Thermoresponsive Ionic Liquid with Different Cation–Anion Pairs as Draw Solutes in Forward Osmosis
Abstract
1. Introduction
2. Results and Discussion
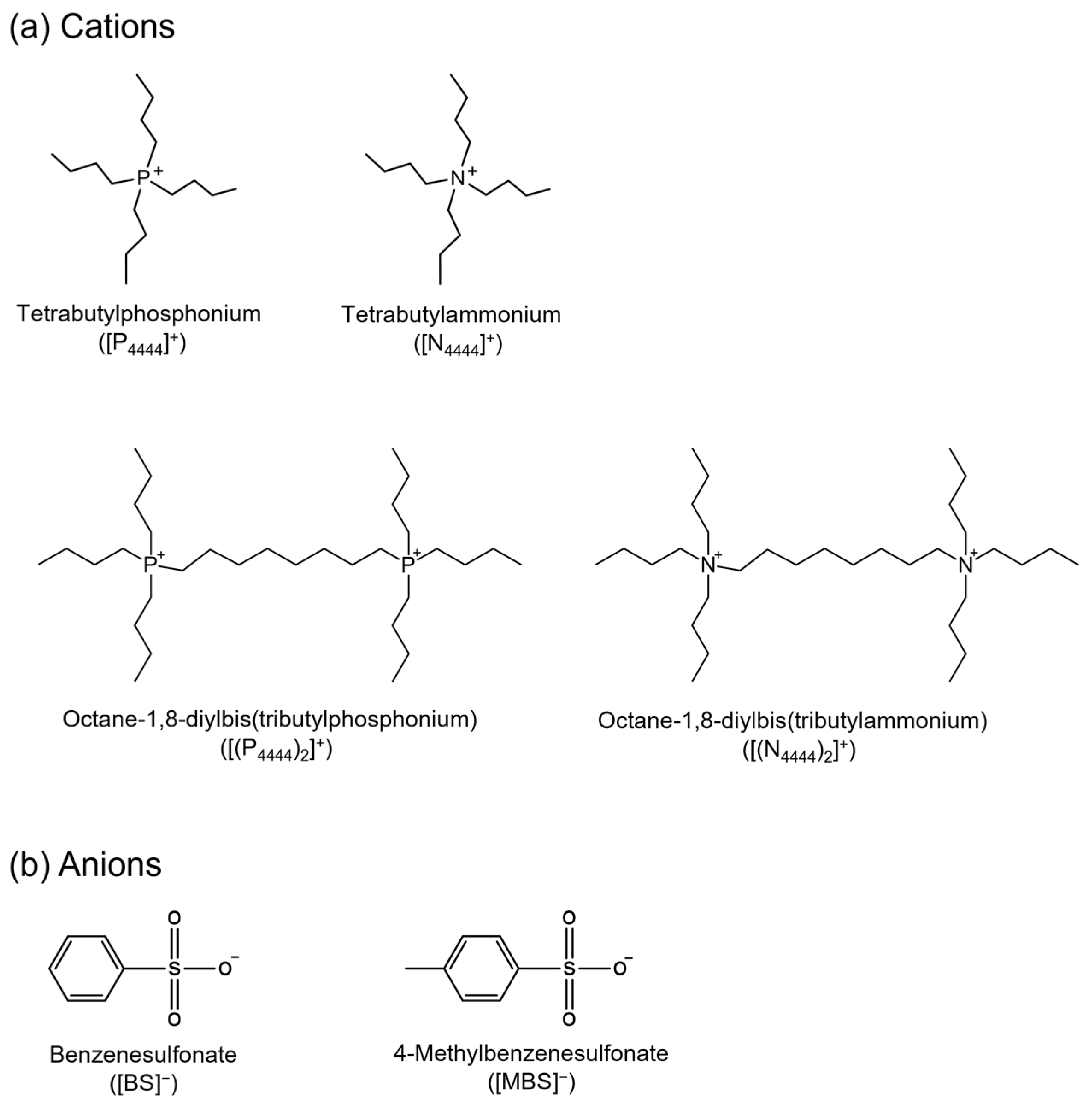
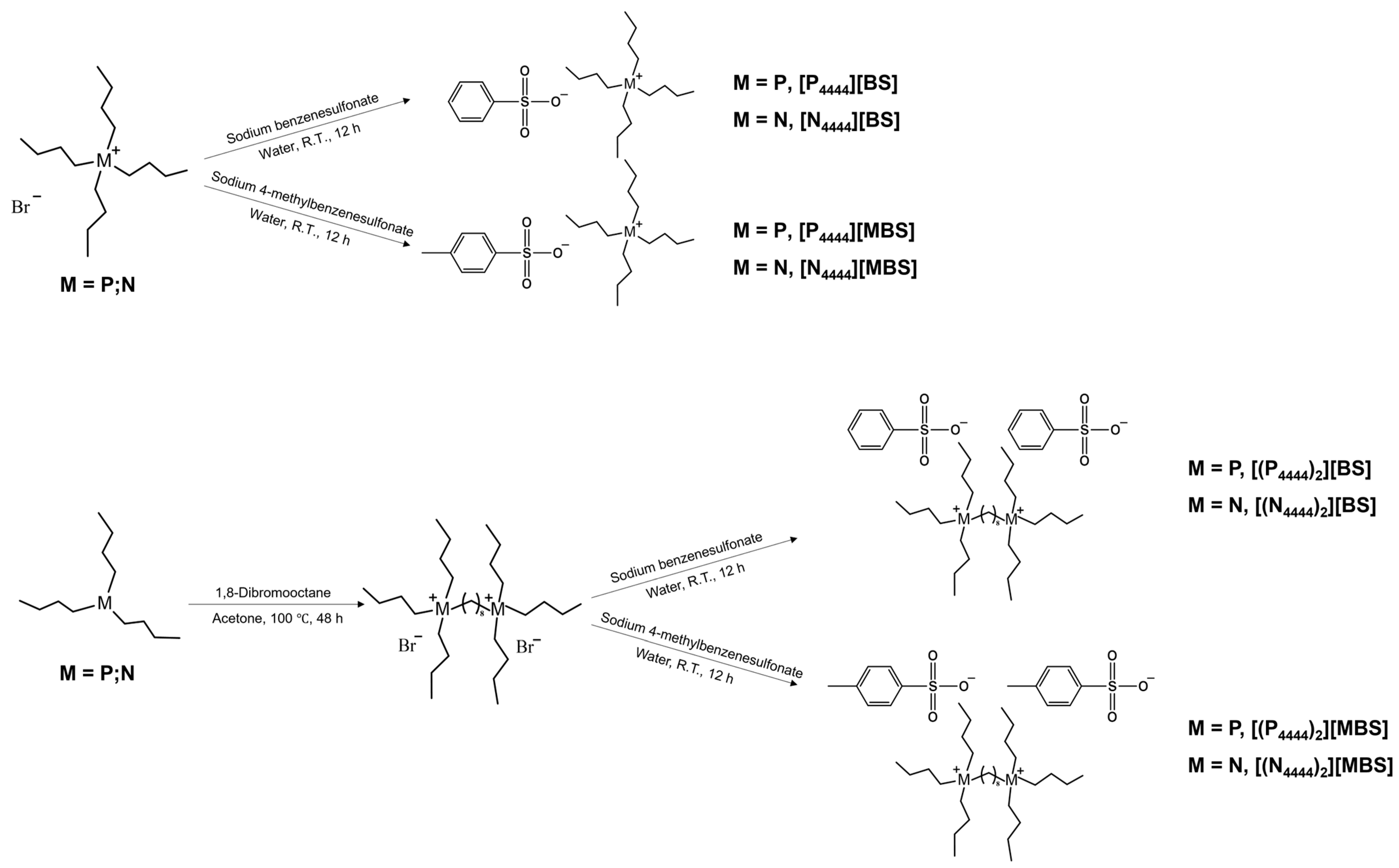
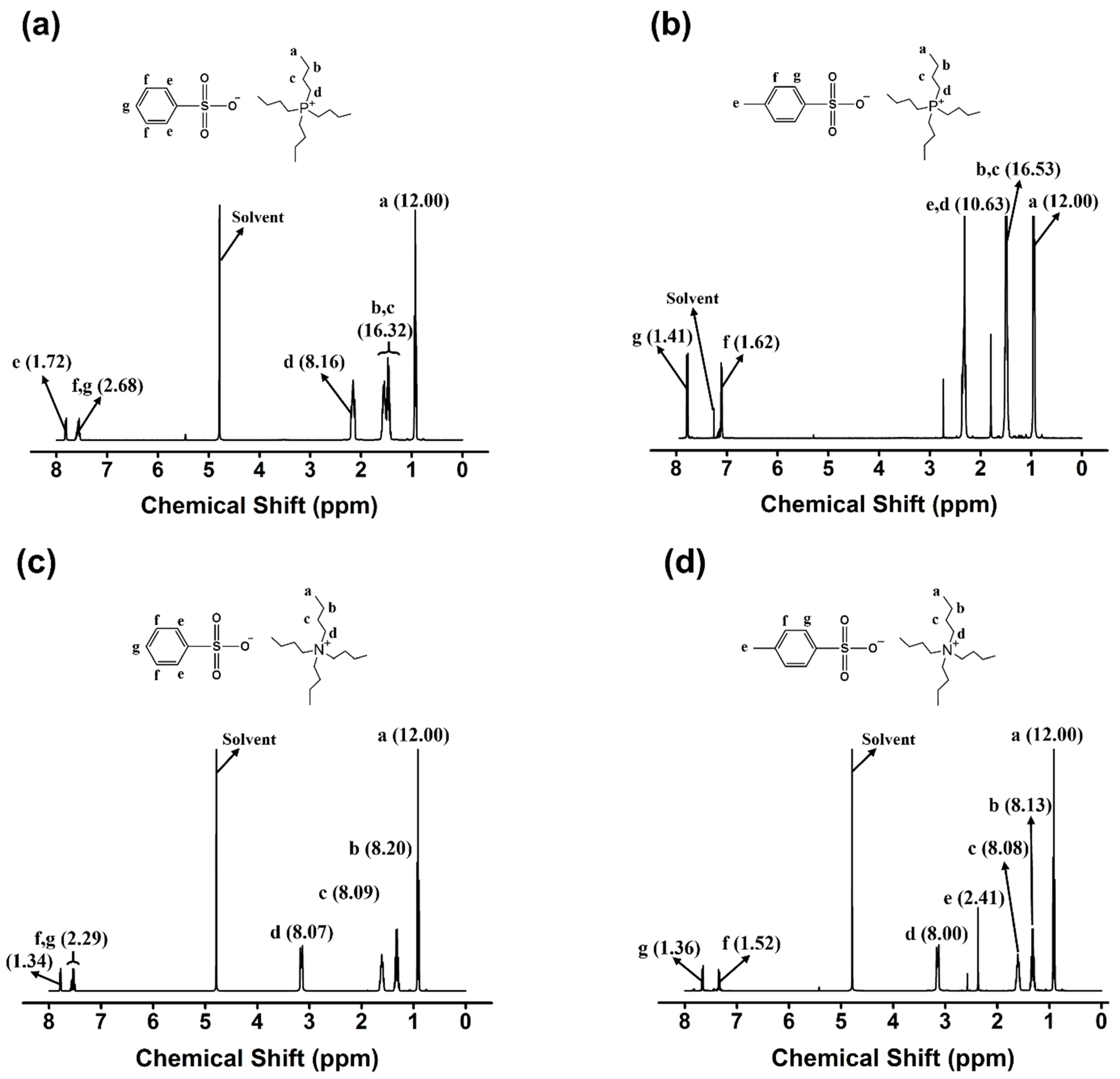
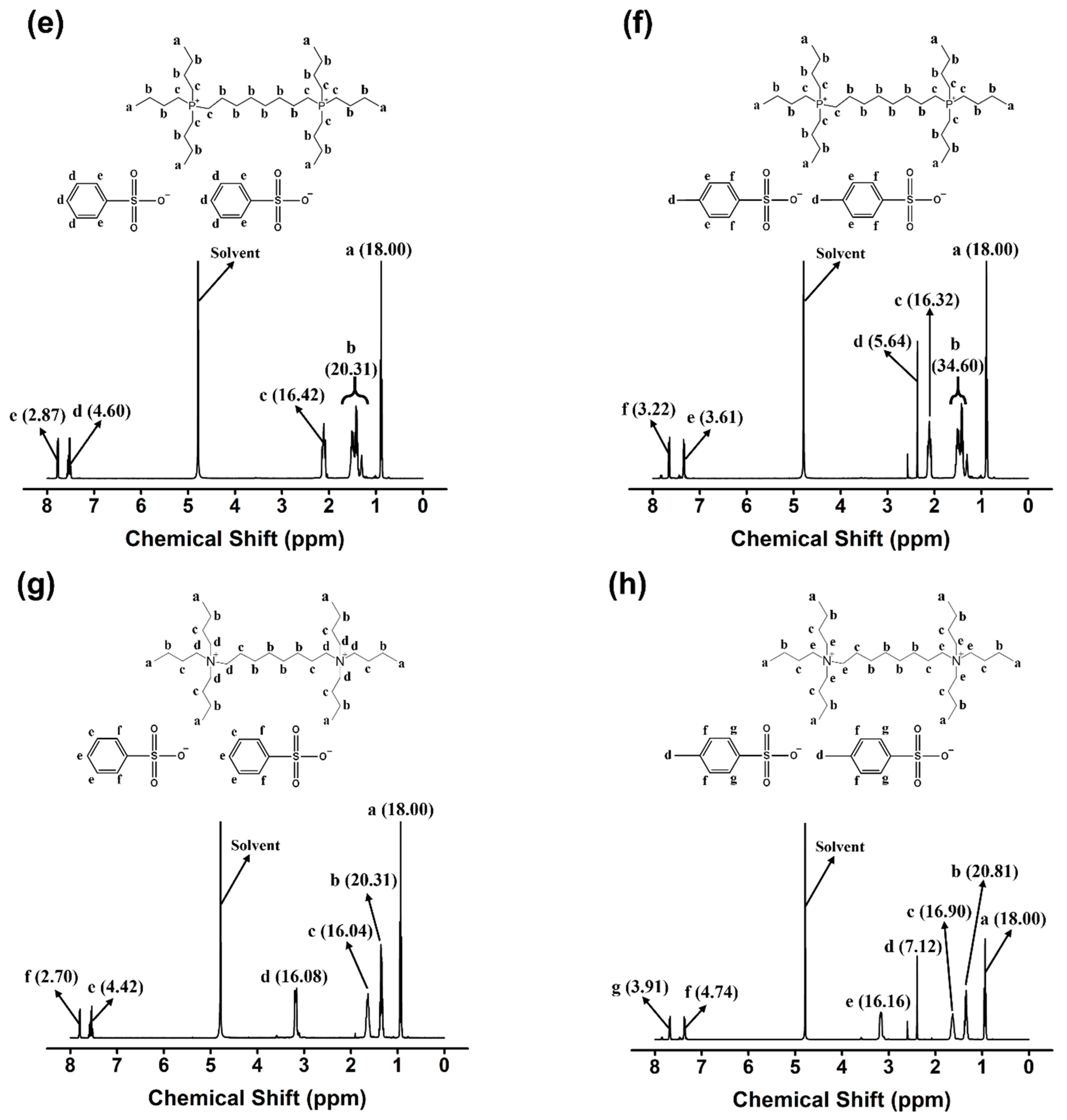
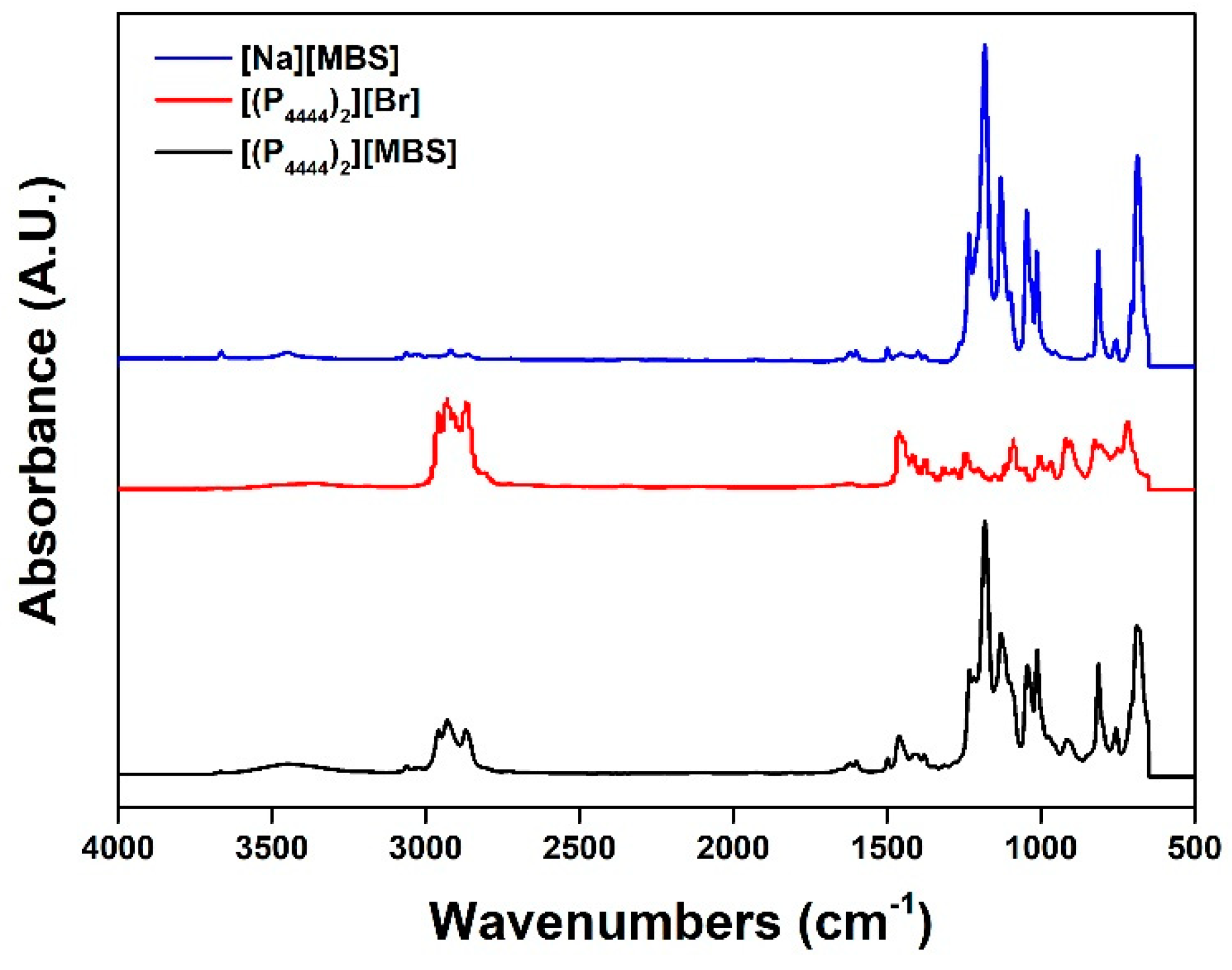
2.1. Synthesis of Monocationic and Dicationic ILs
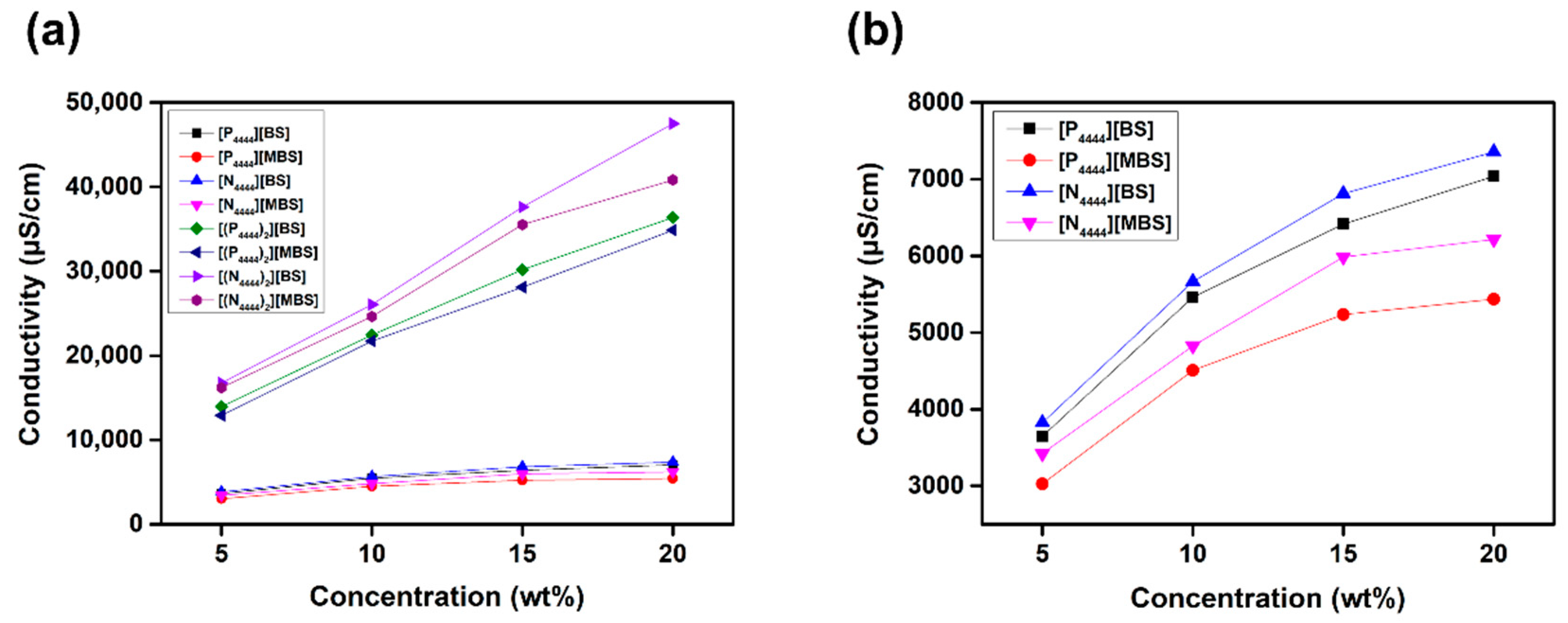
2.2. Conductivity
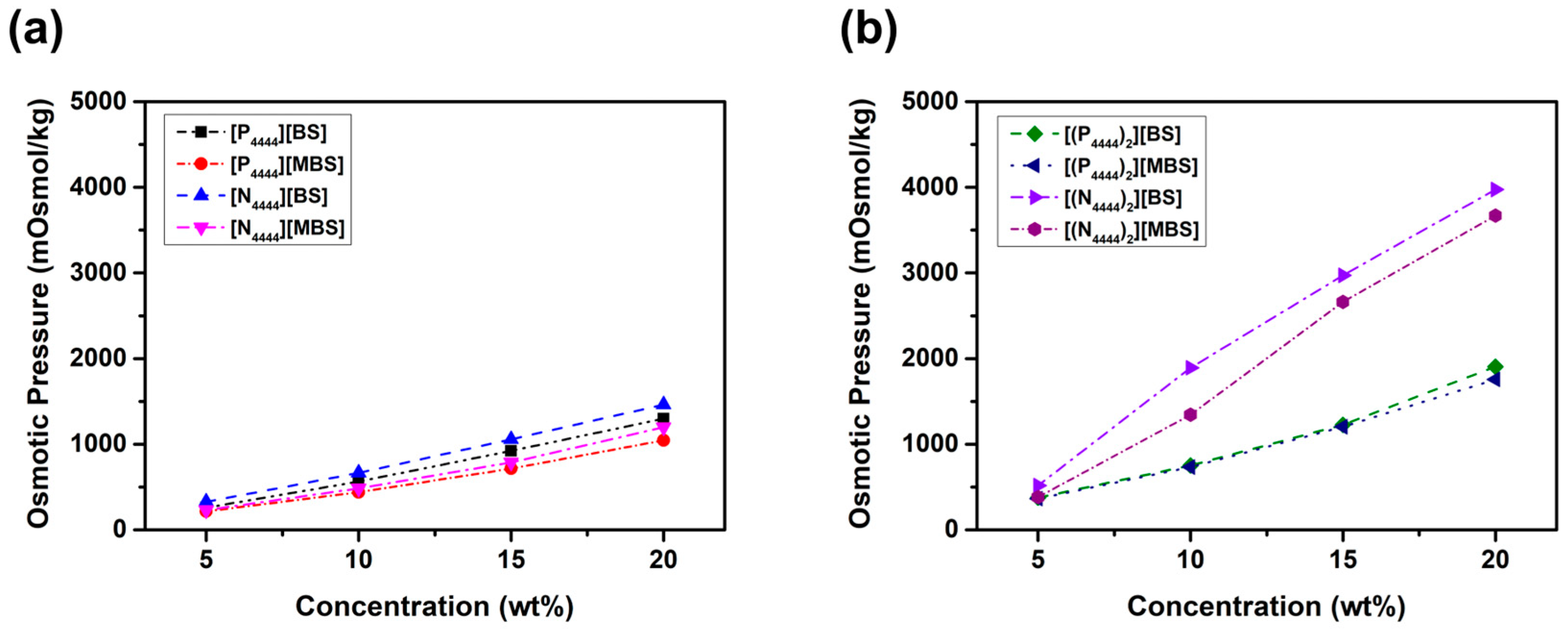
2.3. Osmotic Pressure
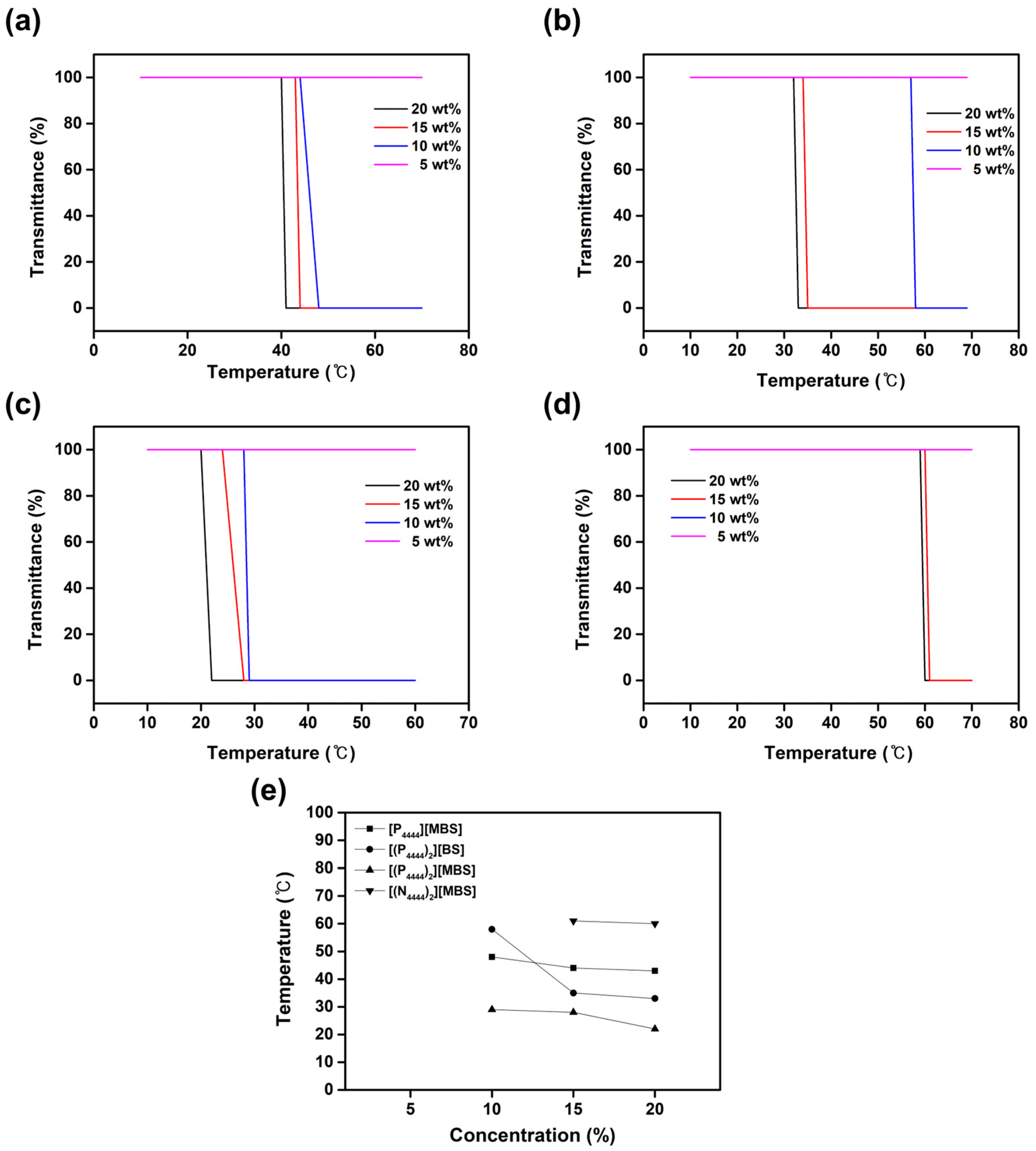
2.4. Thermoresponsive Behavior
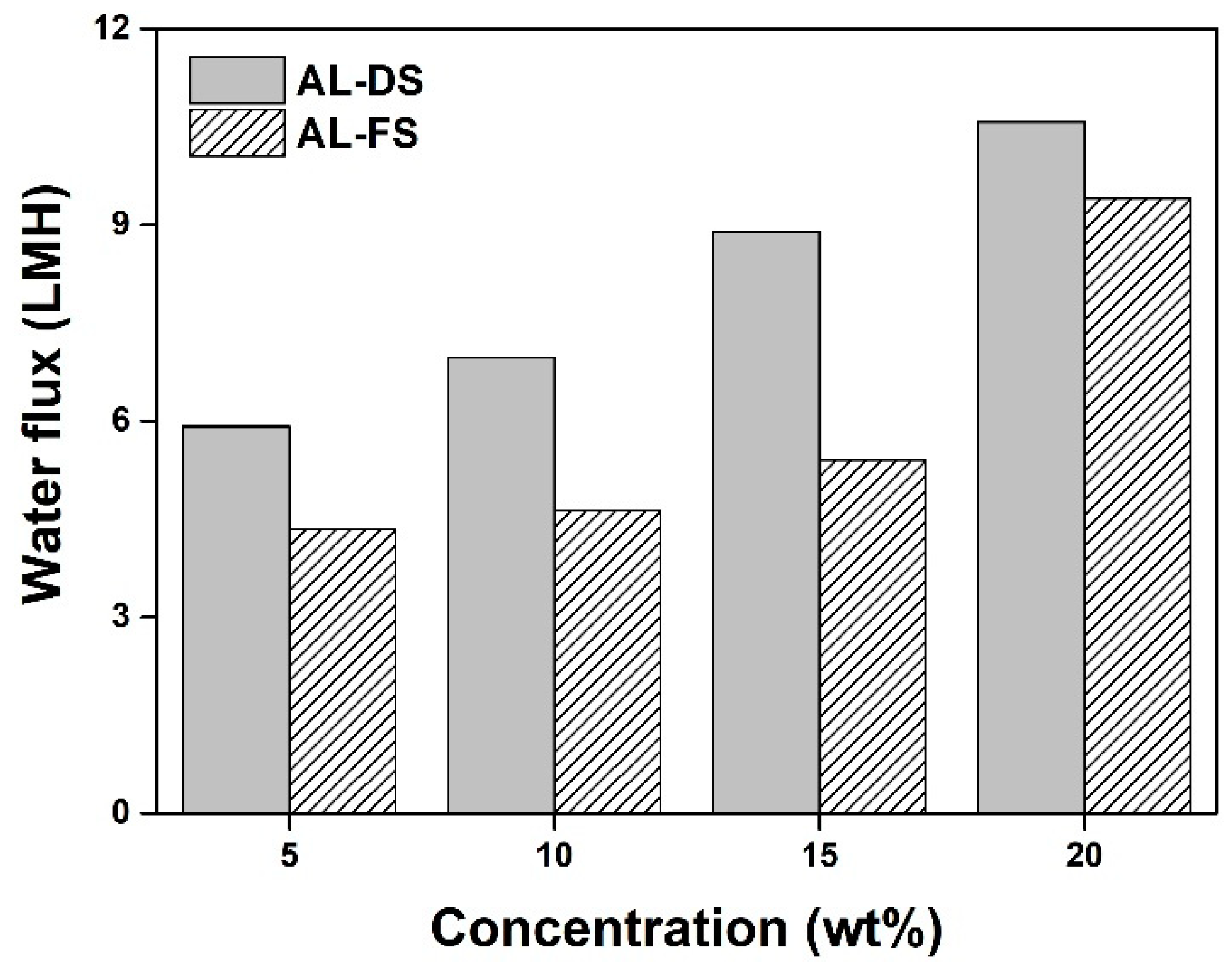
2.5. Water Flux
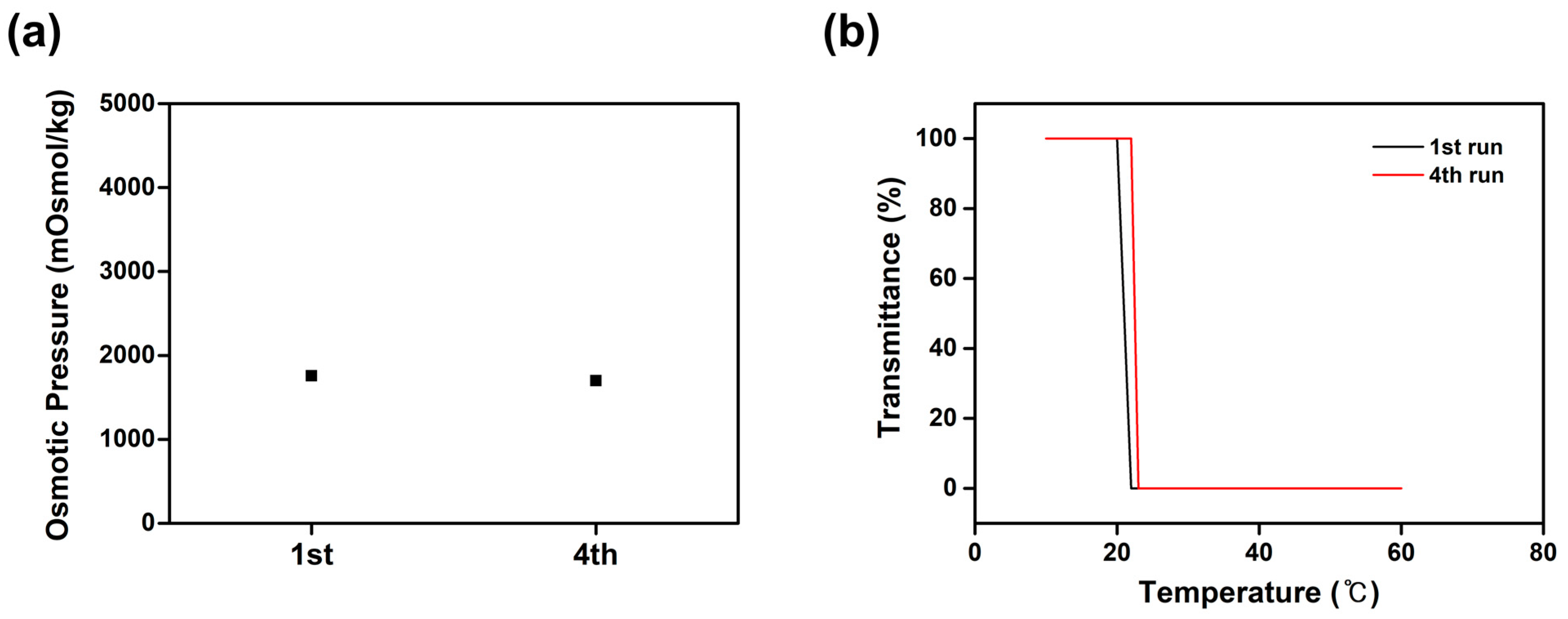
2.6. Recyclability Study of [(P4444)2][MBS]
3. Experimental
3.1. Reagents
3.2. Synthesis of Monocationic and Dicationic ILs
3.2.1. Monocationic Phosphonium-Based ILs
3.2.2. Monocationic Ammonium-Based ILs
3.2.3. Dicationic Phosphonium-Based ILs
3.2.4. Dicationic Ammonium-Based ILs
3.3. Characterization
3.4. Forward Osmosis Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Reimer, J.E.; Maas, C.M.; Galella, J.G.; Utz, R.M.; Duan, S.; Kryger, J.R.; Yaculak, A.M. Freshwater Salinization Syndrome: From Emerging Global Problem to Managing Risks. Biogeochemistry 2021, 154, 255–292. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The Problem of Drinking Water Access: A Review of Disinfection Technologies with an Emphasis on Solar Treatment Methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Zhang, H.; Jiang, Z.; Liu, C.; Cai, W. A Review on Energy, Environment and Economic Assessment in Remanufacturing Based on Life Cycle Assessment Method. J. Clean Prod. 2020, 255, 120160. [Google Scholar] [CrossRef]
- Khodakarami, M.; Bagheri, M. Recent Advances in Synthesis and Application of Polymer Nanocomposites for Water and Wastewater Treatment. J. Clean Prod. 2021, 296, 126404. [Google Scholar] [CrossRef]
- Kehrein, P.; Van, L.M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A Critical Review of Resource Recovery from Municipal Wastewater Treatment Plants–Market Supply Potentials, Technologies and Bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Chen, Y.; Gao, B.; Wang, Z. Scaling Control of Forward Osmosis-Membrane Distillation (FO-MD) Integrated Process for Pretreated Landfill Leachate Treatment. Desalination 2021, 520, 115342. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Ng, B.C.; Abdullah, M.S. Recent Progresses of Forward Osmosis Membranes Formulation and Design for Wastewater Treatment. Water 2019, 11, 2043. [Google Scholar] [CrossRef]
- Mahto, A.; Aruchamy, K.; Meena, R.; Kamali, M.; Nataraj, S.K.; Aminabhavi, T.M. Forward Osmosis for Industrial Effluents Treatment–Sustainability Considerations. Sep. Purif. Technol. 2021, 254, 117568. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; She, Q.; Fane, A.G.; Field, R.W. Exploring the Differences between Forward Osmosis and Reverse Osmosis Fouling. J. Membr. Sci. 2018, 565, 241–253. [Google Scholar] [CrossRef]
- Inada, A.; Yumiya, K.; Takahashi, T.; Kumagai, K.; Hashizume, Y.; Matsuyama, H. Development of Thermoresponsive Star Oligomers with a Glycerol Backbone as the Draw Solute in Forward Osmosis Process. J. Membr. Sci. 2019, 574, 147–153. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward Osmosis: Where are we Now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Rufuss, D.D.W.; Kapoor, V.; Arulvel, S.; Davies, P.A. Advances in Forward Osmosis (FO) Technology for Enhanced Efficiency and Output: A Critical Review. J. Clean Prod. 2022, 356, 131769. [Google Scholar] [CrossRef]
- Abdullah, M.A.M.; Man, M.S.; Abdullah, S.B.; Saufi, S.M. A Glance on Thermo-Responsive Ionic Liquids as Draw Solution in Forward Osmosis System. Desalination Water Treat. 2020, 206, 165–176. [Google Scholar] [CrossRef]
- Inada, A.; Takahashi, T.; Kumagai, K.; Matsuyama, H. Morpholine Derivatives as Thermoresponsive Draw Solutes for Forward Osmosis Desalination. Ind. Eng. Chem. Res. 2019, 58, 12253–12260. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward Osmosis Membranes and Processes: A Comprehensive Review of Research Trends and Future Outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Johnson, D.J.; Suwaileh, W.A.; Mohammed, A.W.; Hilal, N. Osmotic’s Potential: An Overview of Draw Solutes for Forward Osmosis. Desalination 2018, 434, 100–120. [Google Scholar] [CrossRef]
- D’Haese, A.K. Interactions Between Feed Solutes and Inorganic Electrolytic Draw Solutes in Forward Osmosis. J. Membr. Sci. 2020, 597, 117636. [Google Scholar] [CrossRef]
- Siddique, M.S.; Khan, S.J.; Shahzad, M.A.; Nawaz, M.S.; Hankins, N.P. Insight into the Effect of Organic and Inorganic Draw Solutes on the Flux Stability and Sludge Characteristics in the Osmotic Membrane Bioreactor. Bioresour. Technol. 2018, 249, 758–766. [Google Scholar] [CrossRef]
- Ellis, S.N.; Riabtseva, A.; Dykeman, R.R.; Hargreaves, S.; Robert, T.; Champagne, P.; Cunningham, M.F.; Jessop, P.G. Nitrogen Rich CO2-Responsive Polymers as Forward Osmosis Draw Solutes. Ind. Eng. Chem. Res. 2019, 58, 22579–22586. [Google Scholar] [CrossRef]
- Wang, J.; Gao, S.; Tian, J.; Cui, F.; Shi, W. Recent Developments and Future Challenges of Hydrogels as Draw Solutes in Forward Osmosis Process. Water 2020, 12, 692. [Google Scholar] [CrossRef]
- Zeweldi, H.G.; Limjuco, L.A.; Bendoy, A.P.; Kim, H.S.; Park, M.J.; Shon, H.K.; Johnson, E.M.; Lee, H.; Chung, W.J.; Nisola, G.M. The Potential of Monocationic Imidazolium-, Phosphonium-, and Ammonium-Based Hydrophilic Ionic Liquids as Draw Solutes for Forward Osmosis. Desalination 2018, 444, 94–106. [Google Scholar] [CrossRef]
- Bendoy, A.P.; Zeweldi, H.G.; Park, M.J.; Shon, H.K.; Kim, H.; Chung, W.; Nisola, G.M. System using a Response Surface Methodology with Central Composite Design. Desalination 2022, 542, 116067. [Google Scholar] [CrossRef]
- Long, Q.; Jia, Y.; Li, J.; Yang, J.; Liu, F.; Zheng, J.; Yu, B. Recent Advance on Draw Solutes Development in Forward Osmosis. Processes 2018, 6, 165. [Google Scholar] [CrossRef]
- Joafshan, M.; Shakeri, A.; Razavi, S.R.; Salehi, H. Gas Responsive Magnetic Nanoparticle as Novel Draw Agent for Removal of Rhodamine B via Forward Osmosis: High Water Flux and Easy Regeneration. Sep. Purif. Technol. 2022, 282, 119998. [Google Scholar] [CrossRef]
- Ahmed, M.; Kumar, R.; Garudachari, B.; Thomas, J.P. Performance Evaluation of a Thermoresponsive Polyelectrolyte Draw Solution in a Pilot Scale Forward Osmosis Seawater Desalination System. Desalination 2019, 452, 132–140. [Google Scholar] [CrossRef]
- Chaoui, I.; Abderafi, S.; Vaudreuil, S.; Bounahmidi, T. Water Desalination by Forward Osmosis: Draw Solutes and Recovery Methods–Review. Environ. Technol. Rev. 2019, 8, 25–46. [Google Scholar] [CrossRef]
- Kamio, E.; Kurisu, H.; Takahashi, T.; Matsuoka, A.; Yoshioka, T.; Nakagawa, K.; Sun, Y.; Matsuyama, H. Effect of Temperature on the Osmotic Behavior of LCST Type Ionic Liquid Solutions as Draw Solutions in the Forward Osmosis Process. Sep. Purif. Technol. 2021, 275, 119164. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Syu, Z.; Chiao, Y.; Wickramasinghe, S.R.; Ji, Y.; An, Q.; Hung, W.; Hu, C.; Lee, K.; Lai, J. Characterization of a Thermoresponsive Chitosan Derivative as a Potential Draw Solute for Forward Osmosis. Environ. Sci. Technol. 2016, 50, 11935–11942. [Google Scholar] [CrossRef]
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2018, 91, 505–531. [Google Scholar] [CrossRef]
- Zhao, H.; Xia, S.; Ma, P. Use of Ionic Liquids as ‘green’solvents for Extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096. [Google Scholar] [CrossRef]
- Chiappe, C.; Pomelli, C.S.; Rajamani, S. Influence of Structural Variations in Cationic and Anionic Moieties on the Polarity of Ionic Liquids. J. Phys. Chem. B 2011, 115, 9653–9661. [Google Scholar] [CrossRef]
- Freitas, A.A.; Shimizu, K.; Canongia Lopes, J.N. Complex Structure of Ionic Liquids. Molecular Dynamics Studies with Different Cation–Anion Combinations. J. Chem. Eng. Data 2014, 59, 3120–3129. [Google Scholar] [CrossRef]
- Brunel, F.; Lautard, C.; Garzino, F.; Raimundo, J.; Bolla, J.; Camplo, M. Phosphonium-Ammonium-Based Di-Cationic Ionic Liquids as Antibacterial Over the ESKAPE Group. Bioorg. Med. Chem. Lett. 2020, 30, 127389. [Google Scholar] [CrossRef]
- Lungwitz, R.; Strehmel, V.; Spange, S. The Dipolarity/Polarisability of 1-Alkyl-3-Methylimidazolium Ionic Liquids as Function of Anion Structure and the Alkyl Chain Length. New J. Chem. 2010, 34, 1135–1140. [Google Scholar] [CrossRef]
- Russina, O.; Triolo, A.; Gontrani, L.; Caminiti, R. Mesoscopic Structural Heterogeneities in Room-Temperature Ionic Liquids. J. Phys. Chem. Lett. 2012, 3, 27–33. [Google Scholar] [CrossRef]
- Moosavi, M.; Khashei, F.; Sharifi, A.; Mirzaei, M. The Effects of Temperature and Alkyl Chain Length on the Density and Surface Tension of the Imidazolium-Based Geminal Dicationic Ionic Liquids. J. Chem. Thermodyn. 2017, 107, 1–7. [Google Scholar] [CrossRef]
- Talebi, M.; Patil, R.A.; Armstrong, D.W. Physicochemical Properties of Branched-Chain Dicationic Ionic Liquids. J. Mol. Liq. 2018, 256, 247–255. [Google Scholar] [CrossRef]
- Masri, A.N.; Mi, A.M.; Leveque, J. A Review on Dicationic Ionic Liquids: Classification and Application. Ind. Eng. Manag. 2016, 5, 197–204. [Google Scholar] [CrossRef]
- Moosavi, M.; Khashei, F.; Sharifi, A.; Mirzaei, M. Transport Properties of Short Alkyl Chain Length Dicationic Ionic Liquids the Effects of Alkyl Chain Length and Temperature. Ind. Eng. Chem. Res. 2016, 55, 9087–9099. [Google Scholar] [CrossRef]
- Javaherian, M.; Saghanezhad, S.J. Synthesis, Characterization and Applications of Dicationic Ionic Liquids in Organic Synthesis. Mini-Rev. Org. Chem. 2020, 17, 450–464. [Google Scholar] [CrossRef]
- Bender, C.R.; Kuhn, B.L.; Farias, C.A.; Ziembowicz, F.I.; Beck, T.S.; Frizzo, C.P. Thermal Stability and Kinetic of Decomposition of Mono-and Dicationic Imidazolium-Based Ionic Liquids. J. Braz. Chem. Soc. 2019, 30, 2199–2209. [Google Scholar] [CrossRef]
- Kaczmarek, D.K.; Gwiazdowska, D.; Marchwińska, K.; Klejdysz, T.; Wojcieszak, M.; Materna, K.; Pernak, J. Amino Acid-Based Dicationic Ionic Liquids as Complex Crop Protection Agents. J. Mol. Liq. 2022, 360, 119357. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and Purification of Ionic Liquids from Solutions: A Review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef] [PubMed]
- Correia, D.M.; Fernandes, L.C.; Fernandes, M.M.; Hermenegildo, B.; Meira, R.M.; Ribeiro, C.; Ribeiro, S.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid-Based Materials for Biomedical Applications. Nanomaterials 2021, 11, 2401. [Google Scholar] [CrossRef] [PubMed]
- Rajyaguru, Y.V.; Patil, J.H.; Kusanur, R. Ionic Liquids, an Asset in Extraction Techniques—A Comprehensive Review. Rev. Adv. Chem. 2022, 12, 107–122. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and Comparison of Hydrophilic and Hydrophobic Room Temperature Ionic Liquids Incorporating the Imidazolium Cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Schröder, C. Proteins in Ionic Liquids: Current Status of Experiments and Simulations. In Ionic liquids II; Springer: Cham, Switzerland, 2017; pp. 127–152. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E. Antimicrobial Ionic Liquid-based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Thomas, S.; Rayaroth, M.P.; Menacherry, S.P.M.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical Degradation of Benzenesulfonic Acid in Aqueous Medium. Chemosphere 2020, 252, 126485. [Google Scholar] [CrossRef]
- Masoudian, Z.; Salehi-Lisar, S.Y.; Norastehnia, A. Phytoremediation Potential of Azolla Filiculoides for Sodium Dodecyl Benzene Sulfonate (SDBS) Surfactant Considering some Physiological Responses, Effects of Operational Parameters and Biodegradation of Surfactant. Environ. Sci. Pollut. Res. 2020, 27, 20358–20369. [Google Scholar] [CrossRef]
- Kohno, Y.; Saita, S.; Men, Y.; Yuan, J.; Ohno, H. Thermoresponsive Polyelectrolytes Derived from Ionic Liquids. Polym. Chem. 2015, 6, 2163–2178. [Google Scholar] [CrossRef]
- Cui, J.; Li, Y.; Chen, D.; Zhan, T.; Zhang, K. Ionic Liquid-based Stimuli-Responsive Functional Materials. Adv. Funct. Mater. 2020, 30, 2005522. [Google Scholar] [CrossRef]
- Takahashi, T.; Akiya, K.; Niizeki, T.; Matsumoto, M.; Hoshina, T. Tunable Thermoresponsive UCST-Type Alkylimidazolium Ionic Liquids as a Draw Solution in the Forward Osmosis Process. Colloids. Surf. A Physicochem. Eng. Asp. 2022, 639, 128372. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, W.; Wei, J.; Chong, T.H.; Wang, R.; Krantz, W.B.; Fane, A.G.; Hu, X. Energy-Efficient Desalination by Forward Osmosis using Responsive Ionic Liquid Draw Solutes. Environ. Sci. Water Res. Technol. 2015, 1, 341–347. [Google Scholar] [CrossRef]
- Dutta, S.; Nath, K. Prospect of Ionic Liquids and Deep Eutectic Solvents as New Generation Draw Solution in Forward Osmosis Process. J. Water Process Eng. 2018, 21, 163–176. [Google Scholar] [CrossRef]
- Zeweldi, H.G.; Bendoy, A.P.; Park, M.J.; Shon, H.K.; Kim, H.; Johnson, E.M.; Kim, H.; Lee, S.; Chung, W.; Nisola, G.M. Tetrabutylammonium 2,4,6-Trimethylbenzenesulfonate as an Effective and Regenerable Thermo-Responsive Ionic Liquid Drawing Agent in Forward Osmosis for Seawater Desalination. Desalination 2020, 495, 114635. [Google Scholar] [CrossRef]
- Kamio, E.; Takenaka, A.; Takahashi, T.; Matsuyama, H. Fundamental Investigation of Osmolality, Thermo-Responsive Phase Diagram, and Water-Drawing Ability of Ionic-Liquid-Based Draw Solution for Forward Osmosis Membrane Process. J. Membr. Sci. 2019, 570, 93–102. [Google Scholar] [CrossRef]
- Gude, V.G.; Fthenakis, V. Energy Efficiency and Renewable Energy Utilization in Desalination Systems. Prog. Energy 2020, 2, 022003. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, Z.; Li, J.; Tang, Q.; Hu, Y. Modeling and Measurement of Temperature and Draw Solution Concentration Induced Water Flux Increment Efficiencies in the Forward Osmosis Membrane Process. Desalination 2019, 452, 75–86. [Google Scholar] [CrossRef]
- Kim, T.; Ju, C.; Park, C.; Kang, H. Polymer having Dicationic Structure in Dumbbell Shape for Forward Osmosis Process. Polymers 2019, 11, 571. [Google Scholar] [CrossRef]
- Men, Y.; Schlaad, H.; Voelkel, A.; Yuan, J. Thermoresponsive Polymerized Gemini Dicationic Ionic Liquid. Polym. Chem. 2014, 5, 3719–3724. [Google Scholar] [CrossRef]
- Liu, P.; Wang, D.C.; Ho, C.; Chen, Y.; Chung, L.; Liang, T.; Chang, M.; Horng, R. Exploring the Performance-Affecting Factors of Monocationic and Dicationic Phosphonium-Based Thermoresponsive Ionic Liquid Draw Solutes in Forward Osmosis. Desalination Water Treat. 2020, 200, 1–7. [Google Scholar] [CrossRef]
- Raulerson, C.R.; Popat, S.C.; Husson, S.M. Water Recovery from Bioreactor Mixed Liquors using Forward Osmosis with Polyelectrolyte Draw Solutions. Membranes 2021, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Madsen, L.A. Observation of Separate Cation and Anion Electrophoretic Mobilities in Pure Ionic Liquids. J. Chem. Phys. 2014, 140, 084204. [Google Scholar] [CrossRef] [PubMed]
- Narita, A.; Shibayama, W.; Ohno, H. Structural Factors to Improve Physico-Chemical Properties of Zwitterions as Ion Conductive Matrices. J. Mater. Chem. 2006, 16, 1475–1482. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Tu, R.; Shen, Q.; Zhang, X.; Zhang, L. Effect of Lithium Ion Concentration on the Microstructure Evolution and its Association with the Ionic Conductivity of Cubic Garnet-Type Nominal Li7Al0.25La3Zr2O12 Solid Electrolytes. Solid State Ion. 2016, 284, 53–60. [Google Scholar] [CrossRef]
- Alencherry, T.; Naveen, A.R.; Ghosh, S.; Daniel, J.; Venkataraghavan, R. Effect of Increasing Electrical Conductivity and Hydrophilicity on the Electrosorption Capacity of Activated Carbon Electrodes for Capacitive Deionization. Desalination 2017, 415, 14–19. [Google Scholar] [CrossRef]
- Vila, J.; Varela, L.M.; Cabeza, O. Cation and Anion Sizes Influence in the Temperature Dependence of the Electrical Conductivity in Nine Imidazolium Based Ionic Liquids. Electrochim. Acta 2007, 52, 7413–7417. [Google Scholar] [CrossRef]
- Achilli, A.; Cath, T.Y.; Childress, A.E. Selection of Inorganic-Based Draw Solutions for Forward Osmosis Applications. J. Membr. Sci. 2010, 364, 233–241. [Google Scholar] [CrossRef]
- Rodenburg, J.; Dijkstra, M.; van Roij, R. Van’t Hoff’s Law for Active Suspensions: The Role of the Solvent Chemical Potential. Soft Matter 2017, 13, 8957–8963. [Google Scholar] [CrossRef]
- Park, S.Y.; Ahn, H.; Chung, J.W.; Kwak, S. Magnetic Core-Hydrophilic Shell Nanosphere as Stability-Enhanced Draw Solute for Forward Osmosis (FO) Application. Desalination 2016, 397, 22–29. [Google Scholar] [CrossRef]
- Kilduff, J.E.; Mattaraj, S.; Belfort, G. Flux Decline during Nanofiltration of Naturally-Occurring Dissolved Organic Matter: Effects of Osmotic Pressure, Membrane Permeability, and Cake Formation. J. Membr. Sci. 2004, 239, 39–53. [Google Scholar] [CrossRef]
- Liu, C.; An, Y.; Yang, J.; Guo, B.; Yu, H.; Xu, Z. Osmotic Pressure as Driving Force for Recovering Ionic Liquids from Aqueous Solutions. J. Membr. Sci. 2020, 599, 117835. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, W.; Zhang, H.; Zhang, K.; Wang, Z.; Liu, J. Enhanced Performance of Porous Forward Osmosis (FO) Membrane in the Treatment of Oily Wastewater Containing HPAM by the incorporation of palygorskite. RSC Adv. 2021, 11, 22439–22449. [Google Scholar] [CrossRef]
- Itsuki, K.; Kawata, Y.; Sharker, K.K.; Yusa, S. Ultrasound-and Thermo-Responsive Ionic Liquid Polymers. Polymers 2018, 10, 301. [Google Scholar] [CrossRef]
- Nash, M.E.; Carroll, W.M.; Velasco, D.; Gomez, J.; Gorelov, A.V.; Elezov, D.; Gallardo, A.; Rochev, Y.A.; Elvira, C. Synthesis and Characterization of a Novel Thermoresponsive Copolymer Series and their Application in Cell and Cell Sheet Regeneration. J. Biomater. Sci. Polym. Ed. 2013, 24, 253–268. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Pei, Y.; Liu, Z.; Wang, J. Understanding the Mechanism of LCST Phase Separation of Mixed Ionic Liquids in Water by MD Simulations. Phys. Chem. Chem. Phys. 2016, 18, 23238–23245. [Google Scholar] [CrossRef]
- Idziak, I.; Avoce, D.; Lessard, D.; Gravel, D.; Zhu, X.X. Thermosensitivity of Aqueous Solutions of Poly (N,N-Diethylacrylamide). Macromolecules 1999, 32, 1260–1263. [Google Scholar] [CrossRef]
- Clark, K.D.; Emaus, M.N.; Varona, M.; Bowers, A.N.; Anderson, J.L. Ionic Liquids: Solvents and Sorbents in Sample Preparation. J. Sep. Sci. 2018, 41, 209–235. [Google Scholar] [CrossRef]
- Kumari, P.; Pillai, V.; Gobbo, D.; Ballone, P.; Benedetto, A. The Transition from Salt-in-Water to Water-in-Salt Nanostructures in Water Solutions of Organic Ionic Liquids Relevant for Biological Applications. Phys. Chem. Chem. Phys. 2021, 23, 944–959. [Google Scholar] [CrossRef]
- Dong, S.; Heyda, J.; Yuan, J.; Schalley, C.A. Lower Critical Solution Temperature (LCST) Phase Behaviour of an Ionic Liquid and its Control by Supramolecular Host–guest Interactions. Chem. Commun. 2016, 52, 7970–7973. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, X.; Wen, X.; Xu, Q.; Zeng, H.; Zhao, Y.; Liu, M.; Wang, Z.; Hu, X.; Wang, Y. Bio-Responsive Smart Polymers and Biomedical Applications. J. Phys. Mater. 2019, 2, 032004. [Google Scholar] [CrossRef]
- Sugeno, K.; Kokubun, S.; Saito, H. Ucst Type Phase Boundary and Accelerated Crystallization in Ptt/Pet Blends. Polymers 2020, 12, 2730. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, P.; Zhao, Q.; Chen, N.; Lu, X. Thermoresponsive Copolymer-Based Draw Solution for Seawater Desalination in a Combined Process of Forward Osmosis and Membrane Distillation. Desalination 2014, 348, 26–32. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Impact of Support Layer Pore Size on Performance of Thin Film Composite Membranes for Forward Osmosis. J. Membr. Sci. 2015, 483, 25–33. [Google Scholar] [CrossRef]
- Oh, Y.; Lee, S.; Elimelech, M.; Lee, S.; Hong, S. Effect of Hydraulic Pressure and Membrane Orientation on Water Flux and Reverse Solute Flux in Pressure Assisted Osmosis. J. Membr. Sci. 2014, 465, 159–166. [Google Scholar] [CrossRef]
- McCutcheon, J.R.; McGinnis, R.L.; Elimelech, M. Desalination by Ammonia–carbon Dioxide Forward Osmosis: Influence of Draw and Feed Solution Concentrations on Process Performance. J. Membr. Sci. 2006, 278, 114–123. [Google Scholar] [CrossRef]
- Chen, G.; Liu, R.; Shon, H.K.; Wang, Y.; Song, J.; Li, X.; He, T. Open Porous Hydrophilic Supported Thin-Film Composite Forward Osmosis Membrane via co-Casting for Treatment of High-Salinity Wastewater. Desalination 2017, 405, 76–84. [Google Scholar] [CrossRef]
- Wu, B.; Yamashita, Y.; Endo, T.; Takahashi, K.; Castner Jr, E.W. Structure and Dynamics of Ionic Liquids: Trimethylsilylpropyl-Substituted Cations and Bis (Sulfonyl) Amide Anions. J. Chem. Phys. 2016, 145, 244506. [Google Scholar] [CrossRef]
- Zhang, J.L.; Srivastava, R.S.; Misra, R. Core− Shell Magnetite Nanoparticles Surface Encapsulated with Smart Stimuli-Responsive Polymer: Synthesis, Characterization, and LCST of Viable Drug-Targeting Delivery System. Langmuir 2007, 23, 6342–6351. [Google Scholar] [CrossRef]
- Phillip, W.A.; Yong, J.S.; Elimelech, M. Reverse Draw Solute Permeation in Forward Osmosis: Modeling and Experiments. Environ. Sci. Technol. 2010, 44, 5170–5176. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Kang, H. Thermoresponsive Ionic Liquid with Different Cation–Anion Pairs as Draw Solutes in Forward Osmosis. Molecules 2022, 27, 8869. https://doi.org/10.3390/molecules27248869
Yang D, Kang H. Thermoresponsive Ionic Liquid with Different Cation–Anion Pairs as Draw Solutes in Forward Osmosis. Molecules. 2022; 27(24):8869. https://doi.org/10.3390/molecules27248869
Chicago/Turabian StyleYang, DaEun, and Hyo Kang. 2022. "Thermoresponsive Ionic Liquid with Different Cation–Anion Pairs as Draw Solutes in Forward Osmosis" Molecules 27, no. 24: 8869. https://doi.org/10.3390/molecules27248869
APA StyleYang, D., & Kang, H. (2022). Thermoresponsive Ionic Liquid with Different Cation–Anion Pairs as Draw Solutes in Forward Osmosis. Molecules, 27(24), 8869. https://doi.org/10.3390/molecules27248869




