Chemical Composition and Antioxidant and Antibacterial Potencies of the Artemisia ordosica Aerial Parts Essential Oil during the Vegetative Period
Abstract
1. Introduction
2. Results
2.1. Yield of Essential Oil and Chemical Composition
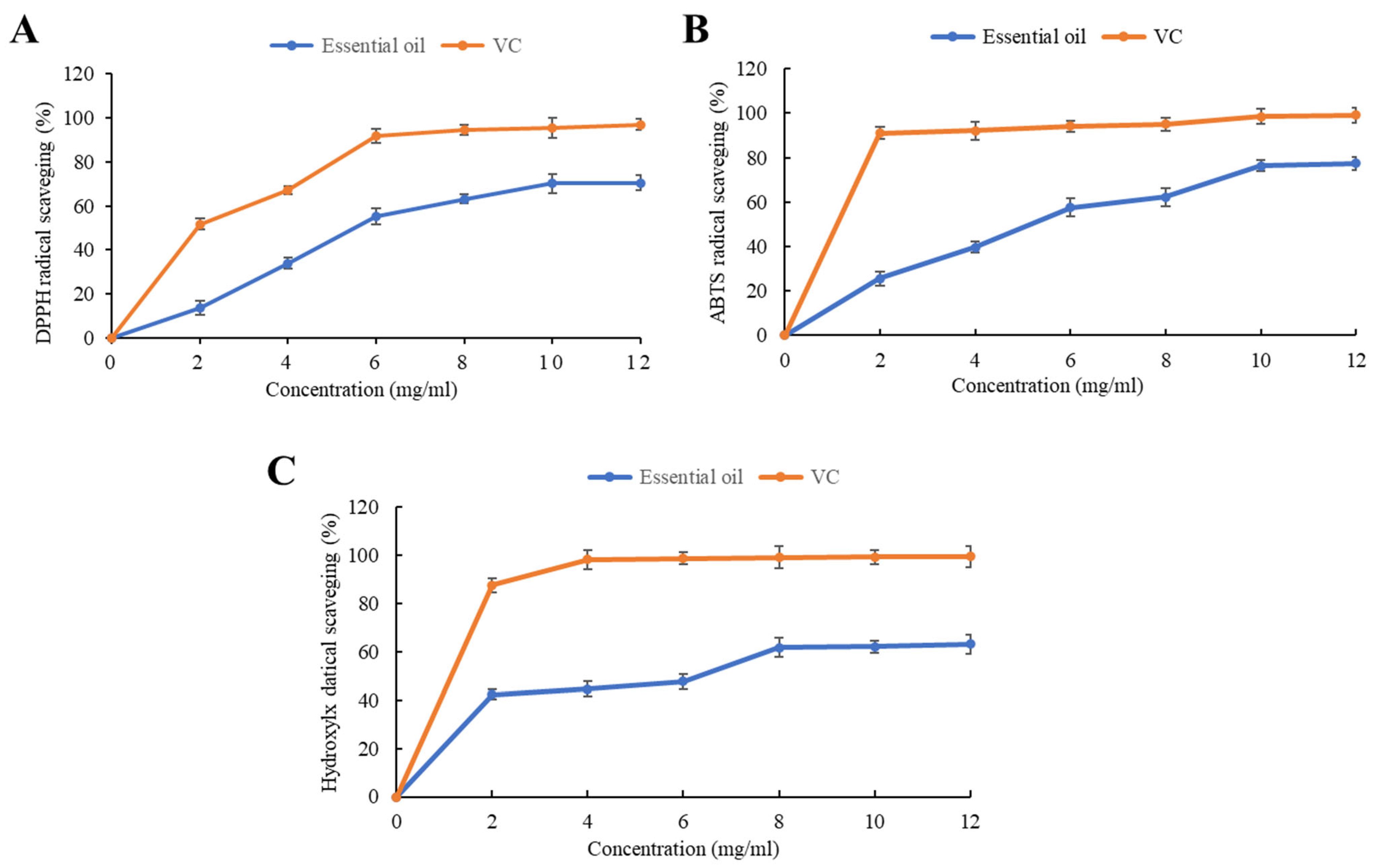
2.2. Antioxidant Activity
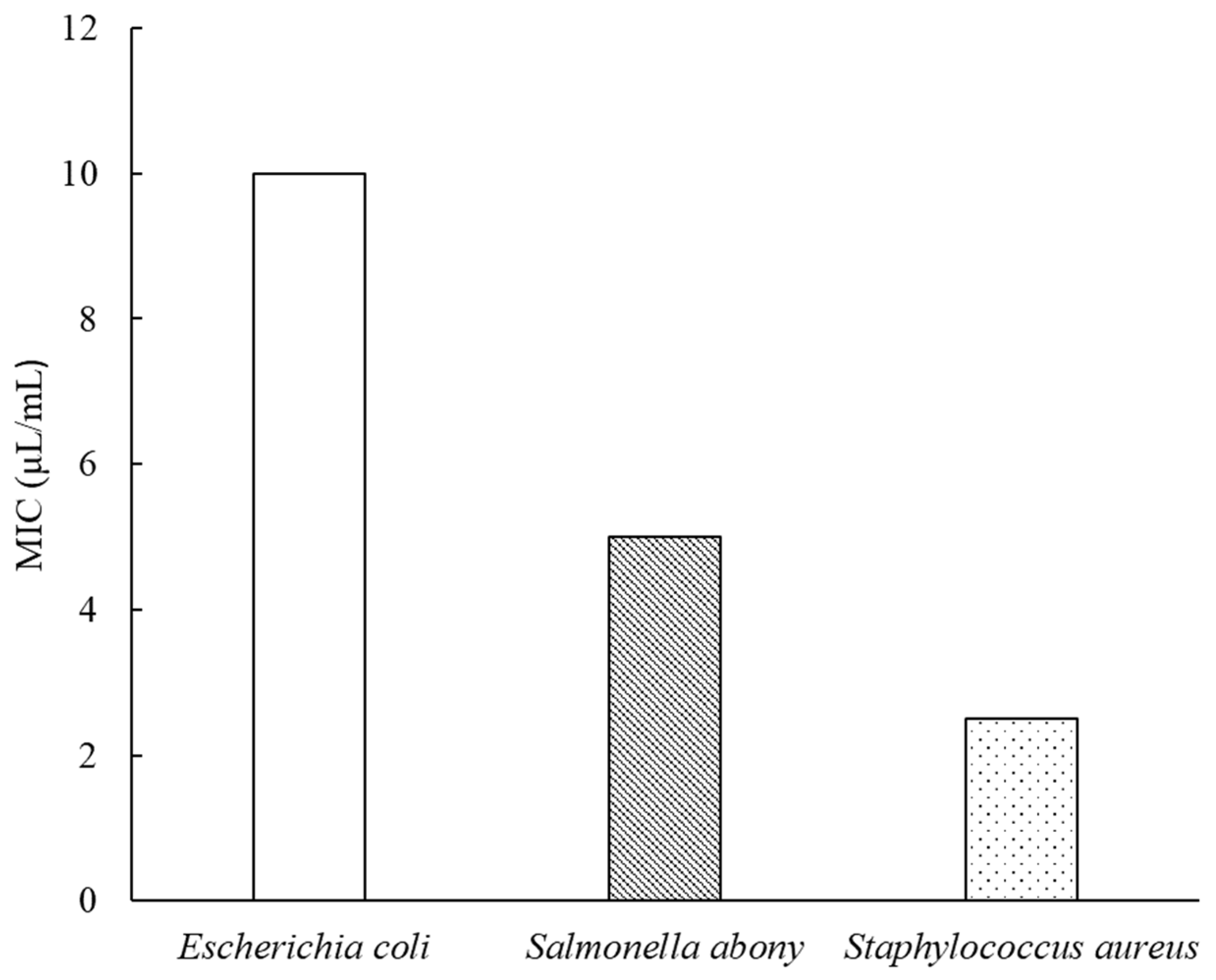
2.3. Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Essential Oil Extraction
4.2. Chemical Composition Analysis of the Essential Oil
4.3. Antioxidant Activity Assay
4.3.1. DPPH Assay
4.3.2. ABTS Assay
4.3.3. OH● Scavenging Activity
4.4. Evaluation of Antibacterial Activity
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bora, K.S.; Sharma, A. The genus Artemisia: A comprehensive review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; Sharifi-Rad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1–17. [Google Scholar]
- Zhao, D.B.; Yang, Y.X.; Zhang, W.; Liu, X.H.; Wang, H.Q. Studies on flavonoids from herb of Artemisia ordosica. Chin. J. Chin. Mater. Med. 2005, 30, 1430–1432. [Google Scholar]
- Zhang, Z.; Guo, S.; Zhang, W.; Geng, Z.; Liang, J.; Du, S.; Wang, C.; Deng, Z. Essential oil and polyacetylenes from Artemisia ordosica and their bioactivities against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Ind. Crops Prod. 2017, 100, 132–137. [Google Scholar] [CrossRef]
- Yang, X.; Deng, S.; De Philippis, R.; Chen, L.; Hu, C.; Zhang, W. Chemical composition of volatile oil from Artemisia ordosica and its allelopathic effects on desert soil microalgae, Palmellococcus miniatus. Plant Physiol. Biochem. 2012, 51, 153–158. [Google Scholar] [CrossRef]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Farhadi, N.; Babaei, K.; Farsaraei, S.; Moghaddam, M.H.; Ghasemi Pirbalouti, A. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crops Prod. 2020, 152, 112570. [Google Scholar] [CrossRef]
- Aminkhani, A.; Sharifi, R.; Dorosti, R. Chemical composition and antimicrobial activity of Achillea tenuifolia Lam. essential oil at different phenological stages from Khoy. Chem. Biodivers. 2019, 16, e1900289. [Google Scholar] [CrossRef]
- Béjaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum Desf. at different phenological stages. J. Med. Food 2013, 16, 1115–1520. [Google Scholar]
- Papageorgiou, V.; Gardeli, C.; Mallouchos, A.; Papaioannou, M.; Komaitis, M. Variation of the chemical profile and antioxidant behavior of Rosmarinus officinalis L. and Salvia fruticosa Miller grown in Greece. J. Agric. Food Chem. 2008, 56, 7254–7264. [Google Scholar] [CrossRef]
- Singh, H.P.; Mittal, S.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chemical composition and antioxidant activity of essential oil from residues of Artemisia scoparia. Food Chem. 2009, 114, 642–645. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Firoznezhad, M.; Craker, L.E.; Akbarzadeh, M.J. Essential oil compositions, antibacterial and antioxidant activities of various populations of Artemisia chamaemelifolia at two phenological stages. Rev. Bras. Farmacogn. 2013, 23, 861–869. [Google Scholar] [CrossRef]
- Akrout, A.; Gonzalez, L.A.; El Jani, H.; Madrid, P.C. Antioxidant and antitumor activities of Artemisia campestris and Thymelaea hirsuta from southern Tunisia. Food Chem. Toxicol. 2011, 49, 342–347. [Google Scholar] [CrossRef]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef]
- Malti, C.E.; El Haci, I.A.; Hassani, F.; Paoli, M.; Gibernau, M.; Tomi, F.; Casanova, J.; Bekhechi, C. Composition, chemical variability and biological activity of Cymbopogon schoenanthus essential oil from central Algeria. Chem. Biodivers. 2020, 17, e2000138. [Google Scholar] [CrossRef]
- de Moraes, Â.A.; de Jesus Pereira Franco, C.; Ferreira, O.O.; Varela, E.L.; do Nascimento, L.D.; Cascaes, M.M.; da Silva, D.R.; Percário, S.; de Oliveira, M.S.; de Aguiar Andrade, E.H. Myrcia paivae O. Berg (Myrtaceae) Essential oil, first study of the chemical composition and antioxidant potential. Molecules 2022, 27, 5460. [Google Scholar] [CrossRef]
- Moghaddam, M.; Ghasemi Pirbalouti, A.; Farhadi, N. Seasonal variation in Juniperus polycarpos var. turcomanica essential oil from northeast of Iran. J. Essent. Oil Res. 2018, 30, 225–231. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Cazella, L.N.; Glamočlija, J.; Soković, M.D.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef]
- Silva, G.N.; Pozzatti, P.; Rigatti, F.; Hörner, R.; Alves, S.H.; Mallmann, C.A.; Heinzmann, B.M. Antimicrobial evaluation of sesquiterpene α-curcumene and its synergism with imipenem. J. Microb. Biotechnol. Food Sci. 2015, 4, 434–436. [Google Scholar] [CrossRef]
- Kuang, C.; Lv, D.; Shen, G.; Li, S.; Luo, Q.; Zhang, Z. Chemical composition and antimicrobial activities of volatile oil extracted from Chrysanthemum morifolium Ramat. J. Food Sci. Technol. 2018, 55, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Forrer, M.; Kulik, E.M.; Filippi, A.; Waltimo, T. The antimicrobial activity of alpha-bisabolol and tea tree oil against Solobacterium moorei, a Gram-positive bacterium associated with halitosis. Arch. Oral Biol. 2013, 58, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Shiraishi, A.; Hada, T.; Hirose, K.; Hamashima, H.; Shimada, J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 2004, 237, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.H.; Kim, S.I.; Cho, M.H.; Lee, J. Anti-biofilm, antihemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef] [PubMed]
- Djihane, B.; Wafa, N.; Elkhamssa, S.; Pedro, D.H.; Maria, A.E.; Mohamed Mihoub, Z. Chemical constituents of Helichrysum italicum (Roth) G. Don essential oil and their antimicrobial activity against Gram-positive and Gram-negative bacteria, filamentous fungi and Candida albicans. Saudi Pharm. J. 2017, 25, 780–787. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Ghavam, M.; Manca, M.L.; Manconi, M.; Bacchetta, G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth. Sci. Rep. 2020, 10, 15647. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial susceptibility and antibacterial mechanism of Limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef]
- He, S.; Zhang, C.; Zhou, P.; Zhang, X.; Ye, T.; Wang, R.; Sun, G.; Sun, X. Herb-induced liver injury: Phylogenetic relationship, structure-toxicity relationship, and herb-ingredient network analysis. Int. J. Mol. Sci. 2019, 20, 3633. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Trigui, M.; Ben Mansour, R.; Mezghani Jarraya, R.; Damak, M.; Jaoua, S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Int. J. Food Microbiol. 2011, 148, 66–72. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Liu, X.Y.; Wang, Y.F.; Jiang, P.P.; Quek, S.Y. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Del Carmen Beristain-Bauza, S.; Hernández-Carranza, P.; Soledad Cid-Pérez, T.; Ávila-Sosa, R.; Israel Ruiz-López, I.; Enrique Ochoa-Velasco, C. Antimicrobial activity of ginger (Zingiber ocinale) and its application in food products. Food Rev. Int. 2019, 41, 1–20. [Google Scholar]
- Vedeanu, N.S.; Voica, C.; Magdas, D.A.; Kiss, B.; Ștefan, M.; Simedrea, R.; Georgiu, C.; Berce, C.; Voștinaru, O.; Boros, R.; et al. Subacute co-exposure to low doses of ruthenium (III) changes the distribution, excretion and biological effects of silver ions in rats. Environ. Chem. 2019, 17, 163–172. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, L.; Li, Y.; Soo Khoo, C.; Han, D.; Liu, Q.; Li, P.; Zhang, X. Antioxidant and immunomodulatory activities of essential oil isolated from anti-upper respiratory tract infection formulation and their chemical analysis. Evid. Based Complement. Alternat. Med. 2022, 2022, 7297499. [Google Scholar] [CrossRef]
- Kaur, N.; Chahal, K.K.; Kumar, A.; Singh, R.; Bhardwaj, U. Antioxidant activity of Anethum graveolens L. essential oil constituents and their chemical analogues. J. Food Biochem. 2019, 43, e12782. [Google Scholar] [CrossRef]
- Ács, K.; Balázs, V.L.; Kocsis, B.; Bencsik, T.; Böszörményi, A.; Horváth, G. Antibacterial activity evaluation of selected essential oils in liquid and vapor phase on respiratory tract pathogens. BMC Complement. Altern. Med. 2018, 18, 227. [Google Scholar] [CrossRef]
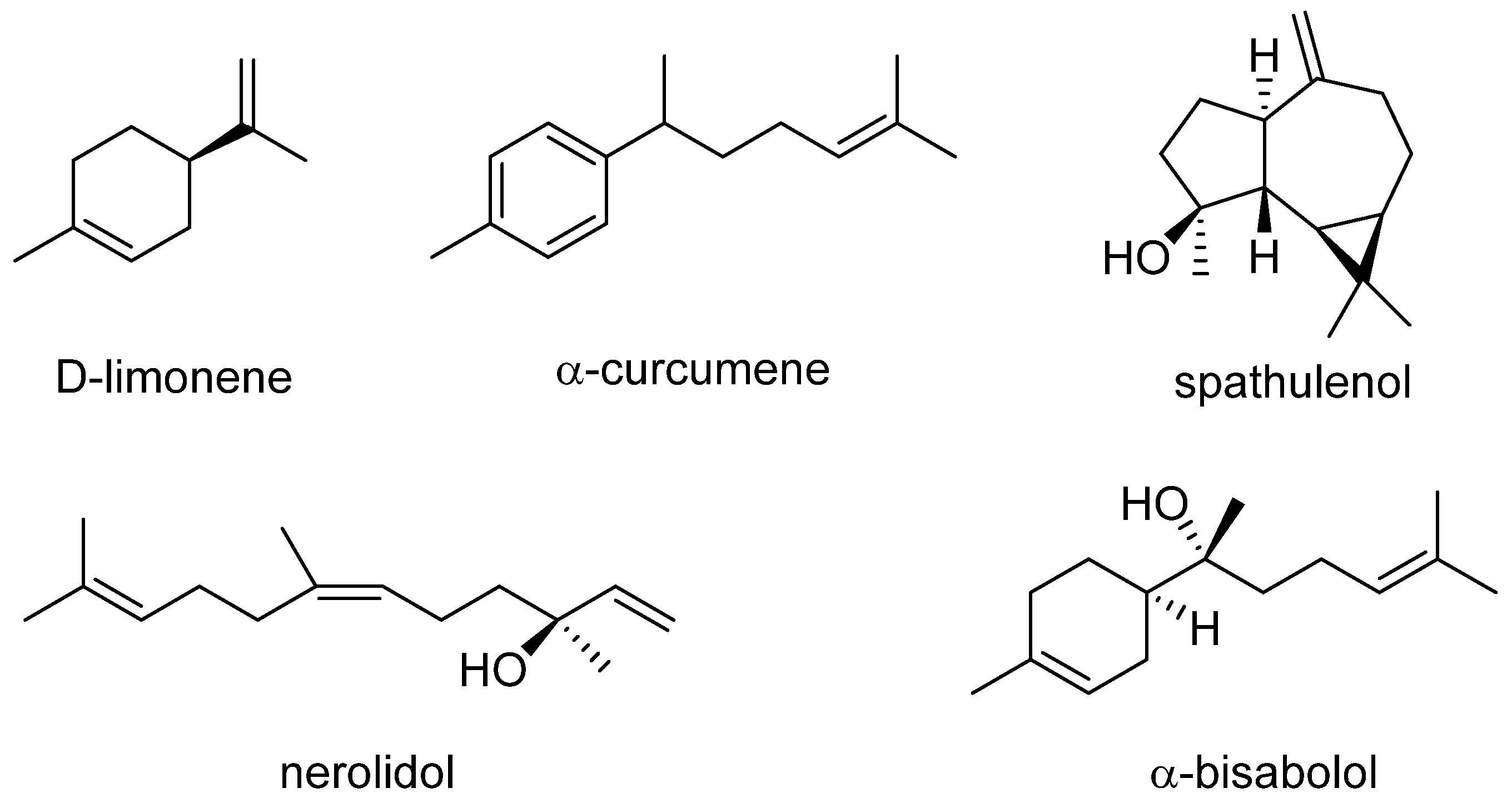

| No. | Compounds | Area (%) | RI |
|---|---|---|---|
| 1 | 4-Carene | 0.53 | 919 |
| 2 | Sulcatone | 0.12 | 938 |
| 3 | β-Pinene | 2.30 | 943 |
| 4 | α-Pinene | 2.34 | 948 |
| 5 | β-Phellandrene | 1.76 | 964 |
| 6 | Cosmene | 0.41 | 966 |
| 7 | Epoxycyclooctane | 0.13 | 970 |
| 8 | (E)-β-Ocimene | 1.35 | 976 |
| 9 | β-Ocimene | 1.06 | 976 |
| 10 | 2,5-Etheno[4.2.2]propella-3,7,9-triene | 0.98 | 976 |
| 11 | Benzaldehyde | 1.69 | 982 |
| 12 | 1-Acetyl-2-methyl-1-cyclopentene | 0.35 | 996 |
| 13 | γ-Terpinene | 1.63 | 998 |
| 14 | d-Limonene | 4.25 | 1018 |
| 15 | 1,3,8-p-Menthatriene | 0.26 | 1029 |
| 16 | δ-Terpinolene | 0.63 | 1052 |
| 17 | 2-Methyl-butyric acid 2-methylbutyl ester | 0.30 | 1054 |
| 18 | Cineol | 0.30 | 1059 |
| 19 | (±)-Cryptone | 0.67 | 1069 |
| 20 | (±)-Linalool | 0.53 | 1082 |
| 21 | Pinocarvone | 0.32 | 1114 |
| 22 | Isovaleric acid pentyl ester | 0.38 | 1118 |
| 23 | α-Phellandren-8-ol | 3.12 | 1125 |
| 24 | 3-Caren-2-ol | 0.91 | 1131 |
| 25 | Verbenol | 0.76 | 1136 |
| 26 | (-)-4-Terpineol | 1.24 | 1137 |
| 27 | cis-Piperitol | 0.13 | 1175 |
| 28 | 1,6-Methanocyclodecapentaene | 0.08 | 1189 |
| 29 | trans-3-Hexenyl butanoate | 0.37 | 1191 |
| 30 | p-Cymene-8-ol | 0.70 | 1197 |
| 31 | 1-Phenylpenta-2,4-diyne | 0.26 | 1206 |
| 32 | Hexyl isovalerate | 0.89 | 1218 |
| 33 | Leaf 2-methylbutyrate | 0.41 | 1226 |
| 34 | Geraniol | 0.76 | 1228 |
| 35 | Perillol | 0.33 | 1261 |
| 36 | Analgit | 0.50 | 1281 |
| 37 | cis-3-Hexenyl pentanoate | 1.21 | 1290 |
| 38 | trans-2-Hexenyl valerate | 0.31 | 1290 |
| 39 | 4-Vinylguaiacol | 0.49 | 1293 |
| 40 | 3a,4,5,6,7,7a-Hexahydro-4,4,7a-trimethyl-1H-inden-1-one | 0.15 | 1343 |
| 41 | Alloaromadendrene | 0.87 | 1386 |
| 42 | Berkheyaradulene | 1.32 | 1416 |
| 43 | Dihydropseudoionone | 0.52 | 1420 |
| 44 | α-Bergamotene | 1.32 | 1430 |
| 45 | Elixene | 0.04 | 1431 |
| 46 | 1-Epibicyclosesquiphellandrene | 0.31 | 1435 |
| 47 | Zingiberene | 1.25 | 1451 |
| 48 | β-Phenylethyl butyrate | 0.17 | 1458 |
| 49 | Capillin | 0.89 | 1461 |
| 50 | 4-Methylene-1-methyl-2-(2-methyl-1-propen-1-yl)-1-vinyl-cycloheptane | 0.04 | 1475 |
| 51 | 1-(1,5-Dimethyl-5-hexenyl)-4-methyl-1,4-cyclohexadiene | 2.33 | 1480 |
| 52 | 4-(2,4,4-Trimethylbicyclo[4.1.0]hept-2-en-3-yl)-(E)-3-buten-2-one | 0.73 | 1480 |
| 53 | γ-Undecalactone | 0.33 | 1483 |
| 54 | α-Bulnesene | 1.14 | 1490 |
| 55 | Citronellyl butyrate | 1.14 | 1501 |
| 56 | α-Curcumene | 9.24 | 1524 |
| 57 | Spathulenol | 9.93 | 1536 |
| 58 | Geranyl butyrate | 3.04 | 1550 |
| 59 | Nerolidol | 5.26 | 1564 |
| 60 | Nerolidol B | 2.34 | 1564 |
| 61 | 11-Tridecyn-1-ol | 0.43 | 1574 |
| 62 | Neryl 2-methyl butyrate | 1.32 | 1586 |
| 63 | β-Bisabolol | 1.08 | 1619 |
| 64 | α-Bisabolol | 6.45 | 1625 |
| 65 | δ-Terpineol pentanoic ester | 0.73 | 1626 |
| 66 | 2-Hydroxy-eudesmane-4,11-diene | 0.34 | 1690 |
| 67 | (-)-α-Bisabolol oxide B | 0.73 | 1707 |
| 68 | Farnesol | 0.18 | 1710 |
| 69 | 1,5,5,8-Tetramethyl-3,7-cycloundecadien-1-ol | 0.55 | 1719 |
| 70 | Nerolidyl acetate | 3.76 | 1754 |
| 71 | 4-Methoxystyrene | 0.19 | 1754 |
| 72 | 3-Hydroxy-humulane-1,6-dien | 1.08 | 1757 |
| 73 | δ-Cuparenol | 0.38 | 1776 |
| 74 | Bisaboloxide A | 0.28 | 1798 |
| Monoterpenes hydrocarbons | 16.51 | ||
| Oxygenated monoterpenes | 11.59 | ||
| Sesquiterpene hydrocarbons | 17.86 | ||
| Oxygenated sesquiterpenes | 33.95 | ||
| Phenolic compounds | 0.49 | ||
| Esters | 9.03 | ||
| Others | 5.10 | ||
| Total | 94.53 |
| Name of Micro-Organism | Concentration of Oil (μL/mL) | |||||
|---|---|---|---|---|---|---|
| 0 | 2.5 | 5 | 10 | 20 | 40 | |
| Staphylococcus aureus (ATCC 6538) | + | − | − | − | − | − |
| Escherichia coli (ATCC 8739) | + | + | + | − | − | − |
| Salmonella abony (NTCC 6017) | + | + | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Pan, Q.; Zhang, X.; Tana. Chemical Composition and Antioxidant and Antibacterial Potencies of the Artemisia ordosica Aerial Parts Essential Oil during the Vegetative Period. Molecules 2022, 27, 8898. https://doi.org/10.3390/molecules27248898
Zhang J, Pan Q, Zhang X, Tana. Chemical Composition and Antioxidant and Antibacterial Potencies of the Artemisia ordosica Aerial Parts Essential Oil during the Vegetative Period. Molecules. 2022; 27(24):8898. https://doi.org/10.3390/molecules27248898
Chicago/Turabian StyleZhang, Jize, Qiang Pan, Xiaoqing Zhang, and Tana. 2022. "Chemical Composition and Antioxidant and Antibacterial Potencies of the Artemisia ordosica Aerial Parts Essential Oil during the Vegetative Period" Molecules 27, no. 24: 8898. https://doi.org/10.3390/molecules27248898
APA StyleZhang, J., Pan, Q., Zhang, X., & Tana. (2022). Chemical Composition and Antioxidant and Antibacterial Potencies of the Artemisia ordosica Aerial Parts Essential Oil during the Vegetative Period. Molecules, 27(24), 8898. https://doi.org/10.3390/molecules27248898






