The Detailed Pharmacodynamics of the Gut Relaxant Effect and GC-MS Analysis of the Grewia tenax Fruit Extract: In Vivo and Ex Vivo Approach
Abstract
1. Introduction
2. Results
2.1. Methanolic Extract Yield (%)
2.2. GC-MS Phytochemical Profiling
2.3. In Vivo Antidiarrheal Effect
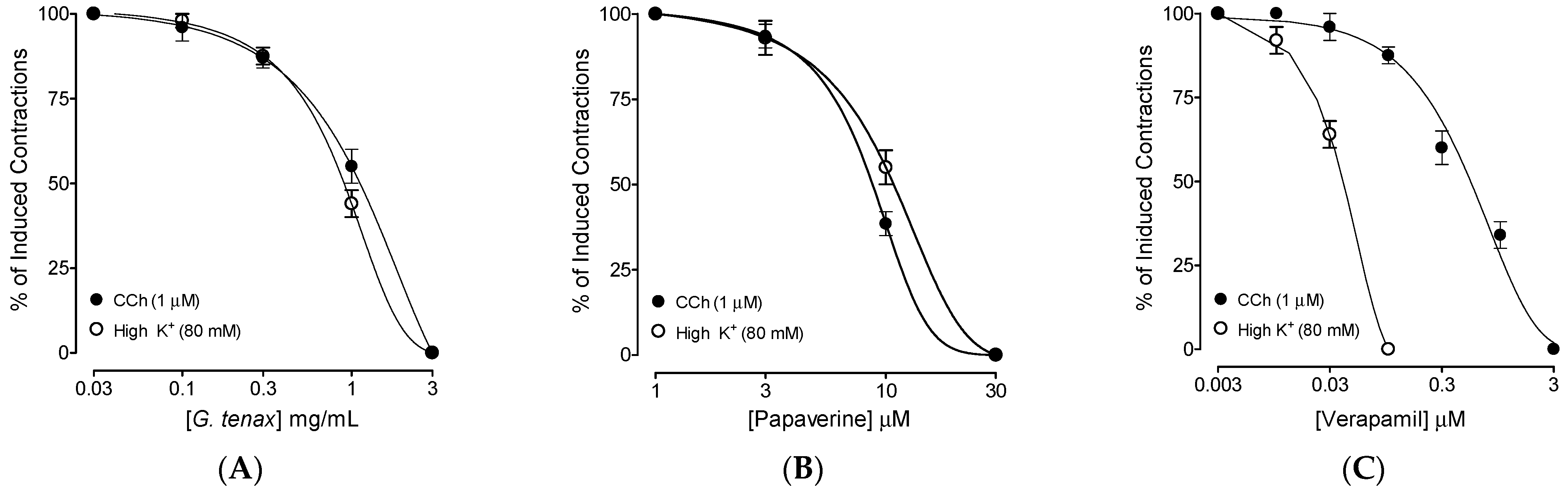
2.4. Ex Vivo Antispasmodic Effects
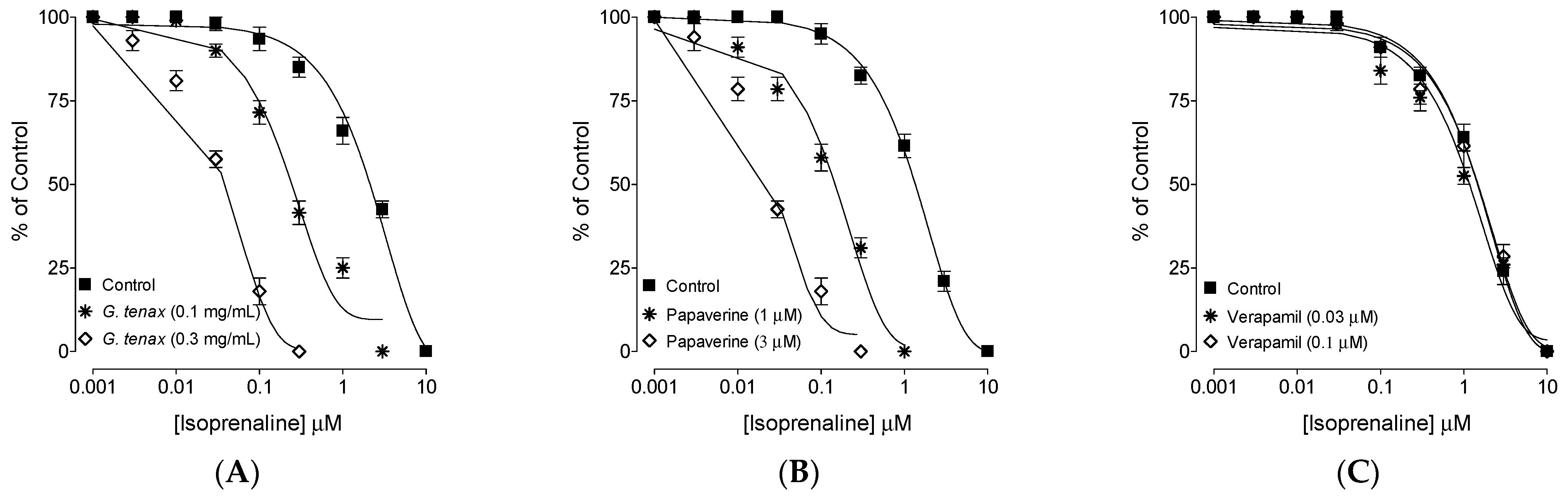
2.5. Phosphodiesterase Enzyme (PDE) Inhibitory-like Effect
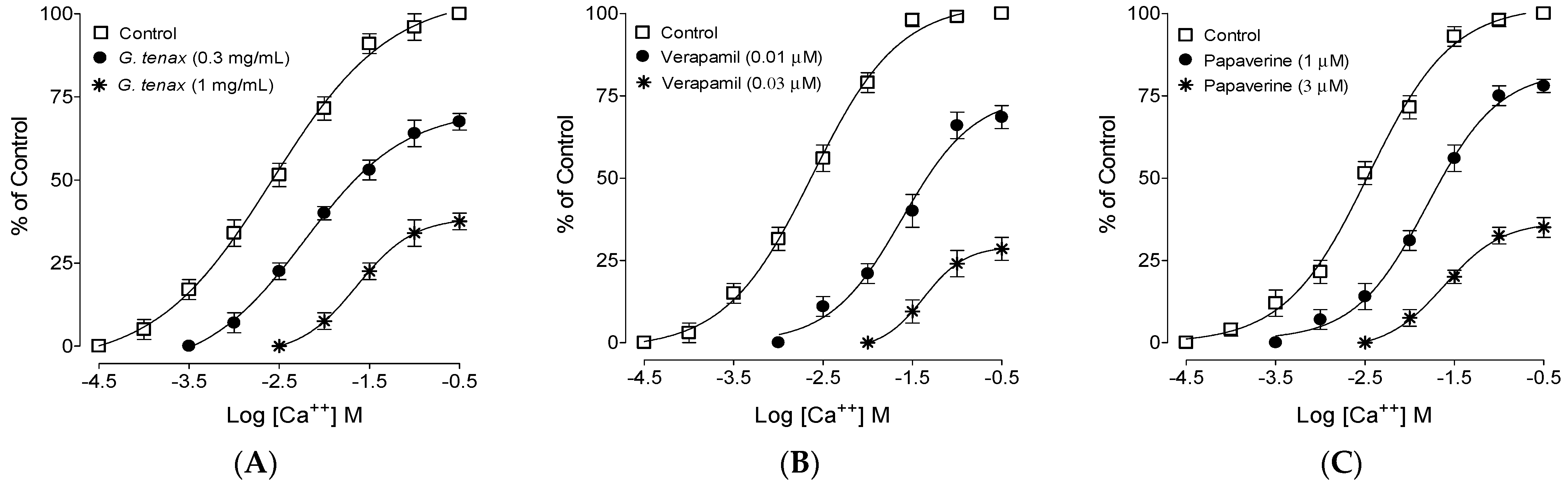
2.6. Calcium Channel Blocking (CCB)-like Effect
3. Discussion
4. Materials and Methods
4.1. Extraction of Plant Material
4.2. Chemicals
4.3. Animals
4.4. GC-MS Analysis
4.5. In Vivo Antidiarrheal Study
4.6. Ex Vivo Experiments on Isolated Rat Ileum
4.7. Ca2+ Inhibitory Confirmation
4.8. PDE Inhibitory Confirmation
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Haque, M.A.; Abdullah, C.S.; Romana, B.; Rafique, M.B.; Zia-ulHuda, G.M.; Hossain, S.F.; Begum, B. Evaluation of anti-diarrheal and anti-diabetic activities of the stem, barks and leaves of the plant Vernonia cinerea (family: Asteraceae). J. Appl. Pharm. Sci. 2013, 3, 69–72. [Google Scholar]
- Sinan, K.I.; Chiavaroli, A.; Orlando, G.; Bene, K.; Zengin, G.; Cziáky, Z.; Jekő, J.; Mahomoodally, M.F.; Picot-Allain, M.; Menghini, L.; et al. Biopotential of Bersama abyssinica fresen stem bark extracts: UHPLC profiles, antioxidant, enzyme inhibitory, and antiproliferative propensities. Antioxidants 2020, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, M.R. An investigation in to the anti-diarrheal property of monteleukast. Int. J. Pharm. Sci. 2014, 6, 147–148. [Google Scholar]
- Sweetser, S. Evaluating the patient with diarrhea: A case-based approach. Mayo Clin. Proc. 2012, 87, 596–602. [Google Scholar] [CrossRef]
- Wansi, S.L.; Nguelefack-Mbuyo, E.P.; Nchouwet, M.L.; Miaffo, D.; Nyadjeu, P.; Wabo, J.P.; Mbiantcha, M.; NKengEfouet, P.A.; Nguelefack, T.B.; Kamanyi, A. Antidiarrheal activity of aqueous extract of the stem bark of Sapium ellipticum (Euphorbiaceae). Trop. J. Pharm. Res. 2014, 13, 929–935. [Google Scholar] [CrossRef][Green Version]
- Alam, A.; Rehman, N.U.; Ansari, M.N.; Palla, A.H. Effects of essential oils of Elettaria cardamomum grown in India and Guatemala on Gram-negative bacteria and gastrointestinal disorders. Molecules 2021, 26, 2546. [Google Scholar] [CrossRef]
- Tadesse, W.T.; Hailu, A.E.; Gurmu, A.E.; Mechesso, A.F. Experimental assessment of antidiarrheal and antisecretory activity of 80% methanolic leaf extract of Zehneria scabra in mice. BMC Complement. Altern. Med. 2014, 14, 460. [Google Scholar] [CrossRef]
- Ansari, M.N.; Bhandari, U. Effect of an Ethanol Extract of Embelia ribes Fruits on Isoproterenol-Induced Myocardial Infarction in Albino Rats. Pharm. Biol. 2008, 46, 928–932. [Google Scholar] [CrossRef]
- Umer, S.; Tekewe, A.; Kebede, N. Antidiarrhoeal and antimicrobial activity of Calpurnia aurea leaf extract. BMC Complement. Altern. Med. 2013, 13, 21. [Google Scholar] [CrossRef]
- Ullah, W.; Uddin, G.; Siddiqui, B.S. Ethnic uses, pharmacological and phytochemical profile of genus Grewia. J. Asian Nat. Prod. Res. 2012, 14, 186–195. [Google Scholar] [CrossRef]
- Gebauer, J.; El-Siddig, K.; El-Tahir, B.A.; Salih, A.A.; Ebert, G.; Hammer, K. Exploiting the potential of indigenous fruit trees: Grewia tenax in Sudan. Genet. Resour. Crop Evol. 2007, 54, 1701–1708. [Google Scholar] [CrossRef]
- Elhassan, G.M.; Yagi, S.M. Nutritional composition of Grewia species (Grewia tenax (Forsk.) Fiori, G. flavescens Juss and G. villosa Willd) fruits. Adv. J. Food Sci. Technol. 2010, 2, 159–162. [Google Scholar]
- Abdelmutti, O.M.S. Biochemical of Nutritional Evaluation of Famine Food of Sudan. Doctor Dissertation, University of Khartoum, Khartoum, Sudan, 1991. [Google Scholar]
- Gupta, M.K.; Sharma, P.K.; Ansari, S.H.; Lagarkha, R. Pharmacognostical evaluation of Grewia asiatica fruits. Int. J. Plant. Sci. 2006, 1, 249. [Google Scholar]
- Sharma, N.; Patni, V. Grewia tenax (frosk.) Fiori.—A traditional medicinal plant with enormous economic prospective. Asian J. Pharm. Clin. Res. 2012, 5, 28–32. [Google Scholar]
- Kehlenbeck, K.; Asaah, E.; Jammadass, R. Diversity of indigenous fruit trees and their contribution to nutrition and livelihoods in sub Saharan Africa: Examples from Kenya and Cameroon. In Diversifying Food and Diets: Using Agricultural Biodiversity to Improve Nutrition and Health Issues in Agricultural Biodiversity; Fanzo, J., Hunter, D., Borelli, T., Mattei, F., Eds.; Earthscan: London, UK, 2013; pp. 257–269. [Google Scholar]
- Goyal, P.K. Phytochemical and pharmacological properties of the genus Grewia: A review. Int. J. Pharm. Pharm. Sci. 2012, 4, 72–78. [Google Scholar]
- Aboagarib, E.A.A.; Yang, R.; Hua, X.; Siddeeg, A. Chemical compositions, nutritional properties and volatile compounds of Guddaim (Grewia tenax. Forssk) Fiori fruits. J. Food Nutr. Res. 2014, 2, 187–192. [Google Scholar] [CrossRef]
- Shekhawat, D.; Batra, A. House hold remedies of Keshavraipatan tehsil in Bundi district, Rajasthan. Indian J. Tradit. Knowl. 2006, 5 (Suppl. 3), 362–367. [Google Scholar]
- Karim, A.M.; Azim, A.I.; Sufian, A. GC-MS analysis and antimicrobial activity of sudanese Grewia tenax forssk (tiliaceae) fixed oil. World J. Pharm. Sci. 2019, 5, 37–41. [Google Scholar]
- Saleh, I.A.; Shams, K.A.; Tawfik, W.A.; Habib, A.A.; Hassan, R.A.; Shahat, A.A.; Aboutab, E.A.; Hammouda, F.M.; Abdel-azim, N.S.; Investigation of the lipid and carbohydrate contents of Grewia tenax forssk. Fruits & evaluation of hepatoprotection activity. Int. J. Pharm. Sci. 2015, 7, 179–182. [Google Scholar]
- Croci, T.; Landi, M.; Elmonds-Alt, X.; Le-Fur, G.; Maffrand, J.P.; Manara, L. Role of tachykinins in castor oil-induced diarrhoea in rats. Br. J. Pharmacol. 1997, 121, 375–380. [Google Scholar] [CrossRef]
- Iwao, I.; Terada, Y. On the mechanism of diarrhea due to castor oil. Jpn. J. Pharmacol. 1962, 12, 137–145. [Google Scholar] [CrossRef]
- Palla, A.; Gilani, A.H.; Bashir, S.; Rehman, N.U. Multiple mechanisms of Flaxseed-effectiveness in Inflammatory Bowel disease. Evid.-Based Complement. Altern. Med. 2020, 2020, 7974835. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.U.; Gilani, A.H.; Khan, A.; Nazneen, M.; El Gamal, A.A.; Fawzy, G.A.; Al-Ati, H.Y.; Abdel-kader, M.S. Antidiarrheal and Antispasmodic Activities of Buddleja polystachya are Mediated Through Dual Inhibition of Ca++ Influx and Phosphodiesterase Enzyme. Phytother. Res. 2015, 29, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, A.U.; Rehman, N.U.; Gilani, A.H. Pharmacological basis for medicinal use of Lens culinaris in gastrointestinal and respiratory disorders. Phytother. Res. 2014, 28, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Imam, F.; Rehman, N.U.; Ansari, M.N.; Qamar, W.; Afzal, M.; Al-Harbi, K.S. Effect of Roflumilast in airways disorders via dual inhibition of Phosphodiesterase and Ca2+-channel. Saudi Pharm. J. 2020, 28, 698–702. [Google Scholar] [CrossRef]
- Ansari, M.N.; Rehman, N.U.; Karim, A.; Bahta, T.; Abujheisha, K.Y.; Ahamad, S.R.; Imam, F. Evaluation of Bronchodilator and antimicrobial activities of Otostegia fruticose: A multi-mechanistic approach. Saudi Pharm. J. 2020, 28, 281–289. [Google Scholar] [CrossRef]
- Fleckenstein, A. Specific pharmacology of Ca++ in myocardium, cardiac pacemakers and vascular smooth muscle. Rev. Pharmacol. Toxicol. 1977, 17, 149–166. [Google Scholar] [CrossRef]
- Downie, J.W.; Twiddy, D.A.; Awad, S.A. Antimuscarinic and non-competitive antagonist properties of dicyclomine hydrochloride in isolated human and rabbit bladder muscle. J. Pharmacol. Exp. Ther. 1977, 201, 662–668. [Google Scholar]
- Choo, L.K.; Mitchelson, F. Antagonism of cholinomimetics by troxypyrrolidinium in guinea-pig atria and longitudinal ileal muscle: Comparison with hemicholinium-3. Eur. J. Pharmacol. 1978, 52, 313–322. [Google Scholar] [CrossRef]
- Boswell-Smith, V.; Spina, D.; Page, C.P. Phosphodiesterase inhibitors. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S252–S257. [Google Scholar] [CrossRef]
- Uddin, S.B.; Mahbub-Uz-Zaman, M.; Akhtar, R.; Ahmed, N.U. Antidiarrheal activity of ethanolic bark extract of Mitragyna diversifolia. Bangladesh J. Pharmacol. 2009, 4, 144–146. [Google Scholar]
- Kaneda, T.; Takeuchi, Y.; Matsui, H.; Shimizu, K.; Urakawa, N.; Nakajyo, S. Inhibitory mechanism of papaverine on carbachol-induced contraction in bovine trachea. J. Pharmacol. Sci. 2005, 98, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.C.; Weir, S.W.; Weston, A.H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br. J. Pharmacol. 1986, 88, 103–111. [Google Scholar] [CrossRef]
- Ahmad, W.; Tamboli, E.T.; Ali, A.; Amir, M.; Zaidi, S.M.A.; Ahmad, S. Didymocarpous pedicellatus R. Br.: Qualitative and Quantitative GCMS Approach for Quality Control in Traditional Poly-herbal Formulation with In vitro Antioxidant and Antimicrobial Activity. Orient. J. Chem. 2019, 35, 648–657. [Google Scholar] [CrossRef]
- Ahmad, W.; Parveen, R.; Mujeeb, M.; Zaidi, S.M.A. Comparative Fingerprint Profiling of Unani Polyherbomineral (Safoof-e-Pathar Phori) Formulation by HPTLC, HPLC, and GC-MS. J. AOAC Int. 2020, 103, 659–668. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996; pp. 1–7. [Google Scholar]
- Khan, W.; Chester, K.; Anjum, V.; Ahmad, W.; Ahmad, S.; Narwaria, A.; Katiyar, C.K. Chromatographic profiling of Pancharishta at different stages of its development using HPTLC, HPLC, GC–MS and UPLC–MS. Phytochem. Lett. 2017, 20, 391–400. [Google Scholar] [CrossRef]
- Rehman, N.U.; Ansari, M.N.; Ahmad, W.; Amir, M. GC–MS Analysis and In Vivo and Ex Vivo Antidiarrheal and Antispasmodic Effects of the Methanolic Extract of Acacia nilotica. Molecules 2022, 27, 2107. [Google Scholar] [CrossRef]
- Rehman, N.U.; Ansari, M.N.; Ahmad, W.; Ahamad, S.R. Dual Inhibition of Phosphodiesterase and Ca++ Channels Explains the Medicinal Use of Balanites aegyptiaca (L.) in Hyperactive Gut Disorders. Plants 2022, 11, 1183. [Google Scholar] [CrossRef]
- Rehman, N.U.; Ansari, M.N.; Samad, A. In Silico, Ex Vivo and In Vivo Studies of Roflumilast as a Potential Antidiarrheal and Antispasmodic agent: Inhibition of the PDE-4 Enzyme and Voltage-gated Ca++ ion Channels. Molecules 2020, 25, 1008. [Google Scholar] [CrossRef]
- Godfraind, T.; Miller, R.; Wibo, M. Calcium antagonism and calcium entry blockade. Pharmacol. Rev. 1986, 38, 321–416. [Google Scholar]
- Gilani, A.H.; Khan, A.; Subhan, F.; Khan, M. Antispasmodic and bronchodilator activities of St. John’s wort are putatively mediated through dual inhibition of calcium influx and phosphodiesterase. Fundam. Clin. Pharmacol. 2005, 19, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.T.; Liao, G.; Bi, X.; Oka, T.; Tamura, S.; Baudry, M. The PDE10A inhibitor, papaverine, differentially activates ERK in male and female rat striatal slices. Neuropharmacology 2011, 61, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Rang, H.P.; Dale, M.M.; Ritter, J.M. Pharmacology, 4th ed.; Churchill Livingstone: New York, NY, USA, 1999; pp. 289–290. [Google Scholar]
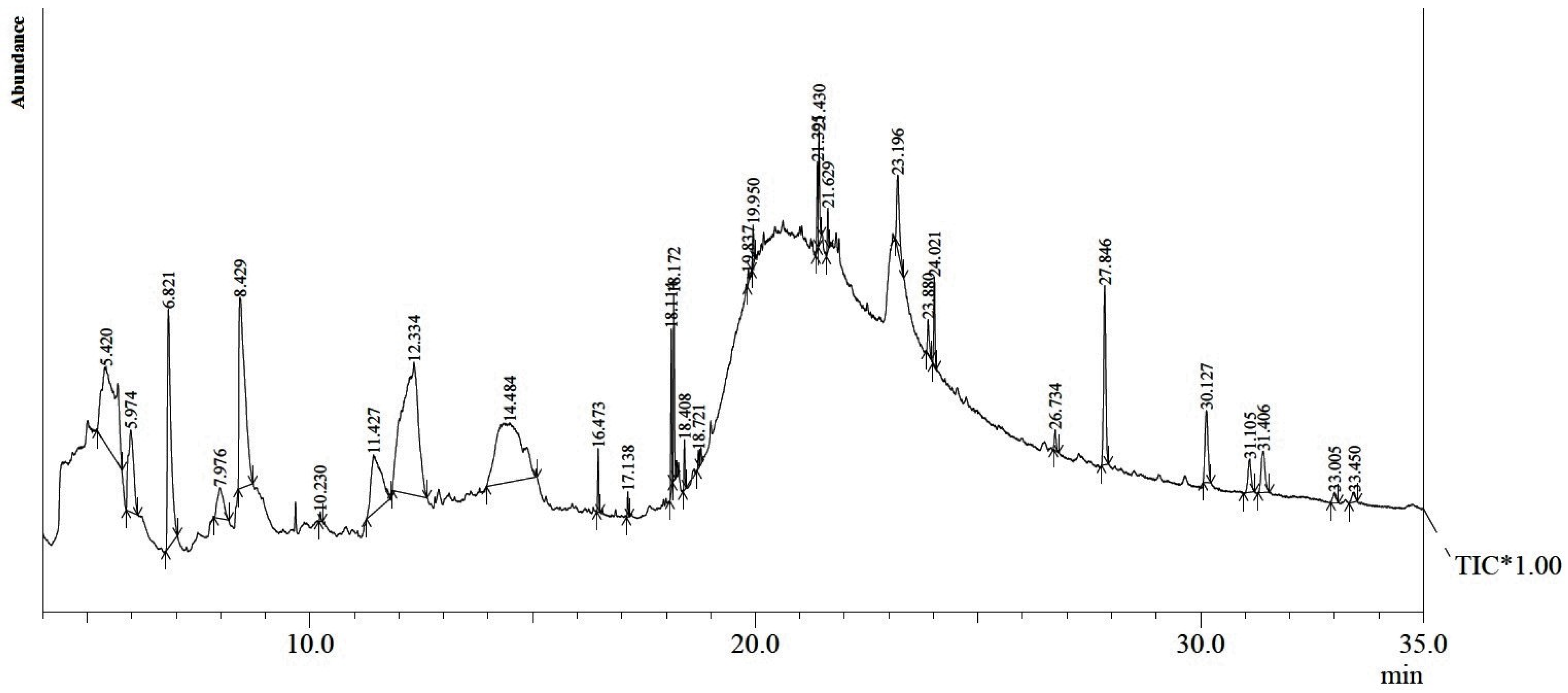
| S. No. | Compound Name | RT (Min) | % Area | Nature of Compound |
|---|---|---|---|---|
| 1 | 1,2,3-Propanetriol | 5.420 | 11.40 | Phenol |
| 2 | 6-Amino-5-nitroso-1H-pyrimidine-2,4-dione | 5.974 | 4.31 | Pyrimidinediones |
| 3 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl | 6.821 | 8.27 | Pyranone |
| 4 | 2-Pentanone | 7.976 | 1.85 | Ketone |
| 5 | 5-Hydroxymethylfurfural | 8.429 | 11.01 | Hydroxymethylfurfural |
| 6 | 1-Tetradecene | 10.230 | 0.11 | Hydrocarbon |
| 7 | 1,2-Cyclobutanedicarboxylic acid | 11.427 | 5.72 | Carboxylic acid |
| 8 | 6,6-Dideutero-nonen-1-ol-3 | 12.334 | 20.98 | Alcohol |
| 9 | 3-Deoxy-d-mannoic lactone | 14.484 | 15.36 | Lactone |
| 10 | Hexadecanoic acid, methyl ester | 16.473 | 0.62 | Fatty Acid Ester |
| 11 | Hexadecanoic acid, ethyl ester | 17.138 | 0.23 | Fatty Acid Ester |
| 12 | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 18.114 | 1.50 | Fatty Acid Ester |
| 13 | 9-Octadecenoic acid, methyl ester, (E)- | 18.172 | 1.84 | Fatty Acid Ester |
| 14 | Octadecenoic acid, methyl ester | 18.408 | 0.49 | Fatty Acid Ester |
| 15 | Ethyl (9Z,12Z)-9,12-octadecadienoate | 18.721 | 0.17 | Fatty Acid |
| 16 | Glycidyl palmitate | 19.950 | 0.44 | Fatty Acid Ester |
| 17 | 1,8,11-Heptadecatriene, (Z,Z)- | 21.395 | 0.87 | Hydrocarbon |
| 18 | Glycidyl oleate | 21.430 | 1.20 | Ester |
| 19 | 15-Hydroxypentadecanoic acid | 21.629 | 0.43 | Fatty Acid |
| 20 | 9,12-Octadecadienoic acid (Z,Z)-, 2-hydroxyl-1-(hydroxymethyl)ethyl ester | 23.19 | 2.05 | Fatty Acid Ester |
| 21 | 9-Octadecenamide, (Z)- | 23.880 | 0.56 | Fatty Acid Primary amide |
| 22 | Squalene | 24.021 | 1.02 | Hydrocarbon |
| 23 | 2H-1-benzopyran-6-OL, 4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltridecyl) | 27.846 | 3.79 | Vitamin E acetate |
| 24 | Beta-sitosterol | 30.127 | 2.12 | Phytosterol |
| 25 | 4,4-Dimethyl-5.alpha.-D1-androstane-3.beta | 31.105 | 1.02 | Acetate |
| 26 | Ergost-5-en-3-ol,(3.beta.) | 31.406 | 1.54 | Steroid |
| 27 | Stigmasta-4,22-dien-3-one | 33.005 | 0.32 | Steroid |
| 28 | 1,1,6-Trimethyl-3-methylene-2-(3,6,10,13,14–pentamethyl-3-ethenyl-pendadec-4-enye)cyclohexane | 33.450 | 0.35 | Sesquiterpenoid |
| 99.66 |
| Treatment (p.o.), Dose (mg/kg) | No. of Mice with Diarrhea | % Protection |
|---|---|---|
| Saline (10 mL/kg) + Castor oil | 5/5 | 0 |
| G. tenax (200 mg/kg) + Castor oil | 3 */5 | 40 |
| G. tenax (400 mg/kg) + Castor oil | 1 */5 | 80 |
| Loperamide (10 mg/kg) + Castor oil | 0 **/5 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, N.U.; Ansari, M.N.; Ahmad, W.; Amir, M. The Detailed Pharmacodynamics of the Gut Relaxant Effect and GC-MS Analysis of the Grewia tenax Fruit Extract: In Vivo and Ex Vivo Approach. Molecules 2022, 27, 8880. https://doi.org/10.3390/molecules27248880
Rehman NU, Ansari MN, Ahmad W, Amir M. The Detailed Pharmacodynamics of the Gut Relaxant Effect and GC-MS Analysis of the Grewia tenax Fruit Extract: In Vivo and Ex Vivo Approach. Molecules. 2022; 27(24):8880. https://doi.org/10.3390/molecules27248880
Chicago/Turabian StyleRehman, Najeeb Ur, Mohd Nazam Ansari, Wasim Ahmad, and Mohd Amir. 2022. "The Detailed Pharmacodynamics of the Gut Relaxant Effect and GC-MS Analysis of the Grewia tenax Fruit Extract: In Vivo and Ex Vivo Approach" Molecules 27, no. 24: 8880. https://doi.org/10.3390/molecules27248880
APA StyleRehman, N. U., Ansari, M. N., Ahmad, W., & Amir, M. (2022). The Detailed Pharmacodynamics of the Gut Relaxant Effect and GC-MS Analysis of the Grewia tenax Fruit Extract: In Vivo and Ex Vivo Approach. Molecules, 27(24), 8880. https://doi.org/10.3390/molecules27248880








