Abstract
The risk of progression of most sporadic neurodegenerative diseases, including Alzheimer’s disease, increases with age. Traditionally, this is associated with a decrease in the efficiency of cell protection systems, in particular, molecular chaperones. Thus, the development of small molecules able to induce the synthesis of chaperones is a promising therapeutic approach to prevent neural diseases associated with ageing. Here, we describe a new compound IA-50, belonging to the class of indolylazines and featured by a low size of topological polar surface area, the property related to substances with potentially high membrane-penetrating activity. We also estimated the absorption, distribution, metabolism and excretion characteristics of IA-50 and found the substance to fit the effective drug criteria. The new compound was found to induce the synthesis and accumulation of Hsp70 in normal and aged neurons and in the hippocampi of young and old mice. The transgenic model of Alzheimer’s disease, based on 5xFAD mice, confirmed that the injection of IA-50 prevented the formation of β-amyloid aggregates, loss of hippocampal neurons and the development of memory impairment. These data indicate that this novel substance may induce the expression of chaperones in neural cells and brain tissues, suggesting its possible application in the therapy of ageing-associated disorders.
1. Introduction
The progression of many neurodegenerative diseases is explained by the formation of toxic oligomers or aggregates consisting of mutant or damaged proteins that are located inside or in close proximity to neurons. The accumulation of such pathogenic structures disturbs the normal functioning of metabolism and signalling and leads to dysfunction and, ultimately, death of neural cells [1]. According to the amyloid hypothesis of Alzheimer’s disease (AD), cytotoxic oligomers and aggregates of Aβ peptide contribute to the progression of the disease [2,3]. Of note, AD as well as many other neurological diseases, progression with age may be due to the reduced ability of the cell protection systems to resist multiple damages in cellular proteostasis. Molecular chaperone Hsp70 constitutes one of the aforementioned protection systems since it has been shown to bind mutant, or damaged proteins/peptides, and prevent their oligomerisation and aggregation [4,5]. In a view of AD pathogenesis, the chaperones were found to target mutant tau and β-amyloid and prevent the formation of their cytotoxic aggregates in AD pathogenesis [6,7].
One of the ways to activate the previously discussed mechanisms is the application of chemical inducers of chaperone synthesis [8], whose protective activity has been proven in a few neurodegeneration models, including AD [9]. However, the bottleneck of this therapeutic approach is that the efficiency of chaperone induction and its cellular concentration is reduced with ageing [10,11]. However, suppression of Hsp70 content and other systems of cellular proteostasis with age has been shown for rat hepatocytes [12], rat and rhesus monkey lymphocytes [13] and human lymphocytes [14,15]. Although it is a common thread in many reviews that the proteostasis system deteriorates with age, we did not find much experimental information about chaperone expression in brain structures, and the data presented in these studies contradict those in other tissues. In the first study, it was demonstrated that both Hsp70 and Hsc70 (constitutive members of the same protein family) increased in the hippocampus, substantia nigra, cerebellum, cortex and striatum during ageing due to the significant changes in the glutathione redox state and lipid peroxidation [16]. In another paper, the authors did not find any difference in Hsp60, Hsp40 or Hsc70 expression in the substantia nigra and striatum during the life span of female rats [17].
The aim of this work was (i) to evaluate the proteostasis status in aged brain structures involved in AD pathogenesis and (ii) to search for the compounds that cause the accumulation of chaperone Hsp70 in older cells or tissues.
Previously, we found that substances with a certain general structure (belonging to pyrrolyl- and indolylazines groups of chemicals) were able to induce the synthesis and accumulation of chaperones, which led to a therapeutic effect in an in vitro model of AD [18]. In the present study, based on in silico calculations, we selected, synthesised and tested a new compound from the indolylazine series, IA-50, which activated the synthesis of the Hsp70 in a culture of aged human neural cells. This compound was also tested on an in vivo model of 5xFAD transgenic mice, which were producing β-amyloid and demonstrating general manifestations of AD.
2. Results
2.1. Analysis of Proteins Regulating Cell Proteostasis in Ageing
The inconsistent data on the regulation of the proteostasis system in ageing brains forced us to carry out an analysis of proteins involved the protein folding control and degradation. We selected mice at the age of 3, 6, 9, 12, 18 and 24 months (n = 5 in each group). To understand what is happening in those regions of the brain where the primary focus of neurodegeneration is located, namely in the hippocampus, as well as in the cerebral cortex, suffering in more advanced stages of the disease, we performed Western blotting of the hippocampal and cortical fractions, using antibodies for the selected proteostasis actors.
The results of Western blotting convinced us that the efficiency of proteostasis systems decreases over the course of a lifetime (Figure 1). The level of molecular chaperones Hsp70 and Hsp40, two proteins functioning in tandem that define the fate of cellular proteins with abnormal conformation, both in the hippocampus and in the cortex, began to decrease after 12 months, and we observed the same pattern for Hsc70, a protein that plays both the role of a cellular chaperone and the main participant in Chaperone mediated autophagy (CMA). Furthermore, in both analysed brain structures, starting from 9–12 months of age, a reduction in the expression of the autophagy marker, Atg5/12, was observed (Figure 1). These data prompted us to search the compounds able to force at least molecular chaperone expression in the aged brains of AD mice.
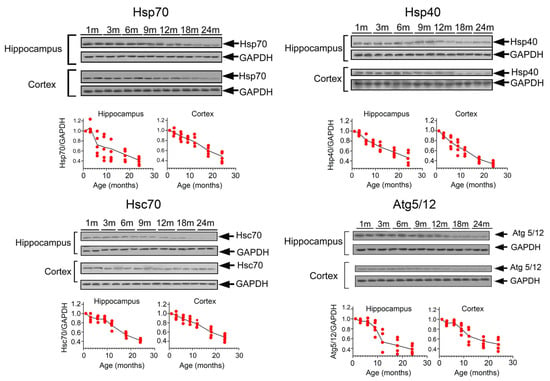
Figure 1.
Expression of proteins involved in proteostasis reduces with mouse ageing. Hippocampus and cortex tissues from untreated mice at ages 1, 3, 6, 9, 12, 18 and 24 months (n = 5 in each age group) were used for Western blotting. On the top of each panel, representative immunoblots of the hippocampus and cortex lysates with antibodies to molecular chaperones and autophagy markers are shown; on the bottom, the calculation of the intensity of the protein zones of interest to the intensity of the zones of the normalizing protein GAPDH. Each point corresponds to one animal.
2.2. In Silico Prediction of the Indolylazine Ability to Penetrate the Cell Membrane of Senescent Neurons
Previously, we established the chemical structure necessary to activate the synthesis and accumulation of molecular chaperones in neural cells. However, most of the chemicals were unable to efficiently induce chaperone synthesis in senescent cells or aged mice (Lazarev et al., unpublished data). This insufficiency was presumably related to changes in membrane permeability in aged cells, due to reduced amounts of polyunsaturated fatty acids [19,20]. One of the key parameters, which is considered to reflect the ability of small molecules to penetrate the cell membrane, is the topological polar surface area (TPSA). The lower the TPSA value—the higher probability of passing a small molecule through the cell membrane [21]. Due to the fact that the compounds synthesized and tested by us did not have a significant effect on the chaperone synthesis in senescent cells, we conducted an in silico analysis of the physicochemical properties of a few of the indole derivatives synthesized by us and compared them with those previously published [18]. We noticed that one of the new compounds, IA-50, stands out with the lowest TPSA value (Table 1), which gave hope for high membrane-penetrating activity of this substance. Of significance, comparing the structure of IA-50 with other relative molecules (Figure 2), we found that it retains the features of heat response—inducing activity and selected the compound for further examinations of its biological properties.

Table 1.
Computationally predicted absorption, distribution, metabolism and excretion (ADME), and other drug-likeness profiles of pyrrolyl- and indolylazine.
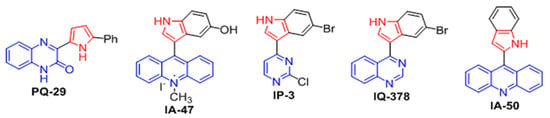
Figure 2.
Examples of pyrrolyl- and indolylazines demonstrating chaperone-inducing activity. The fragments of pyrrole are marked in red.
2.3. IA-50 Is Able to Induce Chaperone Accumulation in Aged Neurons
Since the major goal of this report was to develop a heat shock response inducer in aged neurons, we used human umbilical cord mesenchymal stem cells MSCWJ-2 and reprogrammed them to the neuronal phenotype after the 5th and 15th passages. At the 15th passage, the MSCWJ-2 cells demonstrated senescent phenotype as was proved by approximately 2-fold higher β-galactosidase activity compared to cells of the fifth passage (Figure 3A). Notably, both cells at the 5th and 15th passages were reprogrammed into neurons, that was confirmed by vastly elevated expression of β-3 tubulin and MAP-2 (Figure 3B).
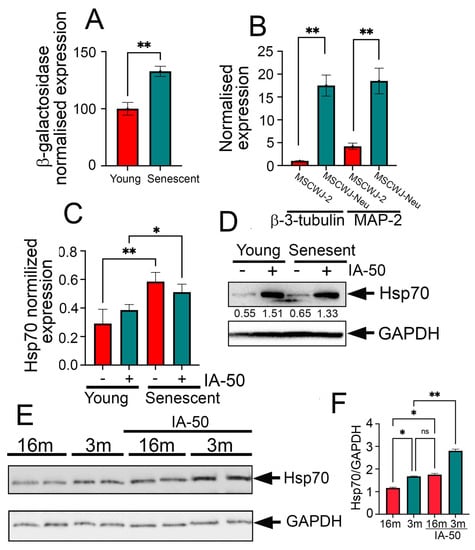
Figure 3.
IA-50 induces transcription of heat shock genes and causes the accumulation of heat shock proteins in senescent neurons and in the hippocampi of aged mice. (A) Data on differentiation of MSCWJ-2 cells into MSCWJ-Neu with a neuronal phenotype. Real-time PCR data are presented. Bars show the relative amount of RNA transcripts with MAP-2 or b-3-tubulin in MSCWJ-2 or MSCWJ-Neu cells. Data from three independent experiments are presented, * p < 0.05; ** p < 0.001. (B) b-galactosidase level in MSCWJ-Neu cells induced into a neuronal phenotype from MSCWJ-2 cells at “5th and 15th passage” (qPCR data). (C) An analysis of the Hsp70 expression in young and senescent MSCWJ-NEU cells after incubation with 1 µM IA-50 for 6 h. The results of RT-PCR. * p < 0.05; ** p < 0.001 (D) Western blotting of young and senescent MSCWJ-NEU cells incubated with 1 µM IA-50 for 24 h with anti-Hsp70 antibody. GAPDH was used as a loading control. (E) Western blot of the hippocampi of 3- or 16-month-old mice treated with IA-50 (10 µg/kg) 24 h after intraperitoneal injection. GAPDH was used as a loading control. The hippocampi of 2 mice were used for each point. (F) Band intensity was measured using StudioLite software (Li Cor, France). * p < 0.05; ** p < 0.001.
Using this model, we tested the ability of IA-50 to induce gene expression of the Hsp70 chaperone in MSCWJ-Neu cells. This was performed with the aid of RT-PCR, using mRNA isolated from young MSCWJ-Neu (5 passages) and aged MSCWJ-Neu (15 passages) cells cultured in normal conditions and after a 6-h treatment with IA-50 at a concentration of 1 µM. We found that the amount of mRNA of the Hsp70 protein gene increased 2-fold in young cells and 1.2-fold in senescent cells (Figure 3C).
Then, we used Western blotting to measure the Hsp70 concentration in young and aged MSCWJ-Neu cells and found that IA-50 was able to cause Hsp70 accumulation in both types of cells (Figure 3D). To estimate the Hsp70-inducing effect in brain tissue, we injected IA-50 (10 µg/kg) in 3- and 16-month-old mice, and 72 h later, the animals were sacrificed, the hippocampi were isolated and subjected to Western blotting. As revealed by Western blotting, IA-50 was able to cause Hsp70 accumulation in the hippocampi of both young and aged mice, suggesting that the substance is able to increase Hsp70 in vitro and in vivo to a similar extent in older and younger animals (Figure 3E).
2.4. The Treatment of 5xFAD Mice with IA-50 Improves Memory Defects and Aβ42 Aggregation
The hallmarks of major AD causalities are paralleled with brain ageing, and therefore, we tested the therapeutic activity of IA-50 using 5xFAD mice, a transgenic model of AD. At the P180 time point, when, according to the literature data, signs of AD can already be observed, such as amyloid deposits in the hippocampus [22,23], the animals were divided into four groups: (1) ‘untreated wt’ mice (n = 10); (2) wt mice treated with IA-50 (‘wt-IA-50’) (n = 10); (3) ‘untreated 5xFAD’ mice (n = 10); (4) 5xFAD mice treated with IA-50 (‘5xFAD-IA-50’) (n = 10). The drug was administered intramuscularly at a dose of 10 mg/kg once a week, and at P360, mice were subjected to different behavioural tests.
Anxiety is considered to be a sign of early AD which progresses throughout the AD pathogenesis due to brain damage and psychosocial factors [24,25]. The marble burying test was employed for the screening of new antidepressants, anxiolytics and antipsychotics [26,27], and the assay was recently found to reveal that 3xTg-AD and Tg-APP/PS1 mice were much less efficient in burying marbles than their healthy peers [28,29]; this prompted us to apply this test in our study. In these experiments, the marbles were placed on the sawdust of the cage bottom as depicted in Figure 4A and left each mouse with marbles for 30 min. Healthy wt mice successfully buried marbles irrespective of whether or not they were treated with IA-50; eight mice in the ‘untreated wt’ group were able to hide all the marbles and two buried only ¾ marbles. ‘Wt-IA-50’ demonstrated similar capacities: seven mice buried all the marbles and three only buried ¾. In the ‘untreated 5xFAD’ group, only three mice were able to hide ¾ of the marbles but treatment with IA-50 increased the mice’s capacity to bury marbles; two mice from this group buried all the marbles, and five hid ¾ of the marbles (Figure 4B).
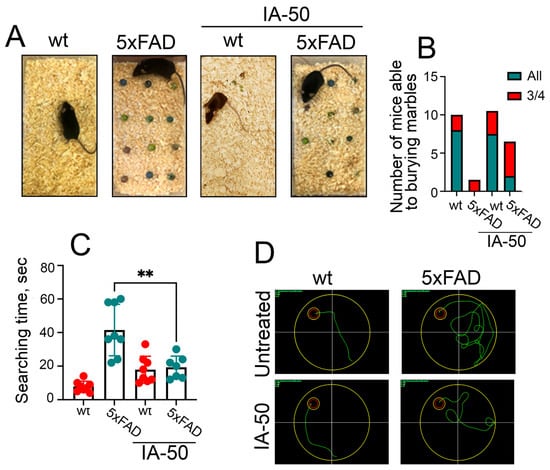
Figure 4.
Behavioural tests of the 5xFAD mice after therapy with IA-50. (A) Marble Burying Assay results in the 5xFAD mice treated with IA-50 at P360. An illustration of the Marble Burying Assay method. The left panel shows a healthy mouse that buried all the marbles; on the second panel a 5xFAD mouse presented that did not receive treatment; and the last two panels demonstrate wt mouse treated with IA-50 that also buried almost all marbles and 5xFAD mouse that received IA-50 treatment and buried several balls at a given time. (B) Test results. The blue bars show the number of animals that buried all the proposed balls, the red bars show the number of mice that buried ¾ of all the balls. (C) Results of the Morris water maze assay performed at P450. The time necessary to find the platform on the 6th day after the start of training is shown. ** p < 0.01. (D) Representative tracks of the wt and 5xFAD mice treated with IA-50. The tracks are in green; the platform itself is in red.
Another well-established behavioural assay, the Morris water maze test, was performed at time point P450 (15 months of age) when we expected a more pronounced effect of ageing and of Hsp70-inducing compounds. The 5xFAD mice that did not receive the IA-50 treatment searching time for the platform was 2.5-fold longer than that of their healthy siblings. The 5xFAD mice treated with IA-50 were able to find the platform 2-fold faster than untreated 5xFAD mice (Figure 4C). The tracks that the 5xFAD mice chose in search of the platform were significantly shorter than those of untreated 5xFAD mice, although their tracks were longer than those in their healthy siblings, independently as to whether or not they received the IA-50 treatment (Figure 4D).
The animals that passed the Morris maze test were sacrificed and their brains were divided into two parts, one was subjected to the histochemical study, and another was used for biochemical tests. We analyzed the frontal sections at the level of the CA1 field of the hippocampus and found that the number of neurons (according to staining for the NeuN marker) in the 5xFAD mice was 57% less than in the healthy ones. At the same time, the loss of neurons in the 5xFAD mice treated with IA-50 was significantly less—only 22% (Figure 5A,B). Finally, using the method of ultrafiltration followed by hybridization with anti-Aβ antibodies, we estimated the accumulation of Aβ42 aggregates in the hippocampus of animals related to different groups. We found a significant number of Aβ42 aggregates in the hippocampus of the 5xFAD mice, while there were no aggregates in the healthy mice. The use of IA-50 resulted in a decrease in the number of aggregates in transgenic animals by approximately 30% (Figure 5C,D).
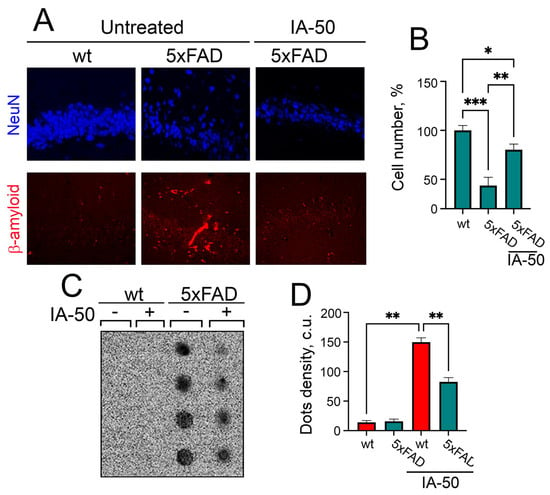
Figure 5.
Histoimmunochemical and biochemical analysis of the hippocampi of the 5xFAD mice after therapy with IA-50 and treated mice. (A) Histoimmunochemical analysis of the hippocampi of the 5xFAD mice after IA-50 therapy. Slices were stained with anti-NeuN antibodies (blue) or anti-Aβ1-42 antibodies (red). (B) The number of neuron nuclei stained with anti-NeuN antibodies was normalised to that in the hippocampus of untreated WT animals and presented as histogram bars. For each group, no less than 500 cells were counted. (C) Results of the Aβ1-42 aggregate amount analysis in the mouse hippocampus by ultrafiltration, followed by hybridisation with specific anti-Aβ1-42 antibodies. Representative image is shown. (D) The result of the quantification of dot intensity obtained from the analysis of the amount of Aβ1-42. The values are presented in conventional units. The mean values ± standard deviation of the results of three independent experiments are shown; differences are significant at * p < 0.05, ** p < 0.01 or *** p < 0.005.
3. Discussion
It is well established that the vast majority of human pathologies transform into more dangerous forms with age, partially due to an inability of numerous protein homeostasis systems to cope with the increasing number of damaged or mutant proteins [30]. The reduction of that capacity was suggested to be particularly negative for molecular chaperones and autophagy; chaperones are able to recognize pathogenic polypeptides through exposed hydrophobic surfaces and convert them into harmless forms or to assist proteolytic machinery to finally degrade the substrate [31]. However, the data on Hsp70 expression in young and aged cells and tissues are very contradictory: there are proofs of enhanced expression of Hsps in aged cells, probably due to their permanent exposure to stress [9,16] or reduced level of chaperones due to damages in transcription/translation machinery [32]. Our analysis of cell proteostasis actors in mice of various ages starting with 3-month-old mice and terminating with 24-month-old mice demonstrated that levels of Hsp70 chaperone and its co-chaperone Hsp40 (Hdj1 or DNAJB1), as well as Hsc70 which could function as a chaperone [33] and a main actor of CMA [34], and autophagy marker Atg5/12, significantly decrease at approximately 12 months of age and decline further over the next 12 months.
Previously, we analysed a few pyrrolyl- and indolylazine compounds and proved their ability to induce a heat shock response [18]. Here, we performed a bioinformatic search aimed at finding the substance with the maximum ability to penetrate living cells with a possibly modified lipid membrane due to ageing [35]. Such a compound, indolylazine IA-50, was able to induce Hsp70 chaperone synthesis in both young and aged reprogrammed human neurons MSCWJ-NEU.
Furthermore, administration of IA-50 was also found to induce Hsp70 expression in the hippocampi of mice, suggesting that the effect remained minimal one day after the IA-50 injection.
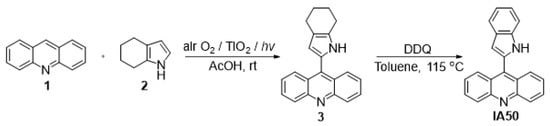
It should be noted that the development of new approaches to the synthesis of bio- and pharmaceutically active compounds has become widespread among organic chemists in order to improve traditional synthetic methods. In this regard, photocatalytic transformations are a topic of interest, for both scientists and practitioners, since it is an environmentally friendly and available alternative to chemical oxidizing and reducing agents [36,37]. An IA-50 derivative was prepared by the C–H/C–H coupling of acridine with 4,5,6,7-tetrahydro-1H-indole in acetic acid at room temperature, using an effective heterogeneous air oxygen/TiO2 oxidative system. All reactions were carried out in a quartz flask under irradiation with a Xe lamp (5000 K, 35 W) for 5 h, according to our previously developed methodology [38].
The treatment with IA-50 was started when the 5xFAD mice were six months old because it was shown to be possible to detect morphological symptoms of AD [39]. The therapy was carried out for the next 9 months, once a week, until the mice were 12 months old; according to Rae et al., this age corresponds to the upper limit of the average age (50–60 years) in humans [39]. The marble burying assay could testify to the elevated anxiety, which is considered to be a sign of early AD [24,25]. The 5xFAD mice were not able to bury marbles; whereas, their healthy siblings demonstrated successful burying efficacy independently of whether or not they were treated with IA-50. However, the results from the 5xFAD mice treated with IA-50 significantly improved in this test. Three months later, when the mice’s ages corresponded to old age in humans (~70–90 years in humans), mice passed through the Morris water maze test; the results of which predictably showed the preservation of spatial memory in the 5xFAD mice treated with IA-50. The data from the immunochemistry and staining with anti-NeuN and anti-amyloid antibodies demonstrated that the IA-50 treatment provided the preservation of the hippocampal neuron number in 5xFAD mice and a reduced number of b-amyloid aggregates. The ultrafiltration assay confirmed the results of the immunochemistry experiments.
To date, the list of Hsp70 inducers includes celastrol, arimoclomol [40], geranylgeranyl-acetone [41], and U-133 [42]; the compounds have undergone pre-clinical trials, and as far as we know, the latter has been subjected to clinical trials as the medicine for orphan lysosome storage disease [43]. We found that IA-50 is capable of coping with the most established attributes of AD pathogenesis and, therefore, believe that the compound, after improving its properties, including solubility and TPSA, may be considered as a candidate for pre-clinical trials.
Similar studies on pre-aged neurons have not been previously performed; nevertheless, it is noteworthy that, according to our data, the response of aged cells to the IA-50 compound was indeed less pronounced compared to the response of young cells. Certain limitations in the use of pyrrolyl- and indolylazine derivatives as neuroprotectors are currently seen in their low water solubility. For this reason, it is not possible to increase the working concentration of the compound, which, given the extremely low toxicity, could be useful. Probably, when conducting further searches for leader compounds from the classes of pyrrolyl- and indolylazines, it will be necessary to focus on compounds with low TPSA values (<40 A) and high water solubility values (Log S > −2).
4. Materials and Methods
4.1. In Silico Assays
The preliminary in silico assessment of pharmacokinetic properties for absorption, distribution, metabolism and excretion properties (ADME) was performed with the SwissADME tool developed by the Swiss Institute of Bioinformatics [44]. The computational analysis included predictions of molecular descriptors, such as solubility, topological polar surface area, gastrointestinal absorption and blood–brain barrier permeability. The SwissADME toolkit contains classification models, which focus on the tendency for a given small molecule to serve as an inhibitor of proteins governing important pharmacokinetic behaviours. Drug-likeness was assessed under Lipinski’s rule of five, which is based on descriptors such as molecular weight, number of hydrogen bond acceptors, number of hydrogen bond donors and octanol/water partition coefficient.
4.2. Synthesis of 9-(1H-indol-2-yl)acridine (IA-50)
The starting 4,5,6,7-tetrahydroindole is a compound available on an industrial scale and can be easily obtained by the reaction of cyclohexanone oxime with acetylene in the superbasic catalytic system KOH/DMSO [45].
A quartz flask containing a solution of acridine 1 (1 mmol), 4,5,6,7-tetrahydro-1H-indole 2 (2 mmol) in acetic acid (20 mL) and a TiO2 catalyst (10 wt.%) was sonicated for 5 min to obtain a suspension. The resulting mixture was irradiated with a Xe lamp (5000 K, 35 W) under air bubbling at room temperature (20 °C). The reaction was completed after 5 h. The reaction mass was concentrated under reduced pressure. The resulting residue was subjected to preparative SiO2 column chromatography. A mixture of hexane:ethyl acetate (8:2) was used as the eluent. The resulting compound of 3 (9-(4,5,6,7-tetrahydro-1H-indol-2-yl)acridine) and DDQ was dissolved in toluene (30 mL) and boiled for 8 h. The solution was concentrated under reduced pressure. The resulting residue was subjected to preparative SiO2 column chromatography (benzene) (Scheme 1).
Scheme 1.
Synthesis of IA-50. Reagents and conditions: (i) CF3COOH/PhH (1/2), rt, 10 h; (ii) BF3 Et2O, CH3CN, rt, 10 h; (iii) air oxygen/TiO2, NaOH, EtOH, rt, 5 h; (iv) air oxygen/TiO2, NaHCO3, MeOH, rt, 5 h. Irradiation was carried out by a cold white lamp (6200 K, 18 W).
9-(4,5,6,7-tetrahydro-1H-indol-2-yl)acridine (3). Yield: 149 mg (50%); yellow solid; Rf = 0.1 (TLC: hexane:ethyl acetate, 8:2). 1H NMR (400 MHz, DMSO-d6): δ = 11.12 (s, N’-H), 8.21–8.23 (d, J = 8.00 Hz, 1-H, 8-H), 8.14–8.16 (d, J = 8.00 Hz, 4-H, 5-H), 7.81–7.84 (m, 3-H, 6-H), 7.55–7.59 (t, J = 8.00 Hz, 2-H, 7-H), 6.26–6.27 (m, 3′-H), 2.69–2.72 (m, 4′-H), 2.61–2.64 (m, 7′-H), 1.81–1.86 (m, 5′-H, 6′-H). 13C NMR (100 MHz, DMSO-d6): δ = 148.39 (C4a, C9a), 139.56 (C9), 129.97 (C3, C6), 129.06 (C1, C8), 127.36 (C4, C5), 125.54 (C2, C7), 124.66 (C8a, C10a), 121.84 (C2′), 117.31 (C3a’, C7a’), 112.03 (C3′), 23.50 (C4), 23.04 (C5), 22.56 (C6), 22.05 (C7). MS (EI): m/z 298 [M]+. Anal. Calcd for C21H18N2 (%): C, 84.82; H, 5.76; N, 9.42. Found (%): C, 84.76; H, 5.83; N, 9.71.
9-(1H-indol-2-yl)acridine (IA-50). Yield: 102 mg (35%); yellow solid; Rf = 0.1 (TLC: benzene); mp 292 °C. 1H NMR (400 MHz, DMSO-d6): δ = 11.84 (1 H, NH), 8.25 (d, J = 8.6 Hz, 2H, 3-H, 6-H), 7.99 (d, J = 8.6 Hz, 2H, 1-H, 8-H), 7.89 (t, J = 7.3 Hz, 2H, 2-H, 7-H), 7.74 (d, J = 7.7 Hz, 1H, 4′-H), 7.60 (t, J = 7.3 Hz, 2H, 4-H, 5-H), 7.52 (d, J = 7.8 Hz, 1H, 6′-H), 7.24 (d, J = 7.8 Hz, 1H, 5′-H), 7.16 (t, J = 7.0 Hz, 1H, 7′-H), 6.88 (s, 1H, 3′-H). 13C NMR (100 MHz, DMSO-d6): δ = 148.75 (C8a, C9a), 138.89 (C9), 137.48 (C3a’), 131.37 (C2′), 130.80 (C2, C7), 129.82 (C3, C6), 128.51 (C7a’), 127.14 (C1, C8), 126.95 (C4, C5), 125.61 (C4a, C10a), 122.48 (C5′), 120.82 (C4′), 120.12 (C7′), 111.99 (C6′), 106.02 (C3′).
NMR, NSQC and HMBC spectrums of IA-50 are presented in Supplementary Materials Figures S1–S8.
4.3. Mimicking the Neuronal Cell Phenotype
For further verification of the chaperone-inducing and neuroprotective properties of PLAs, we used mesenchymal stem cells from Wharton’s jelly of human umbilical cord (MSCWJ-2) cells that was described earlier [46]. The cells were received from the shared research facility “Vertebrate cell culture collection” supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement NO. 075-15-2021-683). Cells were cultured in DMEM/F12 medium (Gibco, Paisley, UK), containing 10% fetal bovine serum (FBS; Gibco, Paisley, UK), 100 units/mL penicillin and 0.1 mg/mL streptomycin (BioloT, St.Petersburg, Russia) at 37 °C and 5% CO2. We reprogrammed these cells into a neuronal phenotype for 5 days in a Neurobasal medium (Gibco, Paisley, UK), containing B27 supplement (Gibco, Paisley, UK), 3% FBS, 100 units/mL penicillin and 0.1 mg/mL streptomycin. Verification of the neuronal phenotype was carried out based on the analysis of the expression of neuronal markers MAP-2 and β-3-tubulin, using a real-time polymerase chain reaction. The cells were named MSCWJ-NEU.
4.4. RNA Isolation and Real-Time PCR
RNA was isolated using TRIzol (Thermo Fisher Scientific, Waltham, MA, USA) and reverse transcribed, using the MMLV RT kit (Evrogen JSC, Moscow, Russia) according to the manufacturer’s instructions. All RT-PCR reactions were performed with a CFX96 Real-Time PCR detection system (BioRad, Hercules, CA, USA), using qPCRmix-HS SYBR (Evrogen JSC, Moscow, Russia) and according to the manufacturer’s protocol. Amplicon authenticity was confirmed by melt curve analysis. The sequence of primers is represented in Table 2.

Table 2.
Sequences of primers used in the study.
GAPDH was used as the normalisation control. All primers were obtained from Evrogen JSC (Moscow, Russia). The parameters of the polymerase chain reaction (PCR) were 5 min of pre-denaturation at 95 °C, followed by 40 cycles of 30 s at 95 °C, 30 s at 65 °C and 30 s at 70 °C. The data were analysed for fold changes (ΔΔCt) using BioRad CFX software.
4.5. Electrophoresis and Immunoblotting
MSCWJ-NEU cells were incubated with IA-50 at a concentration of 1 µM for 24 h. Cells were then lysed by three freeze–thaw cycles in a low RIPA buffer containing 20 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.05% Tween-20, 0.1% SDS, 3 mM EDTA and 1 mM PMSF. To obtain lysates of hippocampal tissues, the mice were sacrificed, and the hippocampus was immediately removed and lysed in a low RIPA buffer. Lysates of MSCWJ-NEU cells or hippocampal tissues were subjected to electrophoresis, following Western. The membrane was subsequently incubated with antibodies against Hsp70, clone 3B5 [47] and glyceraldehyde-3-phosphate dehydrogenase, taken as a loading control (GAPDH, Clone 6C5, Abcam, Cambridge, UK).
4.6. Senescence Analysis
To measure β-galactosidase activity in cells, X-Gal staining solution (Thermo Fisher Scientific, Waltham, MA, USA) was added to the cell culture, according to the manufacturer’s protocol. After 72 h, microscopic photos were collected and analyzed, using ImageJ software, in order to measure the colorimetric staining intensity.
4.7. Transgenic Mice Model of AD
The 5xFAD transgenic mice were genotyped by PCR analysis of DNA extracted from the ear biopsies. The transgenic cassette was detected, using primers 5′-AGGACTGACCACTCGACCAG-3′ and 5′-CGGGGGTCTAGTTCTGCAT-3′, yielding a 377 bp product. Siblings of 5xFAD mice were used as a wild-type (WT); this protocol was described by Peters et al. [48].
WT and 5xFAD male mice at P180 were divided into 4 groups of 10 animals each as follows: (1) WT, untreated; (2) WT, treated with IA-50; (3) 5xFAD, untreated; (4) 5xFAD, treated with IA-50. Treatment involved intraperitoneal injections of IA-50 once a week at a dosage of 1 mg/kg within an additional 180 days.
4.8. Animal Behaviour Tests
All in vivo experiments were carried out following the requirements of the Institute of Cytology of the Russian Academy of Sciences ethics committee (Identification number F18-00380).
5xFAD mice were purchased in the Center of Animal Models in the Institute of Physiologically Active Compounds of Russian Academy of Sciences (Chernogolovka, Moscow Region, Russia). The model is maintained by backcrossing transgenic animals to a B6SJLF1 hybrid at every generation.
4.8.1. Marble Burying Assay
The procedures were performed as described earlier [49] with modification. Briefly, a plastic cage (40 cm × 20 cm × 17 cm) was filled with sawdust up to 5 cm deep, and 12 marbles were placed on top of the sawdust, 3 marbles in four rows (see Figure 3A). Each mouse was placed in the cage and was left with the marbles for 30 min; then, the mouse was removed and the number of buried marbles was counted.
4.8.2. Morris Water Test
Spatial memory impairment in 5xFAD mice from each experimental group (n = 10) was evaluated, using a Morris water maze test [50] of diameter 1.5 m (OpenScience, Krasnogorsk, Russia) on P450. Mice were trained to find the platform for 5 days. Daily training included 4 attempts with an interval of 30 s. During these attempts, the animal was sequentially placed in different sectors (the sequence of sectors was determined randomly). If the mouse did not find the platform, it was forcibly placed on it. In any case, the animal was left on the platform for 15 s. Animal movements were recorded for a minute with a digital video camera, which was located at a height of 450 cm from the floor and connected to a computer via a USB interface. On the 6th day, spatial memory was tested. The mice had one attempt to find the platform. The animal was placed in the pool at the farthest sector from the target sector.
The MWMtrack-9 software was used to build motion tracks and evaluate memory. When evaluating the spatial memory of the mice, the latent release time (s), during which the mouse found the platform and climbed onto it, was calculated. If the animal did not find the platform, then the value of the latent time was taken to be equal to 60 s. The effectiveness of training was assessed by reducing the time spent on the platform and the reduction of movement tracks. The swimming trajectories of animals in experiments were treated according to EthoVision XT14.0 [51].
4.9. Immunohistochemistry
At the end of the Morris water test, the mice were anaesthetized with Zoletil-100 (50 mg/kg, intraperitoneal), perfused with 4% paraformaldehyde and then decapitated. The brain was extracted and examined by confocal microscopy. Brains from all animals used for immunohistochemical assays were fixed in 4% paraformaldehyde and cryoprotected in 20% sucrose before storage in isopentane at −70 °C. Coronal sections (8 μm) were prepared for morphological and immunohistochemical assay with an OTF 6000 cryostat (Bright Instruments, Huntingdon, UK). The frontal slices were collected at the level of the field CA1 of the hippocampus, according to The Mouse Brain Library service (mbl.org, Tennessee, USA). Six alternate series of sections were mounted on SuperFrost Plus slides (Menzel GmbH, Bielefeld, Germany).
For confocal microscopy, sections were pre-incubated in blocking solution (2% bovine serum albumin diluted in PBS with 0.1% Tween-20) for 1 h at room temperature and then hybridized with anti-NeuN (Abcam, Cambridge, UK) or anti-Aβ1-42 antibodies (Elabscience, Houston, TX, USA), following hybridization with secondary fluorescently labelled antibodies (ThermoFisher Scientific, Waltham, MA, USA). Fluorescent images were captured by an Olympus confocal system FV3000 (Olympus, Tokyo, Japan).
4.10. Aggregation Assay
To analyse the amount of aggregates containing Aβ42 in the mouse hippocampus, we used the ultrafiltration method (filter trap assay) previously described [52]. Mouse hippocampal lysates were dissolved in buffer (10 mM Tris-HCl pH 8.0, 150 mM NaCl, 2% sodium dodecyl sulfate) in the amount of 200 µg of total protein, applied to a cellulose acetate membrane, and placed in an ultrafiltration manifold attached to a vacuum pump (BIO-RAD, Hercules, CA, USA). Before and after applying the lysates, the membrane was washed under pressure with a buffer of the following composition: 10 mM Tris-HCl pH 8.0, 150 mM NaCl and 0.1% sodium dodecyl sulfate. The presence of Aβ1-42 in the aggregates was determined using specific antibodies, MAA946Ge21 (Elabscience, Houston, TX, USA), followed by hybridisation with secondary antibodies and labelled with peroxidase (1:10,000; Jackson Laboratory, Bar Harbor, ME, USA). Using TotalLabQuant 1.0 software (TotalLab, Gosforth, UK), we obtained the dot intensity value in conventional units, and then normalized the data to the mean Aβ42 staining intensity in the hippocampus of naive rats.
4.11. Statistical Analysis
All data were expressed as mean ± standard deviation. Data were compared using a non-parametric Mann–Whitney test, using GraphPad Prism 8 software. All experiments, except for animal studies, were repeated at least three times. The statistical difference was determined by p < 0.05.
5. Conclusions
In this work, we synthesised and tested a new compound from the class of indolylazines, IA-50, capable of activating the synthesis and inducing the accumulation of Hsp70 chaperone, both in the culture of aged human neurons and in the hippocampus of old mice, for the first time. The new compound demonstrated therapeutic potential in a transgenic model of AD in the 5xFAD mice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27248950/s1, Figure S1: 1H NMR spectrum for 9-(4,5,6,7-tetrahydro-1H-indol-2-yl)acridine (3); Figure S2: 13C NMR spectrum for 9-(4,5,6,7-tetrahydro-1H-indol-2-yl)acridine (3); Figure S3: Two-dimensional 1H-13C HSQC spectrum for 9-(4,5,6,7-tetrahydro-1H-indol-2-yl)acridine (3); Figure S4: Two-dimensional 1H-13C HMBC spectrum for 9-(4,5,6,7-tetrahydro-1H-indol-2-yl)acridine (3); Figure S5: 1H NMR spectrum for 9-(1H-indol-2-yl)acridine (IA-50); Figure S6: 13C NMR spectrum for 9-(1H-indol-2-yl)acridine (IA-50); Figure S7: Two-dimensional 1H-13C HSQC spectrum for 9-(1H-indol-2-yl)acridine (IA-50); Figure S8: Two-dimensional 1H-13C HMBC spectrum for 9-(1H-indol-2-yl)acridine (IA-50).
Author Contributions
Conceptualization, I.V.G. and V.F.L.; methodology, E.A.D., M.A.T. and I.A.U.; software, E.A.D. and I.A.U.; validation, V.F.L. and I.V.G.; formal analysis, V.F.L.; investigation, E.R.M., E.A.D. and M.A.T.; resources, O.N.C.; data curation, I.V.G. and I.A.U.; writing—original draft preparation, V.F.L., B.A.M. and I.V.G.; writing—review and editing, I.V.G.; supervision I.V.G.; project administration, I.V.G.; funding acquisition, I.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Ministry of Science and Higher Education of Russia, Research Project N 075-15-2020-795, local identifier 13.1902.21.0027.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of the Institute of Cytology of the Russian Academy of Sciences (protocol N 14, 8 November 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors cordially thank Alexander Anisimov (present address: Oslo University, Norway) for the help with in silico prediction analysis and Tatiana Vonts for assistance with illustartion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shepardson, N.; Yang, T.; Chen, G.; Walsh, D.; Selkoe, D.J. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA 2011, 108, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Cascella, R.; Bigi, A.; Cremades, N.; Cecchi, C. Effects of oligomer toxicity, fibril toxicity and fibril spreading in synucleinopathies. Cell Mol. Life Sci. 2022, 79, 174. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Mahanta, S. Association of heat-shock proteins in various neurodegenerative disorders: Is it a master key to open the therapeutic door? Mol. Cell Biochem. 2014, 386, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.F.; Mikhaylova, E.R.; Guzhova, I.V.; Margulis, B.A. Possible Function of Molecular Chaperones in Diseases Caused by Propagating Amyloid Aggregates. Front Neurosci. 2017, 11, 277. [Google Scholar] [CrossRef]
- Lackie, R.E.; Maciejewski, A.; Ostapchenko, V.G.; Marques-Lopes, J.; Choy, W.Y.; Duennwald, M.L.; Prado, V.F.; Prado, M.A.M. The Hsp70/Hsp90 Chaperone Machinery in Neurodegenerative Diseases. Front Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef]
- Shelton, L.B.; Koren, J., 3rd; Blair, L.J. Imbalances in the Hsp90 Chaperone Machinery: Implications for Tauopathies. Front Neurosci 2017, 11, 724. [Google Scholar] [CrossRef]
- Neef, D.W.; Jaeger, A.M.; Thiele, D.J. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat. Rev. Drug Discov. 2011, 10, 930–944. [Google Scholar] [CrossRef]
- Margulis, B.; Tsimokha, A.; Zubova, S.; Guzhova, I. Molecular Chaperones and Proteolytic Machineries Regulate Protein Homeostasis In Aging Cells. Cells 2020, 9, 1308. [Google Scholar] [CrossRef]
- Blake, M.J.; Fargnoli, J.; Gershon, D.; Holbrook, N.J. Concomitant decline in heat-induced hyperthermia and HSP70 mRNA expression in aged rats. Am. J. Physiol. 1991, 260, R663–R667. [Google Scholar] [CrossRef]
- Faassen, A.E.; O’Leary, J.J.; Rodysill, K.J.; Bergh, N.; Hallgren, H.M. Diminished heat-shock protein synthesis following mitogen stimulation of lymphocytes from aged donors. Exp. Cell Res. 1989, 183, 326–334. [Google Scholar] [CrossRef]
- Heydari, A.R.; Wu, B.; Takahashi, R.; Strong, R.; Richardson, A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol. Cell Biol. 1993, 13, 2909–2918. [Google Scholar]
- Pahlavani, M.A.; Harris, M.D.; Moore, S.A.; Weindruch, R.; Richardson, A. The expression of heat shock protein 70 decreases with age in lymphocytes from rats and rhesus monkeys. Exp. Cell Res. 1995, 218, 310–318. [Google Scholar] [CrossRef]
- Rea, I.M.; McNerlan, S.; Pockley, A.G. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp. Gerontol. 2001, 36, 341–352. [Google Scholar] [CrossRef]
- Jin, X.; Wang, R.; Xiao, C.; Cheng, L.; Wang, F.; Yang, L.; Feng, T.; Chen, M.; Chen, S.; Fu, X.; et al. Serum and lymphocyte levels of heat shock protein 70 in aging: A study in the normal Chinese population. Cell Stress Chaperones 2004, 9, 69–75. [Google Scholar] [CrossRef]
- Calabrese, V.; Butterfield, D.A.; Scapagnini, G.; Stella, A.M.; Maines, M.D. Redox regulation of heat shock protein expression by signaling involving nitric oxide and carbon monoxide: Relevance to brain aging, neurodegenerative disorders, and longevity. Antioxid. Redox Signal. 2006, 8, 444–477. [Google Scholar] [CrossRef]
- Gleixner, A.M.; Pulugulla, S.H.; Pant, D.B.; Posimo, J.M.; Crum, T.S.; Leak, R.K. Impact of aging on heat shock protein expression in the substantia nigra and striatum of the female rat. Cell Tissue Res 2014, 357, 43–54. [Google Scholar] [CrossRef]
- Dutysheva, E.A.; Utepova, I.A.; Trestsova, M.A.; Anisimov, A.S.; Charushin, V.N.; Chupakhin, O.N.; Margulis, B.A.; Guzhova, I.V.; Lazarev, V.F. Synthesis and approbation of new neuroprotective chemicals of pyrrolyl- and indolylazine classes in a cell model of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 222, 113577. [Google Scholar] [CrossRef]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef]
- Das, U.N. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 2021, 11, 241. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Ohno, M. Phospho-eIF2α level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice. PLoS ONE 2010, 5, e12974. [Google Scholar] [CrossRef]
- Tanaka, H.; Hashimoto, M.; Fukuhara, R.; Ishikawa, T.; Yatabe, Y.; Kaneda, K.; Yuuki, S.; Honda, K.; Matsuzaki, S.; Tsuyuguchi, A.; et al. Relationship between dementia severity and behavioural and psychological symptoms in early-onset Alzheimer’s disease. Psychogeriatrics 2015, 5, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Baillon, S.; Gasper, A.; Wilson-Morkeh, F.; Pritchard, M.; Jesu, A.; Velayudhan, L. Prevalence and Severity of Neuropsychiatric Symptoms in Early- Versus Late-Onset Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen 2019, 34, 433–438. [Google Scholar] [CrossRef]
- Njung’e, K.; Handley, S.L. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav. 1991, 38, 63–67. [Google Scholar] [CrossRef]
- Kaurav, B.P.; Wanjari, M.M.; Chandekar, A.; Chauhan, N.S.; Upmanyu, N. Influence of Withania somnifera on obsessive compulsive disorder in mice. Asian. Pac. J. Trop. Med. 2012, 5, 380–384. [Google Scholar] [CrossRef]
- Torres-Lista, V.; López-Pousa, S.; Giménez-Llort, L. Impact of Chronic Risperidone Use on Behavior and Survival of 3xTg-AD Mice Model of Alzheimer’s Disease and Mice With Normal Aging. Front. Pharmacol. 2019, 10, 1061. [Google Scholar] [CrossRef]
- Kim, T.K.; Han, H.E.; Kim, H.; Lee, J.E.; Choi, D.; Park, W.J.; Han, P.L. Expression of the plant viral protease NIa in the brain of a mouse model of Alzheimer’s disease mitigates Aβ pathology and improves cognitive function. Exp. Mol. Med. 2012, 44, 740–748. [Google Scholar] [CrossRef]
- Balchin, D.; Hayer-Hartl, M.; Hartl, F.U. In vivo aspects of protein folding and quality control. Science 2016, 353, aac4354. [Google Scholar] [CrossRef]
- Ciechanover, A.; Kwon, Y.T. Protein Quality Control by Molecular Chaperones in Neurodegeneration. Front. Neurosci. 2017, 11, 185. [Google Scholar] [CrossRef]
- Chung, L.; Ng, Y.C. Age-related alterations in expression of apoptosis regulatory proteins and heat shock proteins in rat skeletal muscle. Biochim. Biophys. Acta 2006, 1762, 103–109. [Google Scholar] [CrossRef]
- Hubert, V.; Weiss, S.; Rees, A.J.; Kain, R. Modulating Chaperone-Mediated Autophagy and Its Clinical Applications in Cancer. Cells 2022, 11, 2562. [Google Scholar] [CrossRef]
- Catarino, S.; Pereira, P.; Girão, H. Molecular control of chaperone-mediated autophagy. Essays Biochem. 2017, 61, 663–674. [Google Scholar]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Bagdi, A.K.; Rahman, M.; Bhattacherjee, D.; Zyryanov, G.V.; Ghosh, S.; Chupakhin, O.N.; Hajra, A. Visible light promoted cross-dehydrogenative coupling: A decade update. Green Chem. 2020, 22, 6632–6681. [Google Scholar] [CrossRef]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Utepova, I.A.; Chupakhin, O.N.; Trestsova, M.A.; Musikhina, A.A.; Kucheryavaya, D.A.; Charushin, V.N.; Rempel, A.A.; Kozhevnikova, N.S.; Valeeva, A.A.; Mikhaleva, A.I.; et al. Direct functionalization of the C-H bond in (hetero)arenes: Aerobic photoinduced oxidative coupling of azines with aromatic nucleophiles (SNH-reactions) in the presence of a CdS/TiO2 photocatalyst. Russ. Chem. Bull 2016, 65, 445–450. [Google Scholar] [CrossRef]
- Rae, E.A.; Brown, R.E. The problem of genotype and sex differences in life expectancy in transgenic AD mice. Neurosci. Biobehav. Rev. 2015, 57, 238–251. [Google Scholar] [CrossRef]
- Kalmar, B.; Greensmith, L. Activation of the heat shock response in a primary cellular model of motoneuron neurodegeneration-evidence for neuroprotective and neurotoxic effects. Cell Mol. Biol. Lett. 2009, 14, 319–335. [Google Scholar] [CrossRef]
- Sugano, E.; Endo, Y.; Sugai, A.; Kikuchi, Y.; Tabata, K.; Ozaki, T.; Kurose, T.; Takai, Y.; Mitsuguchi, Y.; Honma, Y.; et al. Geranylgeranyl acetone prevents glutamate-induced cell death in HT-22 cells by increasing mitochondrial membrane potential. Eur. J. Pharmacol. 2020, 883, 173193. [Google Scholar] [CrossRef] [PubMed]
- Ekimova, I.V.; Plaksina, D.V.; Pastukhov, Y.F.; Lapshina, K.V.; Lazarev, V.F.; Mikhaylova, E.R.; Polonik, S.G.; Pani, B.; Margulis, B.A.; Guzhova, I.V.; et al. New HSF1 inducer as a therapeutic agent in a rodent model of Parkinson’s disease. Exp. Neurol. 2018, 306, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, T.; Roth, A.G.; Petersen, N.H.; Mahalka, A.K.; Olsen, O.D.; Moilanen, I.; Zylicz, A.; Knudsen, J.; Sandhoff, K.; Arenz, C.; et al. Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 2010, 463, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Mikhaleva, A.I.; Schmidt, E.Y.; Vasil’tsov, A.M.; Ivanov, A.V.; Protsuk, N.I.; Ryapolov, O.A. A new technology for the synthesis of 4,5,6,7-tetrahydroindole. Dokl. Chem. 2010, 435, 307–310. [Google Scholar] [CrossRef]
- Koltsova, A.M.; Krylova, T.A.; Musorina, A.S.; Zenin, V.V.; Turilova, V.I.; Yakovleva, T.R.; Poljanskaya, G.G. The Dynamics of Cell Properties during Long-Term Cultivation of Two Lines of Mesenchymal Stem Cells Derived from Wharton’s Jelly of Human Umbilical Cord. Cell Tissue Biol. 2018, 121, 7–19. [Google Scholar] [CrossRef]
- Guzhova, I.; Kislyakova, K.; Moskaliova, O.; Fridlanskaya, I.; Tytell, M.; Cheetham, M.; Margulis, B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001, 914, 66–73. [Google Scholar] [CrossRef]
- Peters, O.M.; Shelkovnikova, T.; Tarasova, T.; Springe, S.; Kukharsky, M.S.; Smith, G.A.; Brooks, S.; Kozin, S.A.; Kotelevtsev, Y.; Bachurin, S.O.; et al. Chronic administration of Dimebon does not ameliorate amyloid-β pathology in 5xFAD transgenic mice. J. Alzheimer’s Dis. 2013, 36, 589–596. [Google Scholar] [CrossRef]
- Broekkamp, C.L.; Rijk, H.W.; Joly-Gelouin, D.; Lloyd, K.L. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur. J. Pharmacol. 1986, 126, 223–229. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Sankowski, R.; Huerta, T.S.; Kalra, R.; Klein, T.J.; Strohl, J.J.; Al-Abed, Y.; Robbiati, S.; Huerta, P.T. Large-Scale Validation of the Paddling Pool Task in the Clockmaze for Studying Hippocampus-Based Spatial Cognition in Mice. Front. Behav. Neurosci. 2019, 13, 121. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Dutysheva, E.A.; Komarova, E.Y.; Mikhaylova, E.R.; Guzhova, I.V.; Margulis, B.A. GAPDH-targeted therapy—A new approach for secondary damage after traumatic brain injury on rats. Biochem. Biophys. Res. Commun. 2018, 501, 1003–1008. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).