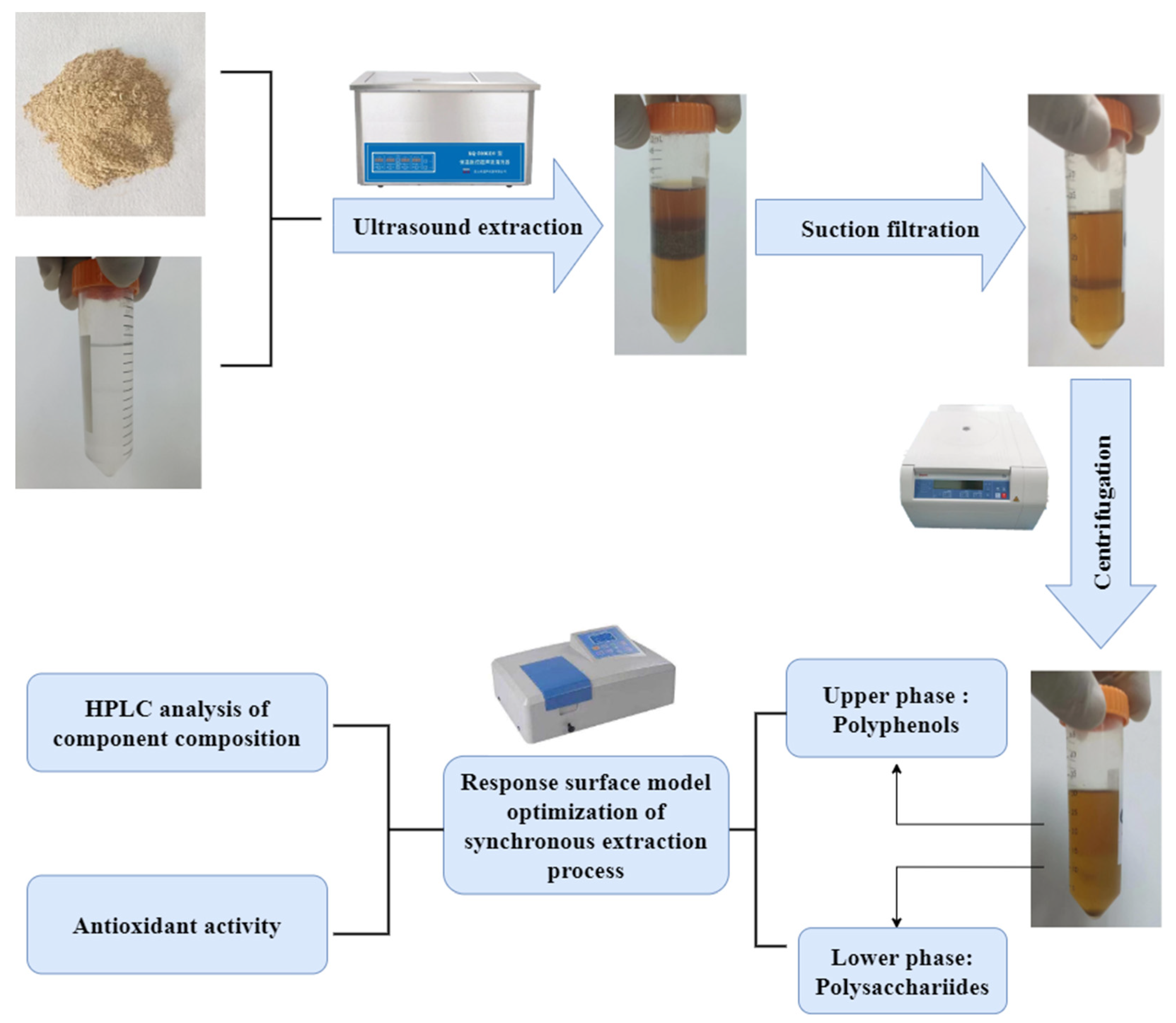
3.2. Synchronous Extraction Process
3.3. Determination of Carbohydrate Content
3.4. Determination of Polyphenol Content
3.5. Single-Factor Experiment
3.6. Response Surface Experiment
3.7. Determination of Antioxidant Activity In Vitro
3.7.1. Determination of ABTS+· Scavenging Capacity
3.7.2. Determination of DPPH· Scavenging Capacity
3.7.3. Determination of Fe3+-Reducing Power
3.8. High-Performance Liquid Chromatography Analysis
3.8.1. Standard Solution Preparation
3.8.2. Sample Solution Preparation
3.8.3. Chromatographic Conditions
3.9. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, Z.S.; Chen, S.M.; Xin, S.Y.; Sheng, H.Y.; Jiang, H.T. Total Phenols and Flavonoids and Antioxidant Activity of Artichoke (Cynara scolymus L.) Bud Juices before and after Gastrointestinal Digestion in Vitro. Food Sci. 2019, 40, 136–142. [Google Scholar]
- Sgroi, F.; Fodera, M.; Di Trapani, A.M.; Tudisca, S.; Testa, R. Profitability of Artichoke Growing in the Mediterranean Area. Hortscience 2015, 50, 1349–1352. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Wang, C.D.; Zhang, R.; Hou, X.J.; Zhao, F.; Zhang, J.J.; Wang, C. Study on literature of artichoke and properties of traditional Chinese medicine. China J. Chin. Mater. Med. 2020, 45, 3481–3488. [Google Scholar]
- Boubaker, M.; El Omri, A.; Blecker, C.; Bouzouita, N. Fibre concentrate from artichoke (Cynara scolymus L.) stem by-products: Characterization and application as a bakery product ingredient. Food Sci. Technol. Int. 2016, 22, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.M.; Di Venere, D.; Linsalata, V.; Fraioli, R.; Miccadei, S. Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer cell line MDA-MB231. J. Cell. Physiol. 2012, 227, 3301–3309. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhang, H.X.; Lo, R. Antifungal activity of Cynara scolymus L. extracts. Fitoterapia 2005, 76, 108–111. [Google Scholar] [CrossRef]
- Wang, M.F.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef]
- Speroni, E.; Cervellati, R.; Govoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M.C. Efficacy of different Cynara scolymus preparations on liver complaints. J. Ethnopharmacol. 2003, 86, 203–211. [Google Scholar] [CrossRef]
- Sisto, A.; Luongo, D.; Treppiccione, L.; De Bellis, P.; Di Venere, D.; Lavermicocca, P.; Rossi, M. Effect of Lactobacillus paracasei Culture Filtrates and Artichoke Polyphenols on Cytokine Production by Dendritic Cells. Nutrients 2016, 8, 635. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Perez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; Lopez-Jimenez, J.A.; Castillo, J.; Zamora, S. Chemical and functional properties of the different by-products of artichoke (Cynara scolymus L.) from industrial canning processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef]
- Hourieh, A. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar]
- Zhao, D.; Liu, X.L.; Zheng, X.Q.; Fan, L. Antioxidant activity of pea protein hydrolysates in vitro and on HepG2 cells. Food Ferment. Ind. 2022, 48, 1–11. [Google Scholar]
- Li, M.R.; Zhou, Y.Z.; Du, G.H.; Qin, X.M. Research progress about the anti-aging effect and mechanism of flavonoids from traditional Chinese medicine. Acta Pharm. Sin. 2019, 54, 1382–1391. [Google Scholar]
- Ademowo, O.S.; Dias, H.K.I.; Burton, D.G.; Griffiths, H.R. Lipid (per) oxidation in mitochondria: An emerging target in the ageing process? Biogerontology 2017, 18, 859–879. [Google Scholar] [CrossRef]
- Li, J.M.; Han, Y.Q.; Wu, T.; Zhang, T.C.; Dong, X.H. Lipid peroxidation in ferroptosis and its relationship with Alzheimer disease. Chin. J. Pathophysiol. 2022, 38, 1142–1147. [Google Scholar]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Idelchik, M.D.S.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Xie, J.H.; Liu, H.M.; Liu, M.H.; Zheng, M.Z.; Xu, Q.; Liu, J.S. Extraction Process Optimization and Antioxidant Activity Analysis of Polyphenols from Azuki Bean Coats (Vigna angularis). J. Chin. Inst. Food Sci. Technol. 2020, 20, 147–157. [Google Scholar]
- Zheng, Q.R.; Li, W.F.; Zhang, H.; Gao, X.X.; Tan, S. Optimizing synchronous extraction and antioxidant activity evaluation of polyphenols and polysaccharides from Ya’an Tibetan tea (Camellia sinensis). Food Sci. Nutr. 2020, 8, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Guo, W.H.; Wang, Z.J.; Deng, L. Optimization of Polysaccharides Extraction from Lycopus Lucidus Turcz. by Response Surface Methodology and Study on Its Antioxidant Activity. Chin. J. Mod. Appl. Pharm. 2021, 38, 2554–2559. [Google Scholar]
- Zhang, Z.Y.; Zhao, Z.H.; Chen, X.; Liu, F.C.; Wang, J.G. Comparison of Polyphenols of Ziziphus Jujuba Extracted by Different Methods. Food Res. Dev. 2020, 41, 153–159. [Google Scholar]
- Wang, Y.H.; Wu, Q.; Chi, Y.L.; Yao, K.; Jia, D.Y. Properties of crude polysaccharides extracted from Tremella fuciformis by acid, alkali and enzyme-assisted methods. Food Sci. Technol. 2019, 44, 200–204. [Google Scholar]
- Jin, W.Q.; Peng, J.Y.; Wang, Y.S.; Tang, Y.J.; Tang, Z.Z.; Chen, H.; Sun, R.; Zheng, T.R.; Liu, M.Y.; Sun, W.J. Optimization of Response Surface Methodology for Microwave-assisted and Ultrasonic-assisted Extraction of Polysaccharides from Amaranthus hybridus L. Genom. Appl. Biol. 2019, 38, 757–765. [Google Scholar]
- Sahin, S.; Samli, R.; Tan, A.S.B.; Barba, F.J.; Chemat, F.; Cravotto, G.; Lorenzo, J.M. Solvent-Free Microwave-Assisted Extraction of Polyphenols from Olive Tree Leaves: Antioxidant and Antimicrobial Properties. Molecules 2017, 22, 1056. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, W.Q.; Cao, Q.Q. Optimization of Enzymatic Extraction Technology of Polysaccharide from Portulaca oleracea by Response Surface Methodology. Food Res. Dev. 2020, 41, 79–84. [Google Scholar]
- Anticona, M.; Blesa, J.; Frigola, A.; Esteve, M.J. High Biological Value Compounds Extraction from Citrus Waste with Non-Conventional Methods. Foods 2020, 9, 811. [Google Scholar] [CrossRef]
- Wang, J.H. Extraction of Flavonoid in Osmanthus Leaves by Aqueous Two-phase System Assisted Inner Ebullition Method. Food Res. Dev. 2022, 43, 22–28. [Google Scholar]
- Zhang, S.M.; Chen, M.Z. Extraction of Polysaccharides from Porphyra haitanensis Using Ethanol-Ammonium Sulfate Aqueous Two-Phase System. Food Sci. 2014, 35, 46–49. [Google Scholar]
- Sadeghi, R.; Jamehbozorg, B. The salting-out effect and phase separation in aqueous solutions of sodium phosphate salts and poly (propylene glycol). Fluid Phase Equilib. 2009, 280, 68–75. [Google Scholar] [CrossRef]
- Rang, F.J.; Liu, W.; Ouyang, Y. Aqueous Two-phase Extraction Process and Antioxidant Activity of the Polyphenols and Polysaccharides of Physalis alkekengi Fruit. Food Res. Dev. 2021, 42, 114–120. [Google Scholar]
- Hou, Q.Z.; Xin, H.J.; Liu, Z.J. Study on Aqueous Two-phase System Extraction of Flavones and Polysaccharides from Pineapple Peel. Food Res. Dev. 2018, 39, 72–76. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144. [Google Scholar]
- Zhao, Y.Y.; Wang, Q.Z.; Zhang, J.H.; Feng, X.; Wang, M. Optimization of Extraction Conditions for Phenolic Acid of Cynara scolymus L. by Orthogonal Design. Food Res. Dev. 2013, 34, 15–17. [Google Scholar]
- Fernandes, P.A.R.; Le Bourvellec, C.; Renard, C.M.G.C.; Wessel, D.F.; Cardoso, S.M.; Coimbra, M.A. Interactions of arabinan-rich pectic polysaccharides with polyphenols. Carbohydr. Polym. 2020, 230, 115644. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Ye, Z.W.; Ye, R.; Hao, D.X.; Ren, S.W.; Li, J.P.; Chen, Q. Optimization of polysaccharide extraction from Xi Pinellia ternate by response surface methodology and its antioxidant activity. China Food Addit. 2022, 33, 90–98. [Google Scholar]
- Su, Y.L.; Zhang, J.H. Optimization of Ultrasonic-assisted Ethanol Extraction of Polyphenols from Kelp and Their Antioxidant Activities. Chin. Condiment 2020, 45, 174–180. [Google Scholar]
- Wu, Y.H.; Lu, W.J.; Liu, M.H.; Zhang, J.P.; Chen, A.H.; Shao, Y.; Wang, C.K.; Liu, E.Q. Optimization of ultrasonic-assisted aqueous two-phase extraction of burdock polysaccharide by response surface design and its antioxidant activities. Food Ferment. Ind. 2020, 46, 215–223. [Google Scholar]
- Liu, L.L.; Li, L.; Zhai, L.M.; Xiao, B.Z. Ultrasonic—assisted Aqueous Two—phase Extraction of Naringin from Pomelo Peel by Orthogonal Experimental Design. Sci. Technol. Food Ind. 2018, 39, 159–163, 171. [Google Scholar]
- Gong, X.H.; Li, M.C.; Xin, M.H.; Zhao, X.J.; Lu, G.; Xu, J.; Zhao, S.Y. Optimization of the Extraction Technology of Tea Polyphenols from Tea Waste by Aqueous Two—phase Systems with Ionic Liquids as Additives. Sci. Technol. Food Ind. 2020, 41, 158–166. [Google Scholar]
- Majd, M.H.; Rajaei, A.; Bashi, D.S.; Mortazavi, S.A.; Bolourian, S. Optimization of ultrasonic-assisted extraction of phenolic compounds from bovine pennyroyal (Phlomidoschema parviflorum) leaves using response surface methodology. Ind. Crops Prod. 2014, 57, 195–202. [Google Scholar] [CrossRef]
- Sahin, S.; Aybastier, O.; Isik, E. Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem. 2013, 141, 1361–1368. [Google Scholar] [CrossRef]
- Guo, C.; Li, C.; Hou, M.M.; Bai, M.Y.; Zhou, C.C.; Zhang, H.Y. Extraction optimization and its inoxidizability and hypoglycemic properties in vitro of polysaccharide from Salvia plebeia R. Br. Sci. Technol. Food Ind. 2022, 43, 211–219. [Google Scholar]
- Li, B.; Lei, Y.; Meng, X.J.; Jiao, X.Y.; Gao, N.X.; Zhao, Y.; Zhang, J.C. Optimization of Ultrasonic-Assisted Extraction of Polyphenols from Haskap Berries (Lonicera caerulea L.) Using Response Surface Methodology and Their Antioxidant Capacity. Food Sci. 2015, 36, 33–39. [Google Scholar]
- Song, S.; He, H.; Tang, X.; Wang, W. Determination of Polyphenols and Chlorogenic Acid in Artichoke. Life Sci. Instrum. 2007, 5, 47–49. [Google Scholar]
- Bao, F.; Yuan, H.Z.; Lin, X.; Liu, L.L.; Zhuang, H.; Zhao, D.S. Comparative study on the different parts of artichoke based on the correlation of “components-antioxidant”. Cereals Oils 2022, 35, 142–145. [Google Scholar]
- Martinez-Morales, F.; Alonso-Castro, A.J.; Zapata-Morales, J.R.; Carranza-Álvarez, C.; Aragon-Martinez, O.H. Use of standardized units for a correct interpretation of IC50 values obtained from the inhibition of the DPPH radical by natural antioxidants. Chem. Pap. 2020, 74, 3325–3334. [Google Scholar] [CrossRef]
- Garbetta, A.; Capotorto, I.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Pizzi, F.; Mineruini, F. Antioxidant activity induced by main polyphenols present in edible artichoke heads: Influence of in vitro gastro-intestinal digestion. Funct. Foods 2014, 10, 456–464. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 28, 15–27. [Google Scholar]

| Levels | Factors | |||
|---|---|---|---|---|
| A Powder Mass (g) | B Ultrasound Time (min) | C Ammonium Sulphate Mass Concentration (g/mL) | D Alcohol–Water Ratio | |
| −1 | 1.0 | 30 | 0.30 | 0.3 |
| 0 | 1.5 | 40 | 0.33 | 0.4 |
| 1 | 2.0 | 50 | 0.36 | 0.5 |
| No. | A | B | C | D | The Content of Polyphenols (mg/g) | The Content of Carbohydrates (mg/g) |
|---|---|---|---|---|---|---|
| 1 | 1 | 30 | 0.3 | 0.3 | 2.17 | 56.61 |
| 2 | 2 | 30 | 0.3 | 0.3 | 1.56 | 26.60 |
| 3 | 1 | 50 | 0.3 | 0.3 | 3.14 | 52.37 |
| 4 | 2 | 50 | 0.3 | 0.3 | 2.68 | 50.80 |
| 5 | 1 | 30 | 0.36 | 0.3 | 1.64 | 57.57 |
| 6 | 2 | 30 | 0.36 | 0.3 | 1.45 | 63.11 |
| 7 | 1 | 50 | 0.36 | 0.3 | 1.82 | 67.23 |
| 8 | 2 | 50 | 0.36 | 0.3 | 1.18 | 56.64 |
| 9 | 1 | 30 | 0.3 | 0.5 | 2.56 | 46.12 |
| 10 | 2 | 30 | 0.3 | 0.5 | 3.91 | 46.85 |
| 11 | 1 | 50 | 0.3 | 0.5 | 2.45 | 48.81 |
| 12 | 2 | 50 | 0.3 | 0.5 | 4.31 | 50.01 |
| 13 | 1 | 30 | 0.36 | 0.5 | 4.53 | 46.76 |
| 14 | 2 | 30 | 0.36 | 0.5 | 3.36 | 53.08 |
| 15 | 1 | 50 | 0.36 | 0.5 | 4.32 | 54.57 |
| 16 | 2 | 50 | 0.36 | 0.5 | 3.46 | 57.61 |
| 17 | 0.5 | 40 | 0.33 | 0.4 | 4.00 | 57.69 |
| 18 | 2.5 | 40 | 0.33 | 0.4 | 1.88 | 44.41 |
| 19 | 1.5 | 20 | 0.33 | 0.4 | 3.14 | 68.72 |
| 20 | 1.5 | 60 | 0.33 | 0.4 | 3.17 | 61.63 |
| 21 | 1.5 | 40 | 0.27 | 0.4 | 2.28 | 66.85 |
| 22 | 1.5 | 40 | 0.39 | 0.4 | 2.06 | 67.61 |
| 23 | 1.5 | 40 | 0.33 | 0.2 | 0.89 | 67.77 |
| 24 | 1.5 | 40 | 0.33 | 0.6 | 4.23 | 47.53 |
| 25 | 1.5 | 40 | 0.33 | 0.4 | 5.21 | 74.77 |
| 26 | 1.5 | 40 | 0.33 | 0.4 | 5.22 | 74.69 |
| 27 | 1.5 | 40 | 0.33 | 0.4 | 5.23 | 74.72 |
| 28 | 1.5 | 40 | 0.33 | 0.4 | 5.28 | 74.76 |
| 29 | 1.5 | 40 | 0.33 | 0.4 | 5.23 | 74.68 |
| 30 | 1.5 | 40 | 0.33 | 0.4 | 5.24 | 74.72 |
| Source | Sum of Squares of Deviation from Mean | Degrees of Freedom | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 53.44 | 14 | 3.82 | 15.99 | <0.0001 |
| A | 1.03 | 1 | 1.03 | 4.29 | 0.0559 |
| B | 0.2091 | 1 | 0.2091 | 0.8755 | 0.3643 |
| C | 0.0888 | 1 | 0.0888 | 0.3719 | 0.5511 |
| D | 16.57 | 1 | 16.57 | 69.38 | 0.0001 |
| AB | 0.0169 | 1 | 0.0169 | 0.0708 | 0.7938 |
| AC | 1.56 | 1 | 1.56 | 6.54 | 0.0218 |
| AD | 0.5929 | 1 | 0.5929 | 2.48 | 0.1359 |
| BC | 0.4160 | 1 | 0.4160 | 1.74 | 0.2066 |
| BD | 0.2070 | 1 | 0.2070 | 0.8670 | 0.3665 |
| CD | 2.18 | 1 | 2.18 | 9.11 | 0.0086 |
| A2 | 8.63 | 1 | 8.63 | 36.13 | <0.0001 |
| B2 | 7.05 | 1 | 7.05 | 29.54 | <0.0001 |
| C2 | 15.57 | 1 | 15.57 | 65.19 | <0.0001 |
| D2 | 11.80 | 1 | 11.80 | 49.41 | <0.0001 |
| Residual | 3.58 | 15 | 0.2388 | — | — |
| Lack of fit | 1.29 | 10 | 0.1290 | 4.23 | 0.1969 |
| Pure Error | 0.0030 | 5 | 0.0006 | — | — |
| Sum error | 57.03 | 29 | — | — | — |
| Source | Sum of Squares of Deviation from Mean | Degrees of Freedom | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 3138.99 | 14 | 224.21 | 3.48 | 0.0112 |
| A | 112.23 | 1 | 112.23 | 1.74 | 0.2069 |
| B | 30.74 | 1 | 30.74 | 0.4766 | 0.5005 |
| C | 266.13 | 1 | 266.13 | 4.13 | 0.0603 |
| D | 190.41 | 1 | 190.41 | 2.95 | 0.1063 |
| AB | 5.64 | 1 | 5.64 | 0.0875 | 0.7715 |
| AC | 72.08 | 1 | 72.08 | 1.12 | 0.3071 |
| AD | 143.52 | 1 | 143.52 | 2.23 | 0.1565 |
| BC | 6.60 | 1 | 6.60 | 0.1024 | 0.7534 |
| BD | 1.54 | 1 | 1.54 | 0.0238 | 0.8793 |
| CD | 89.97 | 1 | 89.97 | 1.40 | 0.2559 |
| A2 | 1449.35 | 1 | 1449.35 | 22.47 | 0.0003 |
| B2 | 383.23 | 1 | 383.23 | 5.94 | 0.0277 |
| C2 | 285.13 | 1 | 285.13 | 4.42 | 0.0528 |
| D2 | 866.06 | 1 | 866.06 | 13.43 | 0.0023 |
| Residual | 967.32 | 15 | 64.49 | — | — |
| Lack of fit | 1.56 | 10 | 0.1560 | 1.62 | 0.3191 |
| Pure Error | 0.0065 | 5 | 0.0013 | — | — |
| Sum error | 4106.32 | 29 | — | — | — |
| Experiment Name | Carbohydrates | Polyphenols | VC |
|---|---|---|---|
| Clear ABTS+ | 0.4141 mg/mL | 0.01108 mg/mL | 0.01294 mg/mL |
| Clear DPPH | 2.6110 mg/mL | 0.04131 mg/mL | 0.17400 mg/mL |
| Fe3+ reducing power | 0.9363 mg/mL | 0.06345 mg/mL | 0.06976 mg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Lu, X.-K.; Zhu, K.-H.; Jiang, X.-Y.; Chen, J.-C.; Yan, P.-Z.; Zhao, D.-S. Synchronous Extraction, Antioxidant Activity Evaluation, and Composition Analysis of Carbohydrates and Polyphenols Present in Artichoke Bud. Molecules 2022, 27, 8962. https://doi.org/10.3390/molecules27248962
Lin X, Lu X-K, Zhu K-H, Jiang X-Y, Chen J-C, Yan P-Z, Zhao D-S. Synchronous Extraction, Antioxidant Activity Evaluation, and Composition Analysis of Carbohydrates and Polyphenols Present in Artichoke Bud. Molecules. 2022; 27(24):8962. https://doi.org/10.3390/molecules27248962
Chicago/Turabian StyleLin, Xiao, Xian-Kun Lu, Kai-Hao Zhu, Xin-Yang Jiang, Jiong-Chao Chen, Pei-Zheng Yan, and Dong-Sheng Zhao. 2022. "Synchronous Extraction, Antioxidant Activity Evaluation, and Composition Analysis of Carbohydrates and Polyphenols Present in Artichoke Bud" Molecules 27, no. 24: 8962. https://doi.org/10.3390/molecules27248962
APA StyleLin, X., Lu, X.-K., Zhu, K.-H., Jiang, X.-Y., Chen, J.-C., Yan, P.-Z., & Zhao, D.-S. (2022). Synchronous Extraction, Antioxidant Activity Evaluation, and Composition Analysis of Carbohydrates and Polyphenols Present in Artichoke Bud. Molecules, 27(24), 8962. https://doi.org/10.3390/molecules27248962






