Synthesis and Antiproliferative Effect of Halogenated Coumarin Derivatives
Abstract
1. Introduction
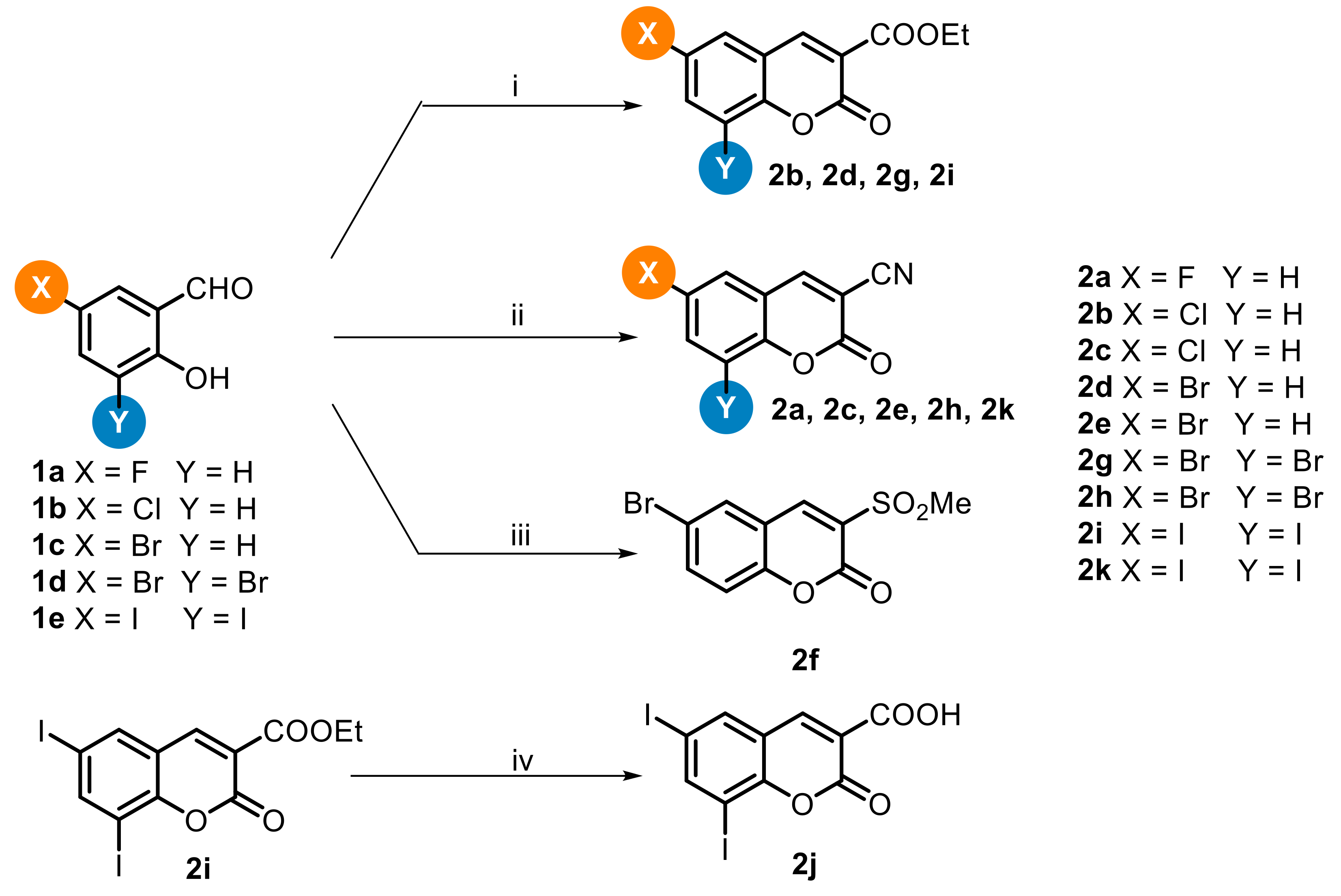
2. Chemistry
3. Results and Discussion
3.1. Antiproliferative Activity of Coumarins 2a–k against a Panel of Cancer and Normal Cell Lines
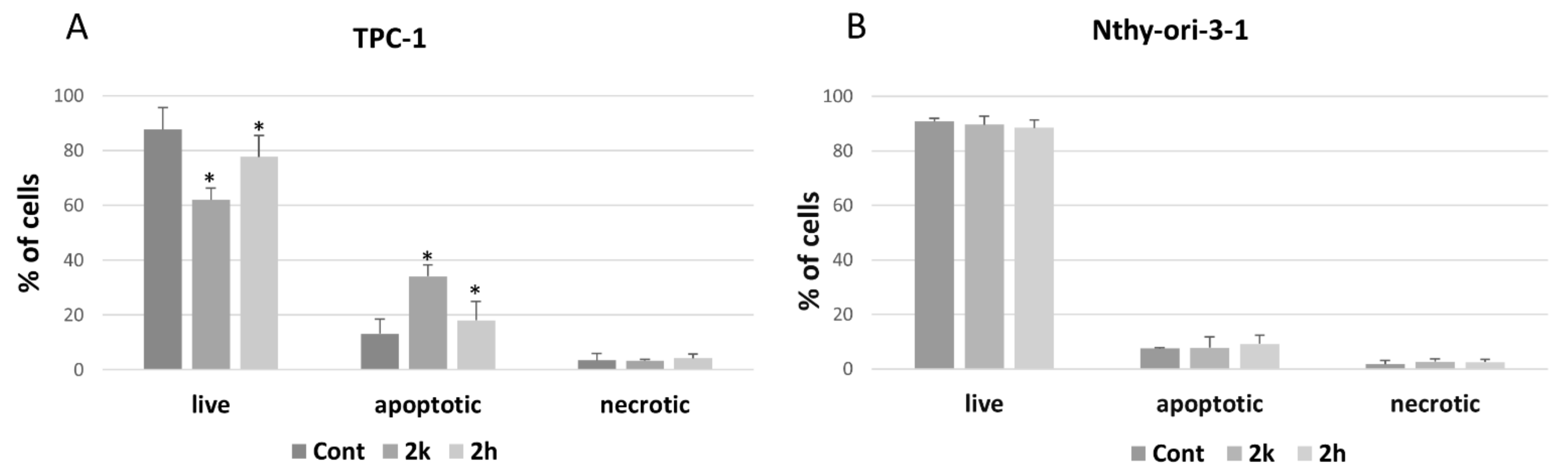
3.2. Apoptosis Induction of Coumarins 2k and 2h
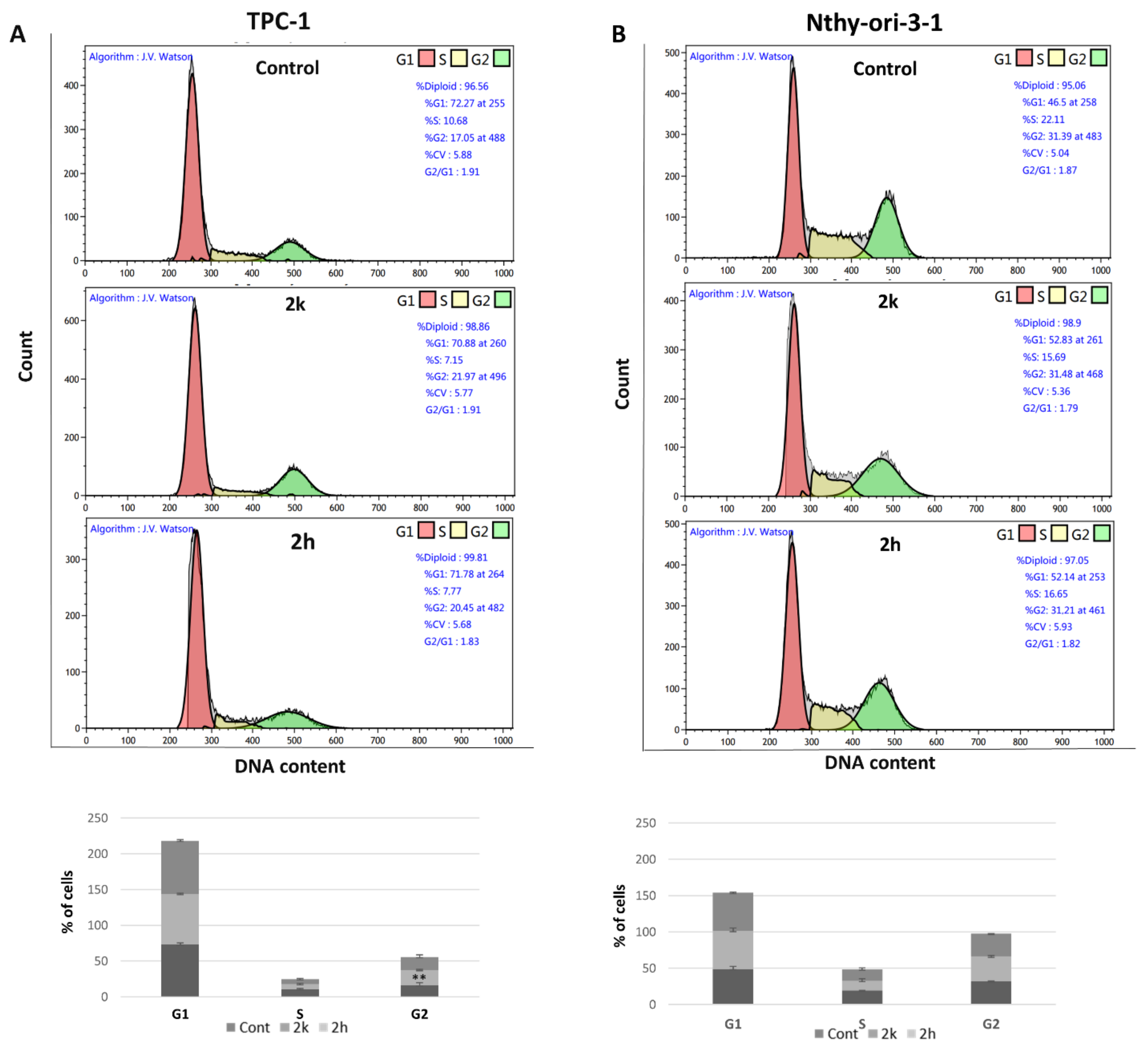
3.3. Effect of Coumarins 2k and 2h on the Different Phases of the Cell Cycle
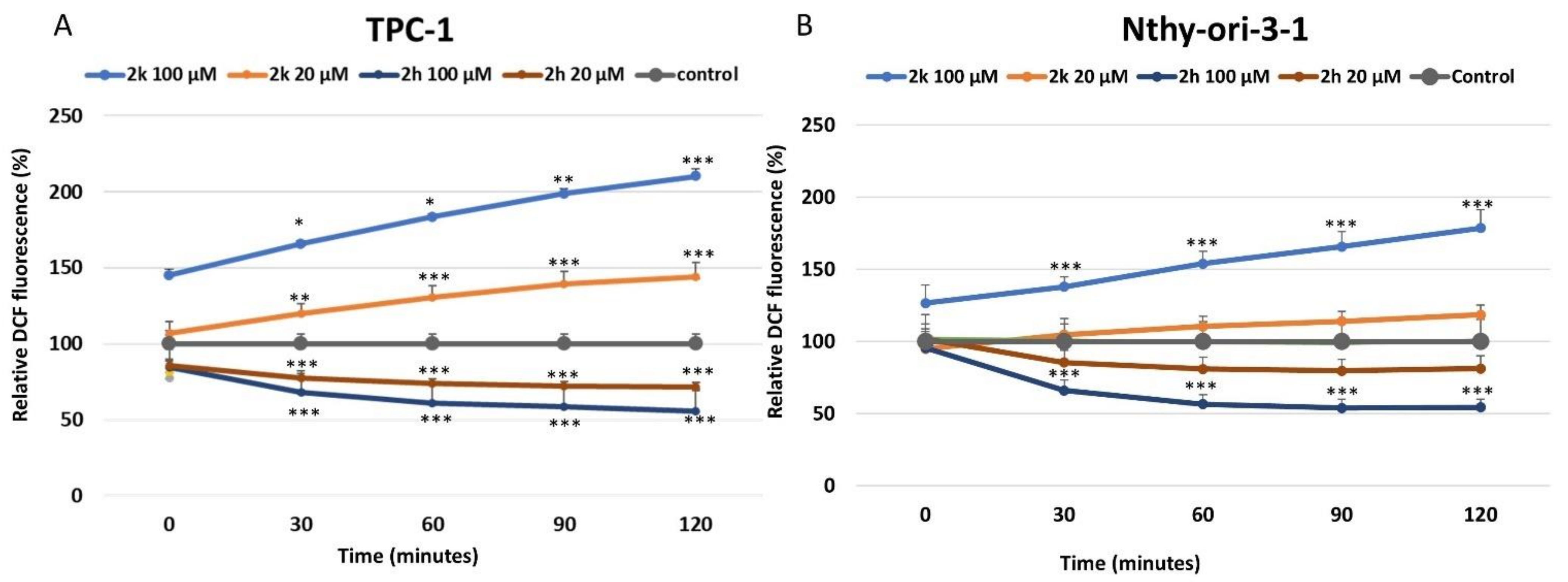
3.4. Effect of Coumarins 2k and 2h on ROS Production and Their Redox Potential
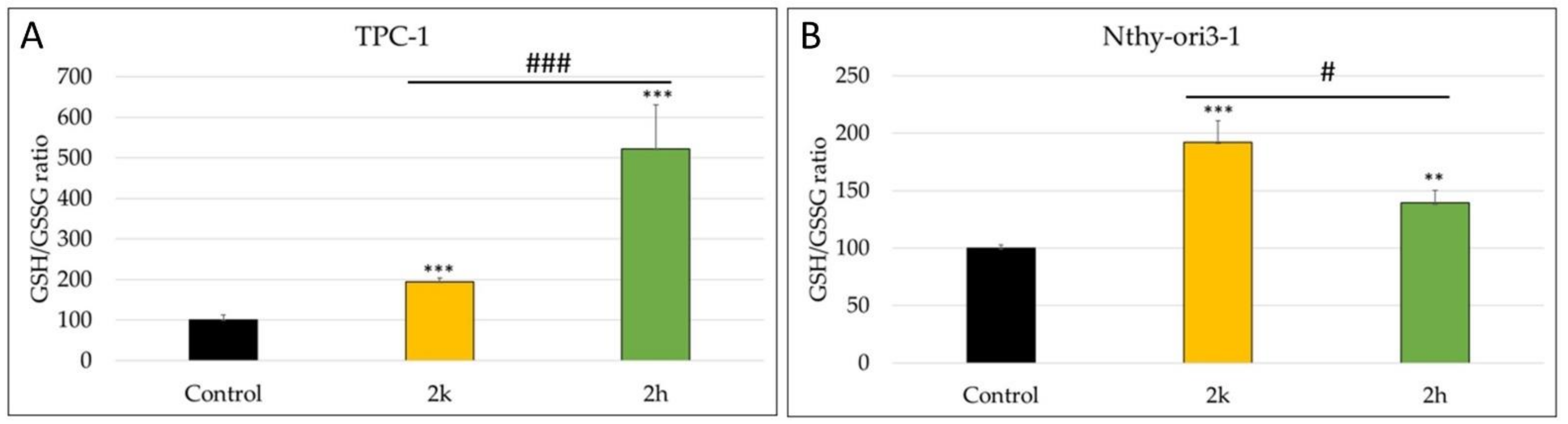
3.5. Modulation of Intracellular Content of Reduced and Oxidized Glutathione (GSH/GSSG)
4. Experimental Section
4.1. Chemistry
4.1.1. General Remarks
4.1.2. General Procedure for the Synthesis of Ethyl 2-oxo-2H-chromene-3-carboxylate derivatives 2b, 2d, 2g, and 2i
Ethyl 6-fluoro-2-oxo-2H-chromene-3-carboxylate (2b)
Ethyl 6-bromo-2-oxo-2H-chromene-3-carboxylate (2d)
Ethyl 6,8-dibromo-2-oxo-2H-chromene-3-carboxylate (2g)
Ethyl 6,8-diiodo-2-oxo-2H-chromene-3-carboxylate (2i)
4.1.3. General Procedure for the Synthesis of 2-oxo-2H-chromene-3-carbonitrile Derivatives 2a, 2c, 2e, 2h and 2k
6-Fluoro-2-oxo-2H-chromene-3-carbonitrile (2a)
6-Chloro-2-oxo-2H-chromene-3-carbonitrile (2c)
6-Bromo-2-oxo-2H-chromene-3-carbonitrile (2e)
Ethyl 6,8-dibromo-2-oxo-2H-chromene-3-carboxylate (2h)
6,8-diiodo-2-oxo-2H-chromene-3-carbonitrile (2k)
4.1.4. 6-Bromo-3-(methylsulfonyl)-2H-chromen-2-one (2f)
4.1.5. 6,8-diiodo-2-oxo-2H-chromene-3-carboxylic Acid (2j)
4.1.6. Electrochemical Measurements
4.2. Biological Evaluations
4.2.1. Cell Lines and Cell Culture
4.2.2. Cell Viability Assays
4.2.3. Cell Cycle Analysis by Flow Cytometry
4.2.4. Cell Apoptosis Assay
4.2.5. Determination of Intracellular ROS Production
4.2.6. Determination of Intracellular Reduced and Oxidized Glutathione
4.2.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cooke, D.; Fitzpatrick, B.; O’Kennedy, R.; McCormack, T.; Egan, D. Coumarin: Biochemical Profile and Recent Developments; John Wiley & Sons: New York, NY, USA, 1997; Volume 3, pp. 311–322. [Google Scholar]
- Annunziata, F.; Pinna, C.; Dallavalle, S.; Tamborini, L.; Pinto, A. An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities. Int. J. Mol. Sci. 2020, 21, 4618. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Coumarin carbonic an-hydrase inhibitors from natural sources. J. Enzym. Inhib. Med. Chem. 2020, 35, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sanchez, E.S.; Capasso, R. Coumarins and coumarin-related compounds in pharmacotherapy of cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Perkin, W. On the artificial production of coumarin and formation of its homologues. J. Chem. Soc. 1868, 21, 53–63. [Google Scholar] [CrossRef]
- Salem, M.A.; Helal, H.M.; Gouda, M.A.; Ammar, Y.A.; El-Gaby, M.S.A.; Abbas, S.Y. An overview on synthetic strategies to coumarins. Synth. Commun. 2018, 48, 1534–1550. [Google Scholar] [CrossRef]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151–186. [Google Scholar] [CrossRef]
- Gulati, S.; Singha, R.; Sangwan, S. A review on convenient synthesis of substituted coumarins using reusable solid acid catalysts. RSC Adv. 2021, 11, 29130–29155. [Google Scholar] [CrossRef]
- Tiana, R.; Renc, X.; Niu, P.; Yang, L.; Sun, A.; Li, Y.; Liu, X.; Wei, L. Development of chromenoquinoline-fused coumarin dyes and their application in bioimaging. Dyes Pigment. 2022, 205, 110530–110536. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Zhou, B.; Niu, G.; Zhang, H.; Wu, J.; Wang, Y.; Ju, W.; Wang, P. Coumarin- and rhodamine-fused deep red fluorescent dyes: Synthesis, photophysical properties, and bioimaging in vitro. J. Org. Chem. 2013, 78, 6121–6130. [Google Scholar] [CrossRef]
- Cocco, A.; Caria, P.; Sanna, G.; Stagi, L.; Cadoni, E.; Corpino, R.; Ricci, P.C.; Carbonaro, C.M.; Secci, F. Synthesis and Photophysical Properties of Fluorescent 6-Aryl-D-π-A Coumarin Derivatives. ACS Omega 2021, 6, 33708–33716. [Google Scholar]
- Luridiana, A.; Pretta, G.L.; Chiriu, D.; Carbonaro, C.M.; Corpino, R.; Secci, F.; Frongia, A.; Stagi, L.; Ricci, P.C. A facile strategy for new organic white LED hybrid devices: Design, features and engineering. RSC Adv. 2016, 6, 22111–22120. [Google Scholar] [CrossRef]
- Rohini, K.; Srikumar, P.S. Therapeutic Role of Coumarins and Coumarin-Related Compounds. J. Thermodyn. Catal. 2014, 5, 130. [Google Scholar] [CrossRef]
- Raj, V.; Lee, J. 2H/4H-Chromenes-a versatile biologically attractive scaffold. Front. Chem. 2020, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- Nasr, T.; Bondock, S.; Youns, M. Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives. Eur. J. Med. Chem. 2014, 76, 539–548. [Google Scholar] [CrossRef]
- Serge, A.T.; Fobofou, K.F.; Brandt, W.; Manzin, A.; Madeddu, S.; Serreli, G.; Sanna, G.; Wessjohann, L.A. Bichromonol, a dimeric coumarin with anti-HIV activity from the stem bark of Hypericum roeperianum. Nat. Prod. Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Vianna, D.R.; Hamerski, L.; Figueiró, F.; Bernardi, A.; Visentin, L.C.; Pires, E.N.S.; Teixeira, H.F.; Salbego, C.G.; Eifler-Lima, V.L.; Battastini, A.M.O.; et al. Selective cytotoxicity and apoptosis induction in glioma cell lines by 5-oxygenated-6,7-methylenedioxycoumarins from Pterocaulon species. Eur. J. Med. Chem. 2012, 57, 268–274. [Google Scholar] [CrossRef]
- Al-Majedy, Y.; Kadhum, A.A.; Ibraheem, H.; Al-Amiery, A.; Moneim, A.A.; Mohamad, A.B. A Systematic Review on Pharmacological Activities of 4-Methylumbelliferon. Syst. Rev. Pharm. 2018, 9, 49–54. [Google Scholar] [CrossRef]
- Morsy, S.A.; Farahat, A.A.; Nasr, M.N.A.; Tantawy, A.S. Synthesis, molecular modeling and anticancer activity of new coumarin containing compounds. Saudi Pharm. J. 2017, 25, 873–883. [Google Scholar] [CrossRef]
- Zhao, J.W.; Wu, Z.H.; Guo, J.W.; Huang, M.J.; You, Y.Z.; Liu, H.M.; Huang, L.H. Synthesis and anti-gastric cancer activity evaluation of novel triazole nucleobase analogues containing steroidal/coumarin/quinoline moieties. Eur. J. Med. Chem. 2019, 181, 111520. [Google Scholar] [CrossRef]
- Emami, S.; Dadashpour, S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015, 102, 611–630. [Google Scholar] [CrossRef]
- Kaur, M.; Kohli, S.; Sandhu, S.; Bansal, Y.; Bansal, G. Coumarin: A promising scaffold for anticancer agents. Anti Cancer Agents Med. Chem. 2015, 15, 1032–1048. [Google Scholar] [CrossRef] [PubMed]
- Dandriyal, J.; Singla, R.; Kumar, M.; Jaitak, V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur. J. Med. Chem. 2016, 119, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singla, R.; Dandriyal, J.; Jaitak, V. Coumarin derivatives as anti-cancer agents for lung cancer therapy: A review. Anti Cancer Agents Med. Chem. 2018, 18, 964–984. [Google Scholar] [CrossRef] [PubMed]
- Warhi, T.A.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef]
- Goud, N.S.; Kumar, P.; Bharath, R.D. Recent developments of target based coumarin derivatives as potential anticancer agents. Mini Rev. Med. Chem. 2020, 20, 1754–1766. [Google Scholar] [CrossRef]
- Song, X.-F.; Fan, X.J.; Liu, L.; Liu, X.F.; Gao, F. Coumarin derivatives with anti-cancer activities: An update. Arch. Pharm. (Weinh.) 2020, 353, e2000025. [Google Scholar] [CrossRef]
- Wang, G.; Sun, S.; Wu, B.; Liu, J. Coumarins as potential anti-drug resistant cancer agents: A mini review. Curr. Top. Med. Chem. 2020, 21, 1725–1736. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Huang, C.; Jia, Y.-M.; Song, B.-A.; Li, J.; Liu, X.-X. Novel coumarin-dihydropyrazole thio-ethanone derivatives: Design, synthesis and anticancer activity. Eur. J. Med. Chem. 2014, 74, 717–725. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.-R.; Liu, H.-S.; Cheng, M.; Xia, P.; Qian, K.; Wu, P.-C.; Lai, C.-Y.; Xia, Y.; Yang, Z.-Y.; et al. Antitumor agents 292. Design, synthesis and pharmacological study of S- and O-substituted 7-mercapto- or hydroxy-coumarins and chromones as potent cytotoxic agents. Eur. J. Med. Chem. 2012, 49, 74–85. [Google Scholar] [CrossRef]
- Secci, D.; Carradori, S.; Bolasco, A.; Chimenti, P.; Yáñez, M.; Ortuso, F.; Alcaro, S. Synthesis and selective human monoamine oxidase inhibition of 3-carbonyl, 3-acyl, and 3-carboxyhydrazido coumarin derivatives. Eur. J. Med. Chem. 2011, 46, 4846–4852. [Google Scholar] [CrossRef]
- Prabhakara, C.T.; Patil, S.A.; Toragalmath, S.S.; Kinnal, S.M.; Badami, P.S. Synthesis, characterization and biological approach of metal chelates of some first row transition metal ions with halogenated bidentate coumarin Schiff bases containing N and O donor atoms. J. Photochem. Photobiol. B Biol. 2016, 157, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, V.K.; Gleiser, R.M.; Kasumbwe, K.; Aldhubiab, B.E.; Attimarad, M.V.; Odhav, B. Evaluation of Halogenated Coumarins for Antimosquito Properties. Sci. World J. 2014, 2014, 189824. [Google Scholar] [CrossRef] [PubMed]
- Voth, A.R.; Ho, P.S. The role of halogen bonding in inhibitor recognition and binding by protein kinases. Curr. Top. Med. Chem. 2007, 14, 1336–1348. [Google Scholar] [CrossRef]
- Rehuman, N.A.; Oh, J.M.; Nath, L.R.; Khames, A.; Abdelgawad, M.A.; Gambacorta, N.; Nicolotti, O.; Ja, R.K.; Kim, H.; Mathew, G. Halogenated coumarin–chalcones as multifunctional monoamine oxidase-B and butyrylcholinesterase inhibitors. ACS Omega 2021, 42, 28182–28193. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, R.; Liu, X.; Zimmermann, M.O.; Rutheford, T.J.; Fersht, A.R.; Joerger, A.C.; Boeckler, F.M. Halogen-enriched fragment libraries as leads for drug rescue of mutant p53. J. Am. Chem. Soc. 2012, 134, 6810–6818. [Google Scholar] [CrossRef]
- Wei, L.; Wang, J.; Zhang, X.; Wang, P.; Zhao, Y.; Li, J.; Hou, T.; Qu, L.; Shi, L.; Liang, X.; et al. Discovery of 2H-chromen-2-one derivatives as G protein-coupled receptor-35 agonists. J. Med. Chem. 2017, 60, 362–372. [Google Scholar] [CrossRef]
- Weng, Y.; Yang, T.; Chen, H.; Chen, Z.; Zhu, M.; Zhan, X. Efficient Synthesis of 3-Aroyl Coumarins in Water via Catalytic Carbopalladation of Nitriles. Chem. Select. 2019, 4, 14233–14236. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Xie, C.; Chen, D.; Li, J. Novel microtubule-targeted agent 6-chloro-4-(methoxyphenyl) coumarin induces G2-M arrest and apoptosis in HeLa cells. Acta Pharmacol. Sin. 2012, 33, 407–417. [Google Scholar] [CrossRef]
- Haghighi, F.; Matin, M.M.; Bahrami, A.R.; Iranshahi, M.; Rassouli, F.B.; Haghighitalab, A. The cytotoxic activities of 7-isopentenyloxycoumarin on 5637 cells via induction of apoptosis and cell cycle arrest in G2/M stage. DARU J. Pharm. Sci. 2014, 22, 3. [Google Scholar] [CrossRef]
- Tronci, L.; Serreli, G.; Piras, C.; Frau, D.V.; Dettori, T.; Deiana, M.; Murgia, F.; Santoru, M.L.; Spada, M.; Leoni, V.P.; et al. Vitamin C Cytotoxicity and Its Effects in Redox Homeostasis and Energetic Metabolism in Papillary Thyroid Carcinoma Cell Lines. Antioxidants 2021, 10, 809. [Google Scholar] [CrossRef]
- Spallarossa, A.; Caneva, C.; Caviglia, M.; Alfei, S.; Butini, S.; Campiani, G.; Gemma, S.; Brindisi, M.; Zisterer, D.M.; Bright, S.A.; et al. Unconventional Knoevenagel-type indoles: Synthesis and cell-based studies for the identification of pro-apoptotic agents. Eur. J. Med. Chem. 2015, 102, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, C.L.; Ferrara, B.; Occhipinti, S.; Boggio, E.; Barrera, G.; Pizzimenti, S.; Giovarelli, M.; Fantozzi, R.; Chiocchetti, A.; Argenziano, M.; et al. Enhanced cytotoxic effect of camptothecin nanosponges in anaplastic thyroid cancer cells in vitro and in vivo on orthotopic xenograft tumors. Drug Deliv. 2017, 24, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Rose, D.M.; Pearson, A.; Ezekewitz, R.A.; Henson, P.M. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 2000, 405, 85–90. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.R.; El-Deiry, W.S. Cell cycle control as a basis for cancer drug development. Int. J. Oncol. 2000, 16, 871–957. [Google Scholar] [CrossRef]
- Ramdani, L.H.; Talhi, O.; Decombat, C.; Vermerie, M.; Berry, A.; Silva, A.; Bachari, K.; Vasson, M.-P.; Delort, L.; Caldefie-Chézet, F. Bis(4-hydroxy-2H-chromen-2-one) coumarin induces apoptosis in MCF-7 human breast cancer cells through aromatase inhibition. Anticancer Res. 2019, 39, 6107–6114. [Google Scholar] [CrossRef]
- Abdelnaby, R.M.; Rateb, H.S.; Ali, O.; Saad, A.S.; Nadeem, R.I.; Abou-Seri, S.M.; Amin, K.M.; Younis, N.S.; Abdelhady, R. Dual PI3K/Akt Inhibitors Bearing Coumarin Thiazolidine Pharmacophores as Potential Apoptosis Inducers in MCF-7 Cells. Pharmaceuticals 2022, 15, 428. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Liu, Y.; Zeng, Y.; Wu, G. A Review on Anti-Tumor Mechanisms of Coumarins. Front. Oncol. 2020, 10, 592853, PMID: 33344242; PMCID: PMC7746827. [Google Scholar] [CrossRef]
- Arora, R.; Sawney, S.; Saini, V.; Steffi, C.; Tiwari, M.; Saluja, D. Esculetin induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to KEAP1. Mol. Cancer 2016, 15, 64. [Google Scholar] [CrossRef]
- Khan, S.; Zafar, A.; Naseem, I. Redox cycling of copper by coumarin-di(2-picolyl)amine hybrid molecule leads to ROS-mediated modulation of redox scavengers, DNA damage and cell death in diethylnitrosamine induced hepatocellular carcinoma. Bioorg. Chem. 2020, 99, 103818. [Google Scholar] [CrossRef]
- Hassanein, E.H.M.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1675957. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The Interplay of Oxidative Stress and Inflammation: Mechanistic Insights and Therapeutic Potential of Antioxidants. Oxid. Med. Cell. Longev 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Kode, A.; Rajendrasozhan, S.; Caito, S.; Yang, S.-R.; Megson, I.L.; Rahman, I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.A.; Neelamohan, R.; Suthagar, E.; Vengatesh, G.; Jayakumar, J.; Chandrasekaran, M.; Banu, S.K.; Aruldhas, M.M. Lipid peroxidation and antioxidants status in human malignant and non-malignant thyroid tumors. Hum. Exp. Toxicol. 2016, 35, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.-Y.; Zhou, Y.-Q.; Yu, N.; Li, Y.-L.; Wei, T.; Peng, L.; Ling, Y.; Jiang, K.; Wei, Y. Deconstructive insertion of oximes into coumarins: Modular synthesis of dihydrobenzofuran-fused pyridines. Org. Lett. 2022, 24, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Dagar, N.; Singh, S.; Roy, S.R. Synergistic effect of cerium in dual photoinduced ligand-to-metal charge transfer and Lewis acid catalysis: Diastereoselective alkylation of coumarins. J. Org. Chem. 2022, 87, 8970–8982. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-C.; Li, J.-L.; Ji, C.-B.; Peng, Y.-Y.; Zeng, X.-P. Construction of cyclopropa[c]coumarins via cascade Michael-alkylation process of 3-cyanocoumarin with 2-bromomalonate. Tetrahedron 2020, 76, 130852. [Google Scholar] [CrossRef]
- Serreli, G.; Incani, A.; Atzeri, A.; Angioni, A.; Campus, M.; Cauli, E.; Zurru, R.; Deiana, M. Antioxidant Effect of Natural Table Olives Phenolic Extract Against Oxidative Stress and Membrane Damage in Enterocyte-Like Cells. J. Food Sci. 2017, 82, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, M.I.; Iqbal, Z.; Shah, Y.; Ahmad, L.; Nazir, S.; Watson, D.G.; Khan, J.A.; Nasir, F.; Khan, A.; et al. A new HPLC method for the simultaneous determination of ascorbic acid and aminothiols in human plasma and erythrocytes using electrochemical detection. Talanta 2011, 84, 789–801. [Google Scholar] [CrossRef]
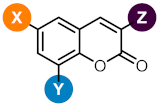 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compd | X | Y | Z | a CC50 (µM) | |||||
| SK-MES-1 | MRC-5 | K-1 | B-CPAP | TPC-1 | Nthy-ori-3-1 | ||||
| 2a | F | H | CN | >100 | >100 | >100 | >100 | >100 | >100 |
| 2b | Cl | H | COOEt | >100 | >100 | >100 | >100 | >100 | >100 |
| 2c | Cl | H | CN | >100 | >100 | >100 | >100 | >100 | >100 |
| 2d | Br | H | COOEt | >100 | >100 | >100 | >100 | >100 | >100 |
| 2e | Br | H | CN | >100 | >100 | >100 | >100 | >100 | >100 |
| 2f | Br | H | SO2Me | >100 | >100 | >100 | >100 | >100 | >100 |
| 2g | Br | Br | COOEt | >100 | >100 | >100 | >100 | >100 | >100 |
| 2h | Br | Br | CN | >100 | >100 | 60 ± 3 | >100 | 90 ± 3.5 | >100 |
| 2i | I | I | COOEt | >100 | >100 | >100 | >100 | >100 | >100 |
| 2j | I | I | COOH | >100 | >100 | >100 | >100 | >100 | >100 |
| 2k | I | I | CN | >100 | >100 | 57 ± 5 | 46 ± 0.2 | 44 ± 5.5 | >100 |
| Camptothecin | - | - | - | 0.017 ± 0.003 | 0.1 ± 0.03 | - | 0.03 ± 0.2 | 0.01 ± 0.02 | 0.01 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dettori, T.; Sanna, G.; Cocco, A.; Serreli, G.; Deiana, M.; Palmas, V.; Onnis, V.; Pilia, L.; Melis, N.; Moi, D.; et al. Synthesis and Antiproliferative Effect of Halogenated Coumarin Derivatives. Molecules 2022, 27, 8897. https://doi.org/10.3390/molecules27248897
Dettori T, Sanna G, Cocco A, Serreli G, Deiana M, Palmas V, Onnis V, Pilia L, Melis N, Moi D, et al. Synthesis and Antiproliferative Effect of Halogenated Coumarin Derivatives. Molecules. 2022; 27(24):8897. https://doi.org/10.3390/molecules27248897
Chicago/Turabian StyleDettori, Tinuccia, Giuseppina Sanna, Andrea Cocco, Gabriele Serreli, Monica Deiana, Vanessa Palmas, Valentina Onnis, Luca Pilia, Nicola Melis, Davide Moi, and et al. 2022. "Synthesis and Antiproliferative Effect of Halogenated Coumarin Derivatives" Molecules 27, no. 24: 8897. https://doi.org/10.3390/molecules27248897
APA StyleDettori, T., Sanna, G., Cocco, A., Serreli, G., Deiana, M., Palmas, V., Onnis, V., Pilia, L., Melis, N., Moi, D., Caria, P., & Secci, F. (2022). Synthesis and Antiproliferative Effect of Halogenated Coumarin Derivatives. Molecules, 27(24), 8897. https://doi.org/10.3390/molecules27248897









