Lignosulfonate-Based Ionic Liquids as Asphaltene Dispersants
Abstract
1. Introduction
2. Results and Discussion
2.1. FTIR
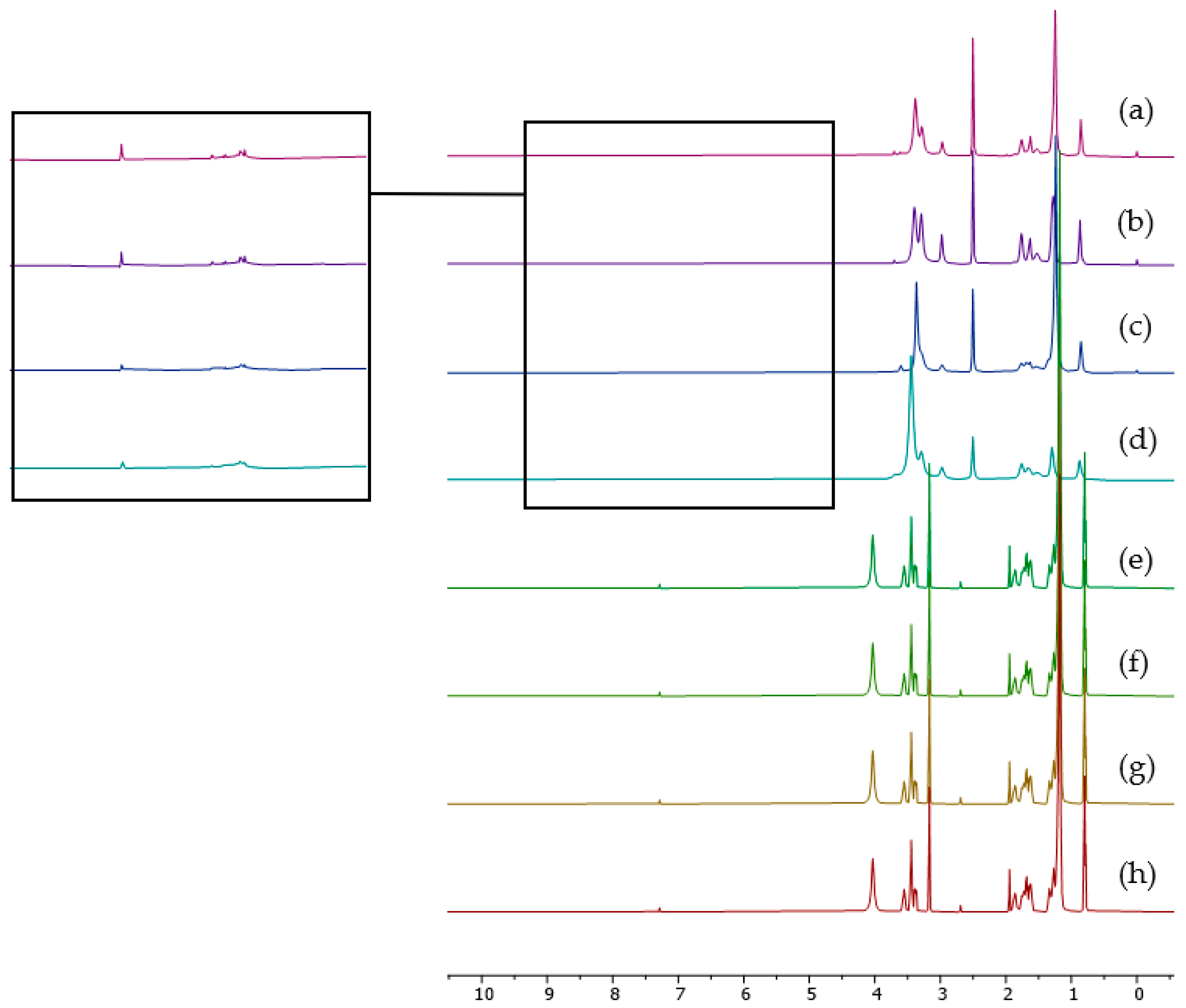
2.2. 1H NMR
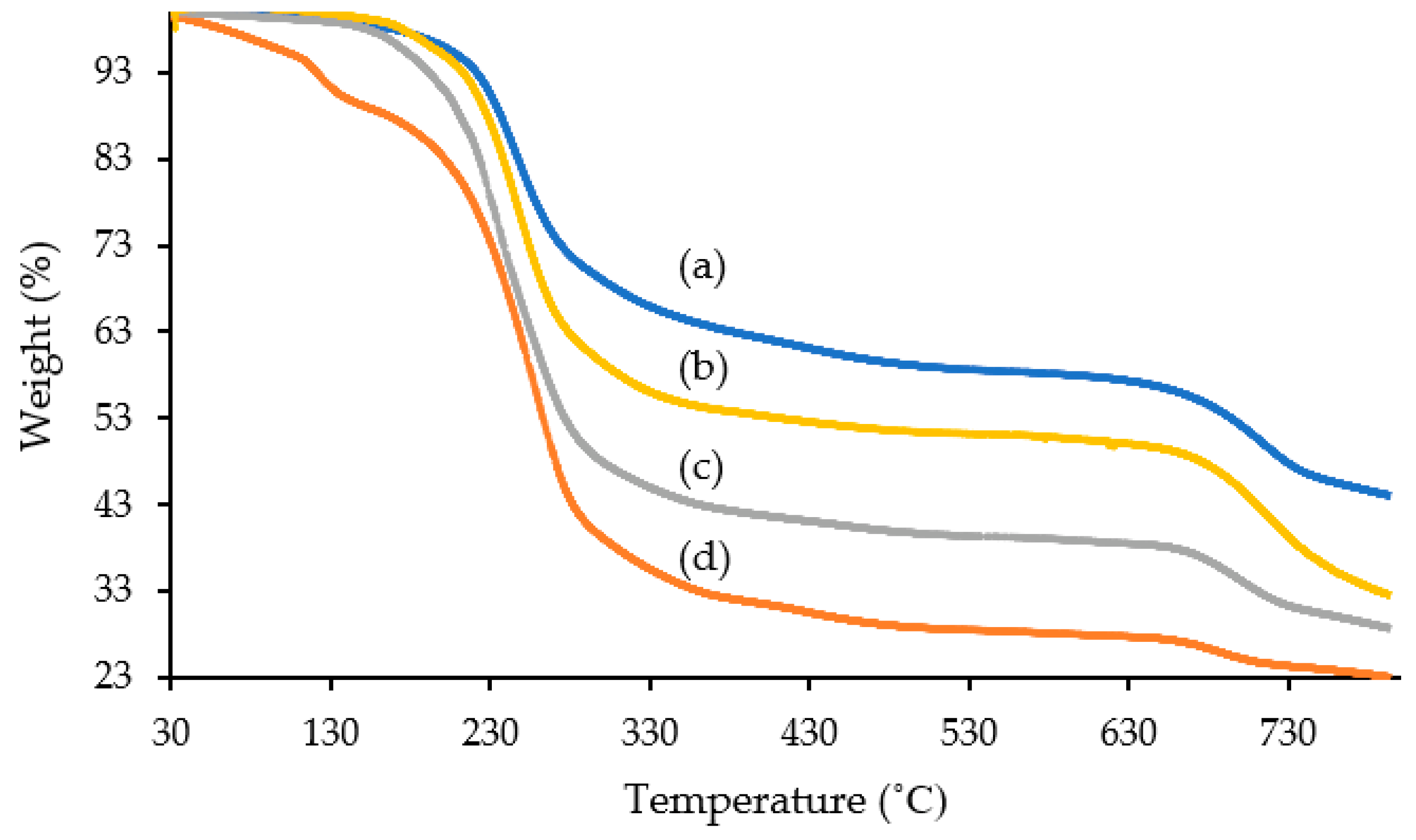
2.3. Thermal Gravimetric Analysis (TGA)
2.4. Asphaltene Dispersion Study
2.4.1. Asphaltene Onset Precipitation
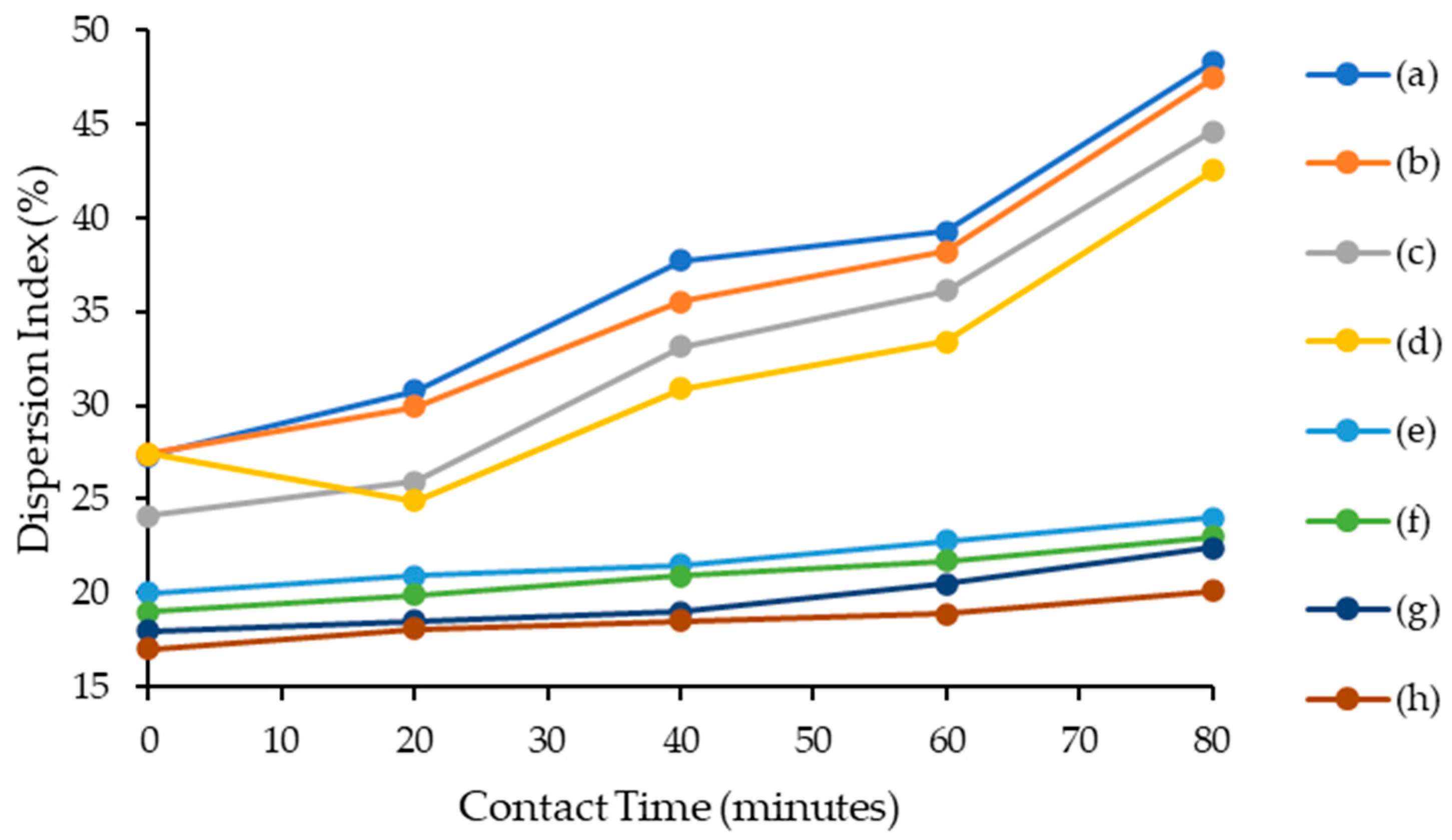
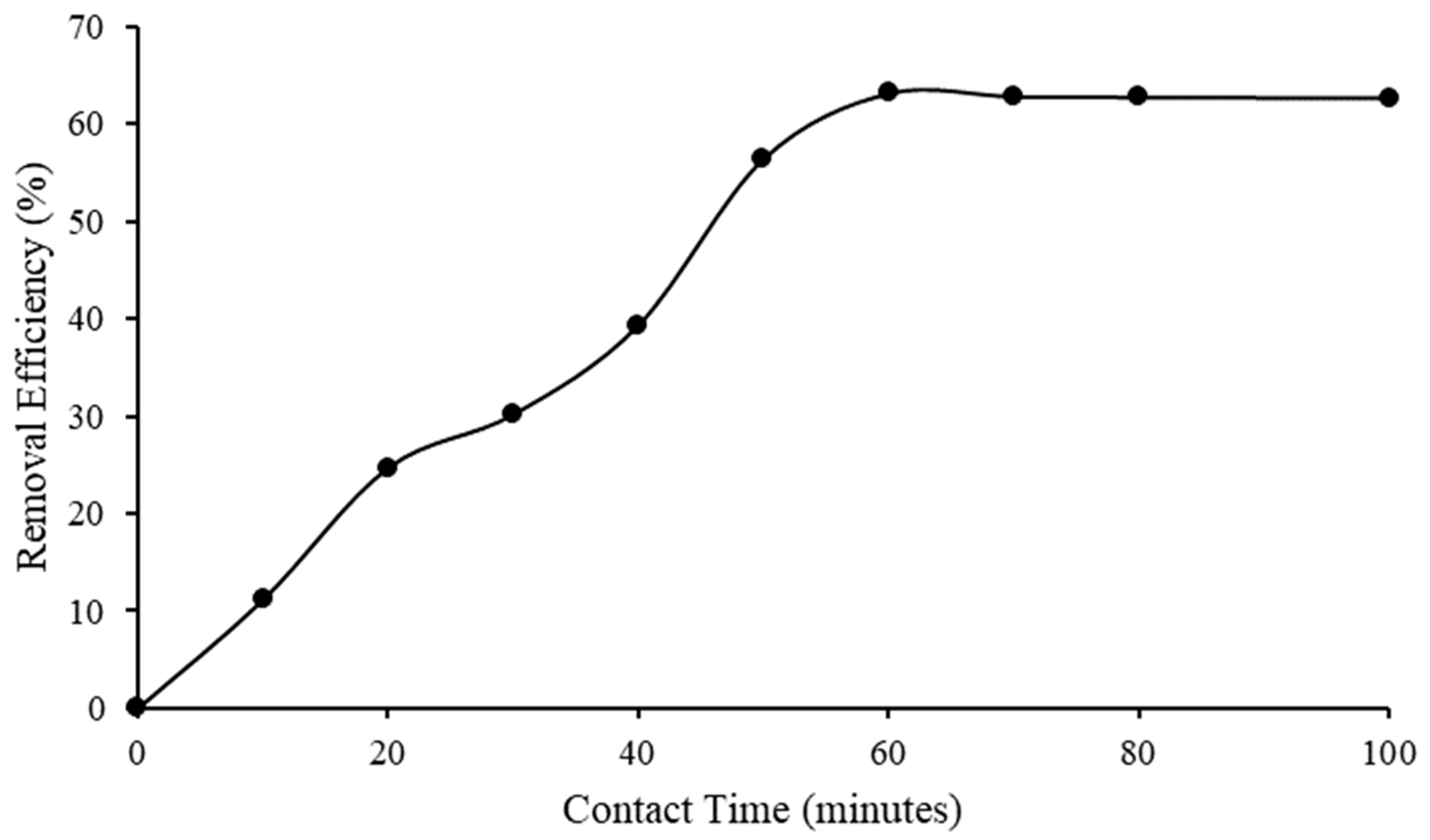
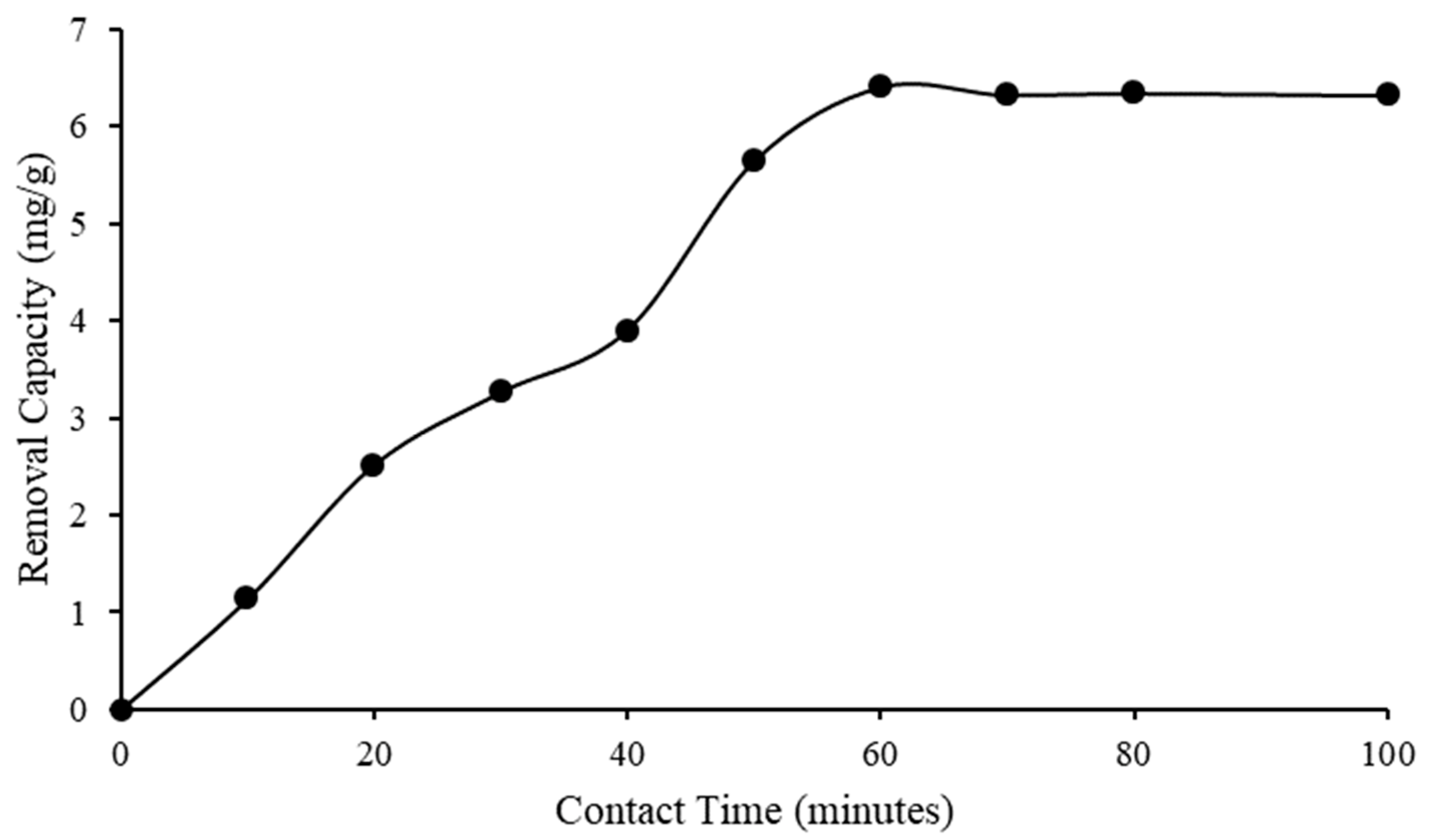
2.4.2. Contact Time Effect
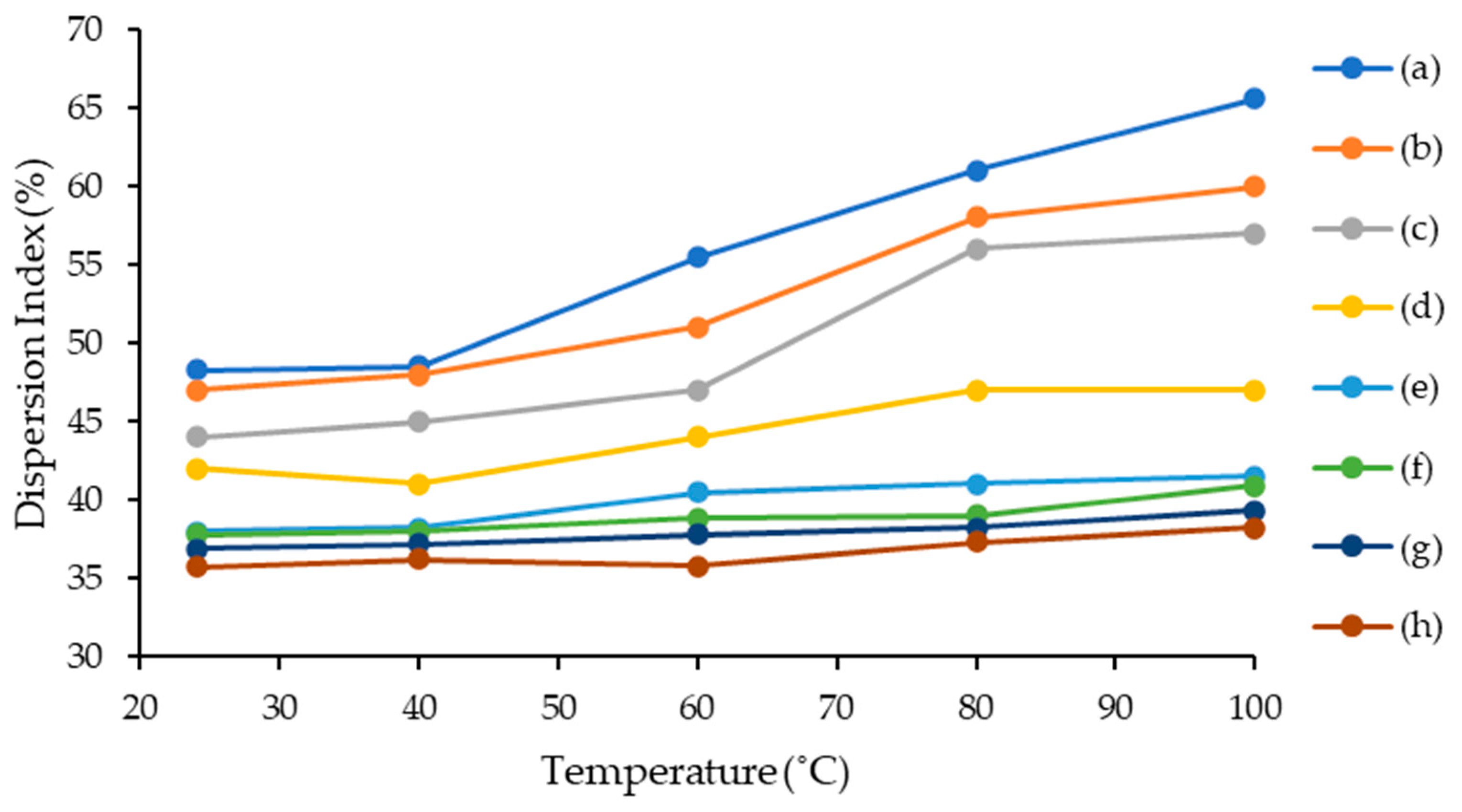
2.4.3. Temperature Effect
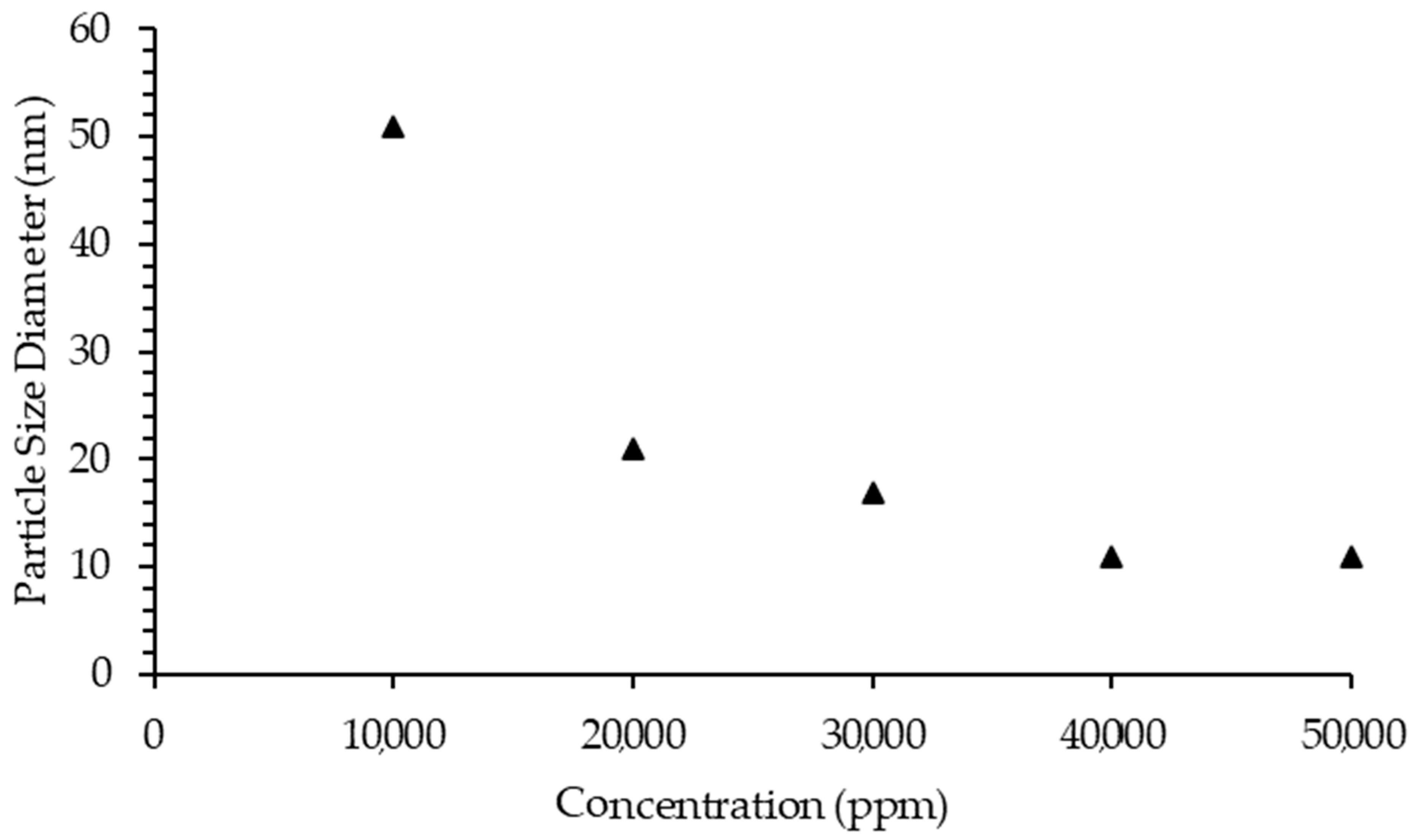
2.4.4. IL Concentration Effect
2.5. Asphaltene Aggregation Study
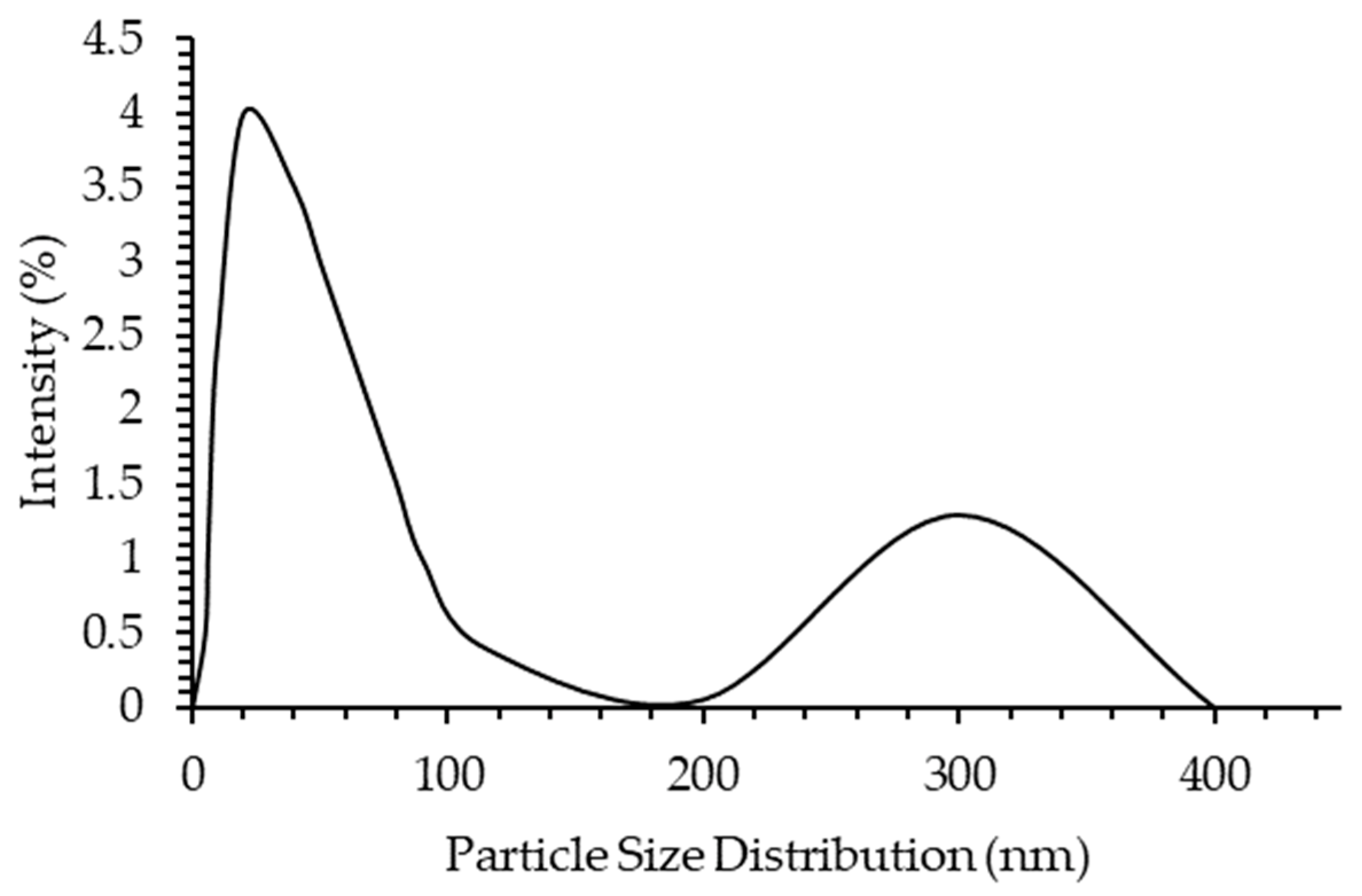
2.5.1. Asphaltene Particle Size Distribution
2.5.2. Asphaltene Particle Size Diameter
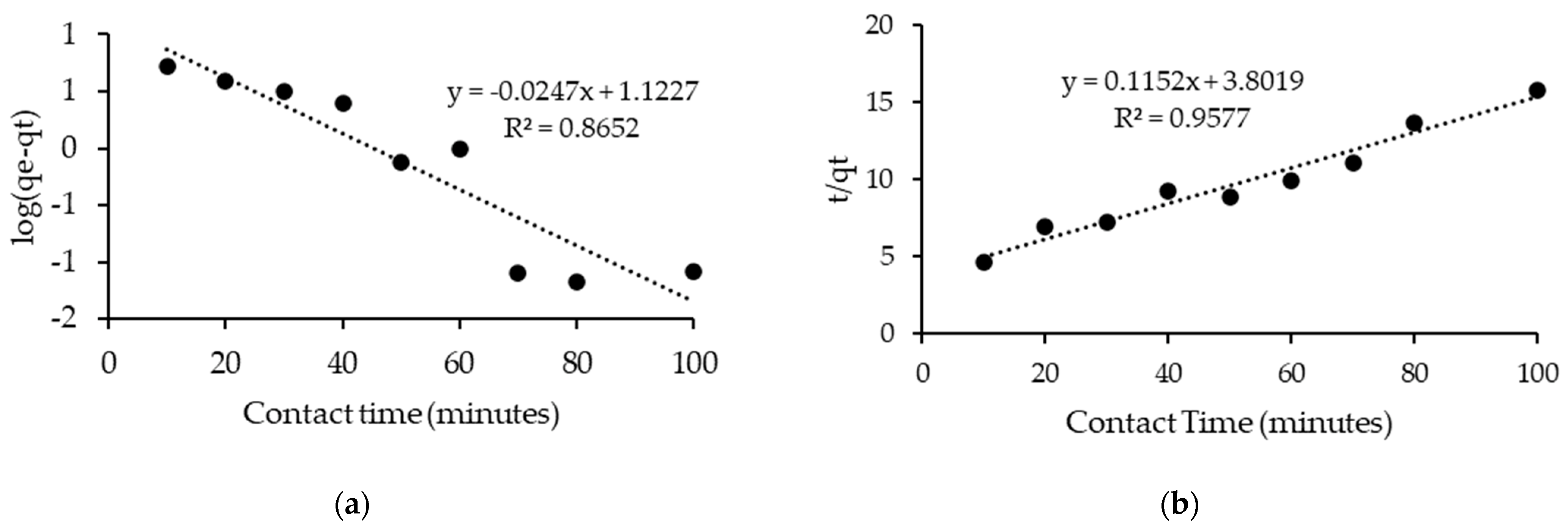
2.6. Kinetic Study
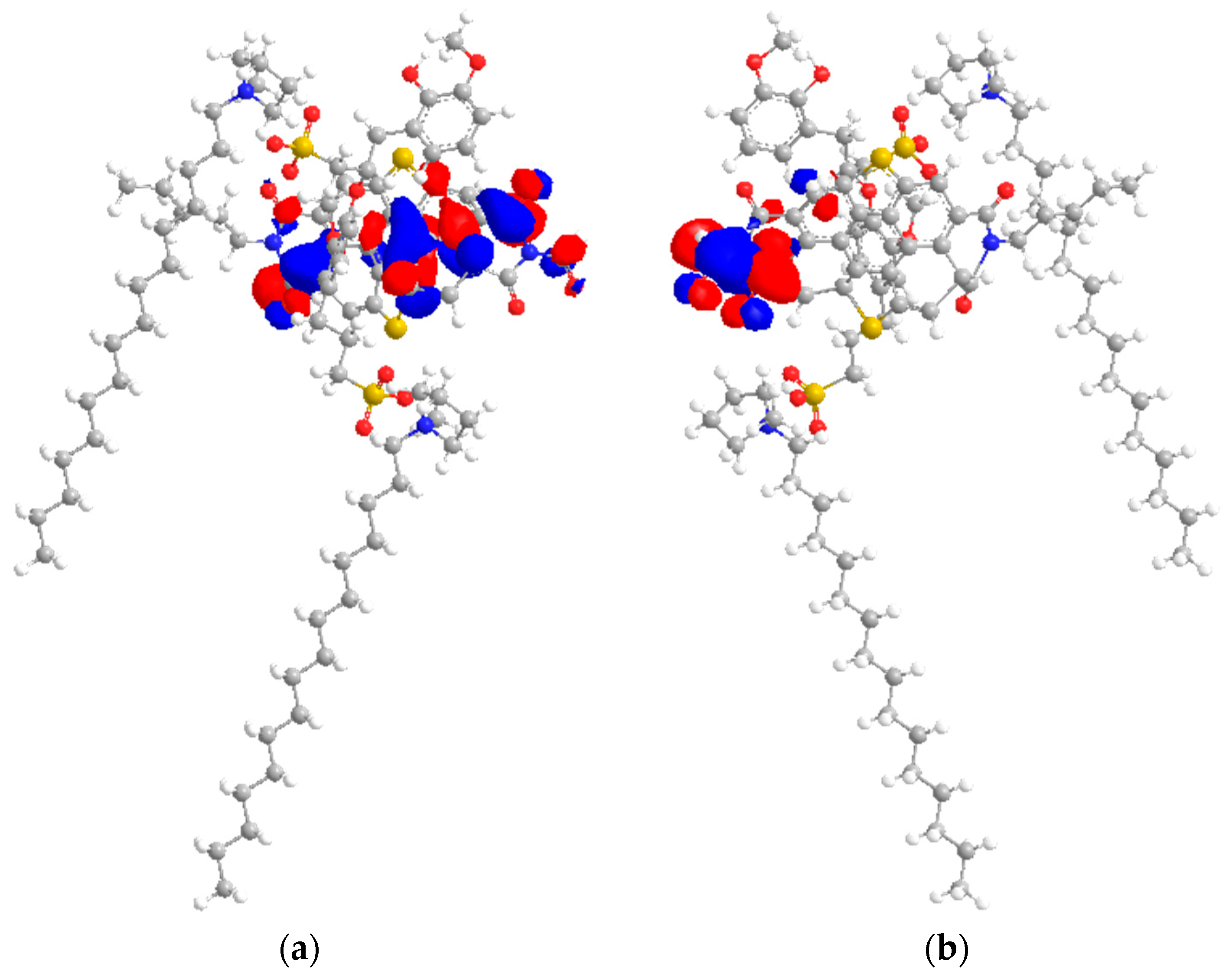
2.7. HOMO–LUMO Study
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Quaternization of N-Methyl-Piperidine
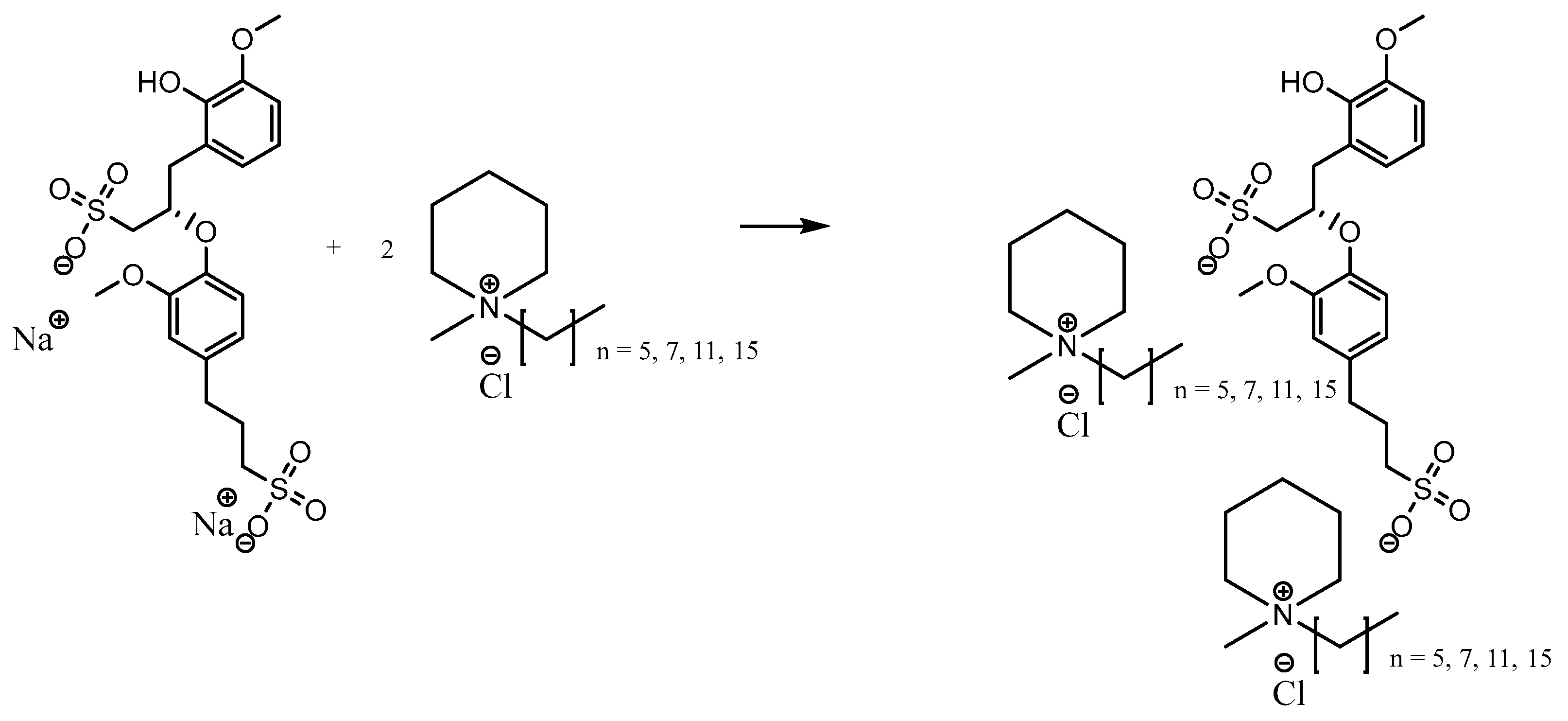
3.2.2. Metathesis of Lignosulfonate-Based Ionic Liquids
3.2.3. Extraction of Asphaltene from Crude Oil
3.2.4. Fourier-Transform Infrared Attenuated Total Reflection (FTIR-ATR)
3.2.5. 1H NMR
3.2.6. TGA
3.2.7. Preparation of Asphaltene Standard Solution Using UV-Vis
3.2.8. Asphaltene Onset Precipitation of [RC1Pip]2[LS] and [RC1Pip][Cl]
3.2.9. Contact Time Effect of [RC1Pip]2[LS] and [RC1Pip][Cl] on Asphaltene Model Oil
3.2.10. Temperature Effect of [RC1Pip]2[LS] and [RC1Pip][Cl] on Asphaltene Model Oil
3.2.11. [RC1Pip]2[LS] and [RC1Pip][Cl] Concentration Effect on Asphaltene Model Oil
3.2.12. Dispersion Calculation
3.2.13. Particle Size Analysis
3.2.14. Kinetic Study
3.2.15. HOMO–LUMO Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mozaffari, S.; Tchoukov, P.; Mozaffari, A.; Atias, J.; Czarnecki, J.; Nazemifard, N. Capillary driven flow in nanochannels—Application to heavy oil rheology studies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 178–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Siskin, M.; Gray, M.R.; Walters, C.C.; Rodgers, R.P. Mechanisms of Asphaltene Aggregation: Puzzles and a New Hypothesis. Energy Fuels 2020, 34, 9094–9107. [Google Scholar] [CrossRef]
- Moud, A.A. Asphaltene induced changes in rheological properties: A review. Fuel 2022, 316, 123372. [Google Scholar] [CrossRef]
- Gray, M.R.; Yarranton, H.W.; Chacón-Patiño, M.L.; Rodgers, R.P.; Bouyssiere, B.; Giusti, P. Distributed Properties of Asphaltene Nanoaggregates in Crude Oils: A Review. Energy Fuels 2021, 35, 18078–18103. [Google Scholar] [CrossRef]
- Mullins, O.C. The Modified Yen Model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Gharfeh, S.; Yen, A.; Asomaning, S.; Blumer, D. Asphaltene Flocculation Onset Determinations for Heavy Crude Oil and Its Implications. Pet. Sci. Technol. 2007, 22, 37–41. [Google Scholar] [CrossRef]
- Goual, L.; Schabron, J.F.; Turner, T.F.; Towler, B.F. On-Column Separation of Wax and Asphaltenes in Petroleum Fluids. Energy Fuels 2008, 75, 4019–4028. [Google Scholar] [CrossRef]
- Baghani, A.N.; Sorooshian, A.; Heydari, M.; Sheikhi, R.; Golbaz, S.; Ashournejad, Q.; Kermani, M.; Golkhorshidi, F.; Barkhordari, A.; Jafari, A.J.; et al. A case study of BTEX characteristics and health effects by major point sources of pollution during winter in Iran. Environ. Pollut. 2019, 247, 607–617. [Google Scholar] [CrossRef]
- Hu, Y.-F.; Guo, T.-M. Effect of the Structures of Ionic Liquids and Alkylbenzene-Derived Amphiphiles on the Inhibition of Asphaltene Precipitation from CO2-Injected Reservoir Oils. Langmuir 2005, 21, 8168–8174. [Google Scholar] [CrossRef]
- Boukherissa, M.; Mutelet, F.; Modarressi, A.; Dicko, A.; Dafri, D.; Rogalski, M. Ionic Liquids as Dispersants of Petroleum Asphaltenes. Energy Fuels 2009, 23, 2557–2564. [Google Scholar] [CrossRef]
- Dhakal, P.; Shah, J.K. Developing machine learning models for ionic conductivity of imidazolium-based ionic liquids. Fluid Phase Equilibria 2021, 549, 113208. [Google Scholar] [CrossRef]
- Yunus, N.M.M.; Dhevarajan, S.; Wilfred, C.D. Studies on the effect of sulfonate based ionic liquids on asphaltenes. J. Mol. Liq. 2022, 360, 119567. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, X.; Chen, Y.; Mu, T. Water Sorption in Amino Acid Ionic Liquids: Kinetic, Mechanism, and Correlations between Hygroscopicity and Solvatochromic Parameters. ACS Sustain. Chem. Eng. 2014, 2, 138–148. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y.; Lu, L.; Xue, Z.; Mu, T. Water Sorption in Functionalized Ionic Liquids: Kinetics and Intermolecular Interactions. Ind. Eng. Chem. Res. 2013, 52, 2073–2083. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Clarke, C.J.; Bui-Le, L.; Hallett, J.P.; Licence, P. Thermally-Stable Imidazolium Dicationic Ionic Liquids with Pyridine Functional Groups. ACS Sustain. Chem. Eng. 2020, 8, 8762–8772. [Google Scholar] [CrossRef]
- Barulli, L.; Mezzetta, A.; Brunetti, B.; Guazzelli, L.; Ciprioti, S.V.; Ciccioli, A. Evaporation thermodynamics of the tetraoctylphosphonium bis(trifluoromethansulfonyl)imide([P8888]NTf2) and tetraoctylphosphonium nonafluorobutane-1-sulfonate ([P8888]NFBS) ionic liquids. J. Mol. Liq. 2021, 333, 115892. [Google Scholar] [CrossRef]
- Wu, H.-B.; Zhang, B.; Liu, S.-H.; Chen, C.-C. Flammability estimation of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide. J. Loss Prev. Process. Ind. 2020, 66, 104196. [Google Scholar] [CrossRef]
- Wolny, A.; Chrobok, A. Silica-Based Supported Ionic Liquid-like Phases as Heterogeneous Catalysts. Molecules 2022, 27, 5900. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Gao, Y.-R.; Xue, R.; Nguyen, W.; Chen, W.; Wang, J.-H.; Shu, Y. Liquid formulations based on ionic liquids in biomedicine. Mater. Today Phys. 2023, 30, 100925. [Google Scholar] [CrossRef]
- Tang, X.; Lv, S.; Jiang, K.; Zhou, G.; Liu, X. Recent development of ionic liquid-based electrolytes in lithium-ion batteries. J. Power Sources 2022, 542, 231792. [Google Scholar] [CrossRef]
- Ullah, H.; Wilfred, C.D.; Shaharun, M. Ionic liquid-based extraction and separation trends of bioactive compounds from plant biomass. Sep. Sci. Technol. 2018, 54, 559–579. [Google Scholar] [CrossRef]
- Paucar, N.E.; Kiggins, P.; Blad, B.; De Jesus, K.; Afrin, F.; Pashikanti, S.; Sharma, K. Ionic liquids for the removal of sulfur and nitrogen compounds in fuels: A review. Environ. Chem. Lett. 2021, 19, 1205–1228. [Google Scholar] [CrossRef]
- Thulasiraman, S.; Yunus, N.M.M.; Kumar, P.; Kesuma, Z.R.; Norhakim, N.; Wilfred, C.D.; Roffi, T.M.; Hamdan, M.F.; Burhanudin, Z.A. Effects of Ionic Liquid, 1-Ethyl-3-methylimidazolium Chloride ([EMIM]Cl), on the Material and Electrical Characteristics of Asphaltene Thin Films. Materials 2022, 15, 2818. [Google Scholar] [CrossRef]
- Xiao, J.; Shreeve, J.M. Synthesis of 2,2′-Biimidazolium-Based Ionic Liquids: Use as a New Reaction Medium and Ligand for Palladium-Catalyzed Suzuki Cross-Coupling Reactions. J. Org. Chem. 2005, 2343, 3072–3078. [Google Scholar] [CrossRef] [PubMed]
- Ohira, K.; Yoshida, K.; Hayase, S.; Itoh, T. Amino Acid Ionic Liquid as an Efficient Cosolvent of Dimethyl Sulfoxide to Realize Cellulose Dissolution at Room Temperature. Chem. Lett. 2012, 41, 987–989. [Google Scholar] [CrossRef]
- Wendler, F.; Todi, L.-N.; Meister, F. Thermostability of imidazolium ionic liquids as direct solvents for cellulose. Thermochim. Acta 2012, 528, 76–84. [Google Scholar] [CrossRef]
- Vatti, A.K.; Dey, P.; Acharya, S.; Kundarapu, L.K.; Puttapati, S.K. Role of Ionic Liquid in Asphaltene Dissolution: A Combined Experimental and Molecular Dynamics Study. Energy Fuels 2022, 36, 9111–9120. [Google Scholar] [CrossRef]
- El-Hefnawy, M.E.; Atta, A.M.; El-Newehy, M.; Ismail, A.I. Synthesis and characterization of imidazolium asphaltenes poly (ionic liquid) and application in asphaltene aggregation inhibition of heavy crude oil. J. Mater. Res. Technol. 2020, 9, 14682–14694. [Google Scholar] [CrossRef]
- Ghosh, B.; Sulemana, N.; Banat, F.; Mathew, N. Ionic liquid in stabilizing asphaltenes during miscible CO2 injection in a high pressure oil reservoir. J. Pet. Sci. Eng. 2019, 180, 1046–1057. [Google Scholar] [CrossRef]
- Rashid, Z.; Wilfred, C.D.; Iyyaswami, R.; Appusamy, A.; Thanabalan, M. Investigating the solubility of petroleum asphaltene in ionic liquids and their interaction using COSMO-RS. J. Ind. Eng. Chem. 2019, 79, 194–203. [Google Scholar] [CrossRef]
- Becherini, S.; Mezzetta, A.; Chiappe, C.; Guazzelli, L. Levulinate amidinium protic ionic liquids (PILs) as suitable media for the dissolution and levulination of cellulose. New J. Chem. 2019, 43, 4554–4561. [Google Scholar] [CrossRef]
- Boissou, F.; Mühlbauer, A.; Vigier, K.D.O.; Leclercq, L.; Kunz, W.; Marinkovic, S.; Estrine, B.; Nardello-Rataj, V.; Jérôme, F. Transition of cellulose crystalline structure in biodegradable mixtures of renewably-sourced levulinate alkyl ammonium ionic liquids, γ-valerolactone and water. Green Chem. 2014, 16, 2463–2471. [Google Scholar] [CrossRef]
- Razik, A.H.A.; Khor, C.S.; Elkamel, A. A model-based approach for biomass-to-bioproducts supply Chain network planning optimization. Food Bioprod. Process. 2019, 118, 293–305. [Google Scholar] [CrossRef]
- Saldarriaga-Hernández, S.; Velasco-Ayala, C.; Flores, P.L.-I.; Rostro-Alanis, M.D.J.; Parra-Saldivar, R.; Iqbal, H.M.; Carrillo-Nieves, D. Biotransformation of lignocellulosic biomass into industrially relevant products with the aid of fungi-derived lignocellulolytic enzymes. Int. J. Biol. Macromol. 2020, 161, 1099–1116. [Google Scholar] [CrossRef] [PubMed]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. Chemsuschem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Ekielski, A.; Mishra, P.K. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int. J. Mol. Sci. 2020, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Nowakowski, D.J.; Bridgwater, A.V. A systematic study of the kinetics of lignin pyrolysis. Thermochim. Acta 2010, 498, 61–66. [Google Scholar] [CrossRef]
- Lai, B.; Bai, R.; Gu, Y. Lignosulfonate/Dicationic Ionic Liquid Composite as a Task-Specific Catalyst Support for Enabling Efficient Synthesis of Unsymmetrical 1,3-Diynes with A Low Substrate Ratio. ACS Sustain. Chem. Eng. 2018, 6, 17076–17086. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Achyuthan, A.M.; Adams, P.D.; Dirk, S.M.; Harper, J.C.; Simmons, B.A.; Singh, A.K. Supramolecular Self-Assembled Chaos: Polyphenolic Lignin’s Barrier to Cost-Effective Lignocellulosic Biofuels. Molecules 2010, 15, 8641–8688. [Google Scholar] [CrossRef]
- Li, G.; Hou, X.; Ma, W.; Wang, F. Mechanical Properties of Loess Treated by Calcium Lignosulfonate. In Recent Advances in Geo-Environmental Engineering, Geomechanics and Geotechnics, and Geohazards; Springer: Berlin/Heidelberg, Germany, 2019; pp. 287–289. [Google Scholar] [CrossRef]
- Liu, S.; Van Muyden, A.P.; Bai, L.; Cui, X.; Fei, Z.; Li, X.; Hu, X.; Dyson, P.J. Metal-Sulfide Catalysts Derived from Lignosulfonate and their Efficient Use in Hydrogenolysis. Chemsuschem 2019, 12, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Pourmahdi, M.; Nasiri, A.R. Synthesis and characterization of lignosulfonate/acrylamide graft copolymers and their application in the environmentally friendly water- based drilling fluid. J. Pet. Sci. Eng. 2018, 171, 484–494. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, B.; Guo, Y.; Wang, T.; Zhang, L.; Zhong, C.; Wang, H. The role of oxidizer in the flotation separation of chalcopyrite and galena using sodium lignosulfonate as a depressant. Miner. Eng. 2021, 172, 107160. [Google Scholar] [CrossRef]
- Li, J. Influence of Sodium Lignosulfonate on the Corrosion-Inhibition Behavior of Q235 Steel in Simulated Concrete Pore Solutions. Int. J. Electrochem. Sci. 2020, 15, 7136–7151. [Google Scholar] [CrossRef]
- Simon, S.; Saadat, M.; Ruwoldt, J.; Dudek, M.; Ellis, R.J.; Øye, G. Lignosulfonates in Crude Oil Processing: Interactions with Asphaltenes at the Oil/Water Interface and Screening of Potential Applications. ACS Omega 2020, 5, 30189–30200. [Google Scholar] [CrossRef]
- Ghorbani-Choghamarani, A.; Taherinia, Z.; Tyula, Y.A. Efficient biodiesel production from oleic and palmitic acid using a novel molybdenum metal–organic framework as efficient and reusable catalyst. Sci. Rep. 2022, 12, 10338. [Google Scholar] [CrossRef] [PubMed]
- Bahrpaima, K.; Fatehi, P. Synthesis and Characterization of Carboxyethylated Lignosulfonate. Chemsuschem 2018, 11, 2967–2980. [Google Scholar] [CrossRef]
- Ghanem, A.; Alharthy, R.D.; Desouky, S.M.; El-Nagar, R.A. Synthesis and Characterization of Imidazolium-Based Ionic Liquids and Evaluating Their Performance as Asphaltene Dispersants. Materials 2022, 15, 1600. [Google Scholar] [CrossRef] [PubMed]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]
- Popovicheva, O.B.; Kireeva, E.D.; Shonija, N.K.; Vojtisek-Lom, M.; Schwarz, J. FTIR analysis of surface functionalities on particulate matter produced by off-road diesel engines operating on diesel and biofuel. Environ. Sci. Pollut. Res. 2015, 22, 4534–4544. [Google Scholar] [CrossRef]
- Dos Santos, P.S.; Erdocia, X.; Gatto, D.A.; Labidi, J. Characterisation of Kraft lignin separated by gradient acid precipitation. Ind. Crop. Prod. 2014, 55, 149–154. [Google Scholar] [CrossRef]
- Liu, Z.; Fatehi, P.; Jahan, M.S.; Ni, Y. Separation of lignocellulosic materials by combined processes of pre-hydrolysis and ethanol extraction. Bioresour. Technol. 2011, 102, 1264–1269. [Google Scholar] [CrossRef]
- Janus, R.; Wądrzyk, M.; Lewandowski, M.; Zaborowska, K.; Korzeniowski, Ł.; Plata, M. A novel capillary forces-founded accessory for reliable measurements of ATR-FTIR spectra of volatile liquids. Microchem. J. 2023, 185, 108219. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B. Chemical and Thermal Characteristics of Ion-Exchanged Lignosulfonate. Molecules 2023, 28, 2755. [Google Scholar] [CrossRef] [PubMed]
- Clough, M.T.; Geyer, K.; Hunt, P.A.; Mertes, J.; Welton, T. Thermal decomposition of carboxylate ionic liquids: Trends and mechanisms. Phys. Chem. Chem. Phys. 2013, 15, 20480–20495. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yuan, Y.; He, M.; Zhang, X.; Li, L.; Zhang, Y.; Li, B. Development of a multifunctional food packaging for meat products by incorporating carboxylated cellulose nanocrystal and beetroot extract into sodium alginate films. Food Chem. 2023, 415, 135799. [Google Scholar] [CrossRef] [PubMed]
- Montanino, M.; Carewska, M.; Alessandrini, F.; Passerini, S.; Appetecchi, G.B. The role of the cation aliphatic side chain length in piperidinium bis(trifluoromethansulfonyl)imide ionic liquids. Electrochimica Acta 2011, 57, 153–159. [Google Scholar] [CrossRef]
- Lima, R.B.; Raza, R.; Qin, H.; Li, J.; Lindström, M.E.; Zhu, B. Direct lignin fuel cell for power generation. RSC Adv. 2013, 3, 5083–5089. [Google Scholar] [CrossRef]
- Sun, R. Lignin Source and Structural Characterization. Chemsuschem 2020, 13, 4385–4393. [Google Scholar] [CrossRef]
- Domínguez, J.; Oliet, M.; Alonso, M.; Gilarranz, M.; Rodríguez, F. Thermal stability and pyrolysis kinetics of organosolv lignins obtained from Eucalyptus globulus. Ind. Crop. Prod. 2007, 27, 150–156. [Google Scholar] [CrossRef]
- Majumdar, R.D.; Gerken, M.; Mikula, R.; Hazendonk, P. Validation of the Yen–Mullins Model of Athabasca Oil-Sands Asphaltenes using Solution-State 1H NMR Relaxation and 2D HSQC Spectroscopy. Energy Fuels 2013, 27, 6528–6537. [Google Scholar] [CrossRef]
- Dastgerdi, Z.H.; Meshkat, S.S.; Hosseinzadeh, S.; Esrafili, M.D. Application of Novel Fe3O4–Polyaniline Nanocomposites in Asphaltene Adsorptive Removal: Equilibrium, Kinetic Study and DFT Calculations. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1160–1170. [Google Scholar] [CrossRef]
- Hizaddin, H.F.; Hashim, M.A.; Anantharaj, R. Evaluation of Molecular Interaction in Binary Mixture of Ionic Liquids + Heterocyclic Nitrogen Compounds: Ab Initio Method and COSMO-RS Model. Ind. Eng. Chem. Res. 2013, 52, 18043–18058. [Google Scholar] [CrossRef]
- Ogunlaja, A.S.; Hosten, E.; Tshentu, Z.R. Dispersion of Asphaltenes in Petroleum with Ionic Liquids: Evaluation of Molecular Interactions in the Binary Mixture. Ind. Eng. Chem. Res. 2014, 53, 18390–18401. [Google Scholar] [CrossRef]
- Rashid, Z.; Wilfred, C.D.; Gnanasundaram, N.; Arunagiri, A.; Murugesan, T. Screening of ionic liquids as green oilfield solvents for the potential removal of asphaltene from simulated oil: COSMO-RS model approach. J. Mol. Liq. 2018, 255, 492–503. [Google Scholar] [CrossRef]
- Mi, Y.-F.; Xu, G.; Guo, Y.-S.; Wu, B.; An, Q.-F. Development of antifouling nanofiltration membrane with zwitterionic functionalized monomer for efficient dye/salt selective separation. J. Membr. Sci. 2020, 601, 117795. [Google Scholar] [CrossRef]





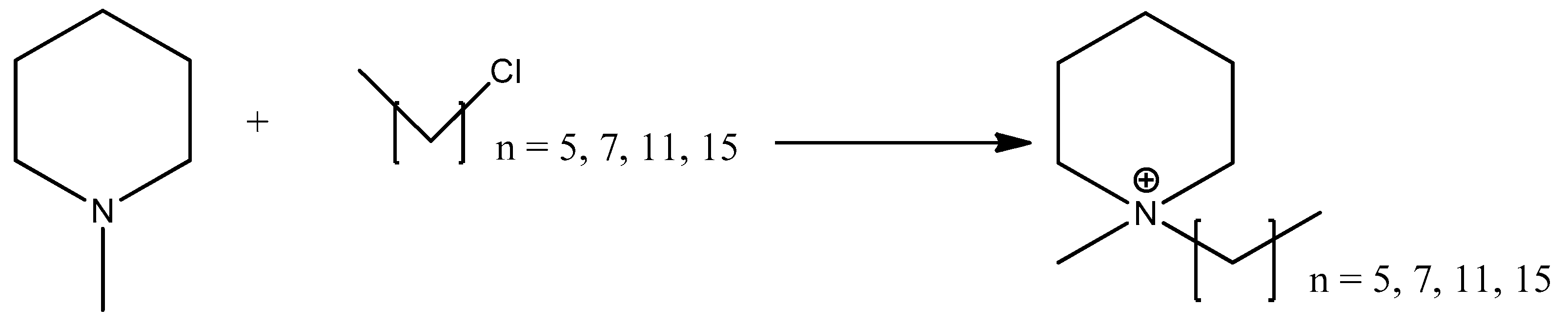
| ILs | Findings | Reference |
|---|---|---|
| 1-butyl-3-methyl-imidazolium hexafluorophosphate, [BMIM][PF6] | The IL possessed a higher affinity towards asphaltene to disperse the particles in non-polar media. | [28] |
| 1,3 diheptyl-2-hydroxyphenyl-imidazolium asphaltene carboxylate, AHIL | The hydrophobic groups in the cation can increase the dispersion of asphaltenes in n-alkanes. | [29] |
| 1-butyl-3-methyl-imidazolium bromide, [BMIM][Br] | A larger ionic radius exhibits better steric stabilization of IL–asphaltene complexes. | [30] |
| 1-methyl-1-hexyl-piperidinium, [C6C1Pip]+, 1-methyl-1-hexyl-pyrrolidinium, [C6C1Pyr]+, 1-hexyl-quinolinium, [C6q]+, beryllium tetrafluoride [BF4]− phenyl benzoate [PhBenz]-, benzoate [Benz]- | A high non-polarity cation can enhance the charge-sharing interaction with poly-aromatic molecules. An anion with high hydrophobicity can enhance the dispersing strength of asphaltene molecules. | [31] |
| Methyl-triooctyl-ammonium dodecyl sulfate [MTOA][DDS] | The amphiphilic property of IL provides interactions between polar and non-polar sites of asphaltene molecules. | [12] |
| Lignosulfonate-Based | Studies | Reference |
|---|---|---|
| Calcium lignosulfonate | Effect of mechanical properties on treated loess | [41] |
| Lignosulfonate derivative of metal-sulfide catalyst | Efficiency of the hydrogenolysis process | [42] |
| Lignosulfonate reagent | Drilling fluid | [43] |
| Sodium lignosulfonate | Flotation separation of chalcopyrite and galena | [44] |
| Sodium lignosulfonate | Corrosion inhibition of Q235 steel in concrete pore solutions. | [45] |
| Assignments | Wavenumber (cm−1) | Reference | ||
|---|---|---|---|---|
| [Na]2[LS] | [RC1Pip][Cl] | [RC1Pip]2[LS] | ||
| O-H stretch | 3250 | - | 3250 | [48] |
| N-H stretch | - | 3250 | 3250 | [49] |
| C-H stretch | 2930 | 2930 | 2930 | [50] |
| Unsaturated C=C stretch | 1560 | 1560 | [51] | |
| C-H bend in aromatic | 1460 | 1460 | [52] | |
| C-H bend in aliphatic | 1460 | 1460 | [53] | |
| CH3 bend | 1350 | 1350 | 1350 | [54] |
| S=O stretch | 1110 | 1110 | [55] | |
| C-O stretch of methoxy | 1050 | 1050 | ||
| Lignosulfonate-Based ILs | Onset Degradation Temperature (°C) | Degradation Temperature Range (°C) | ||
|---|---|---|---|---|
| First Stage | Second Stage | Third Stage | ||
| [C6C1Pip]2[LS] | 118.24 | 118.24–207.21 | 207.21–305.49 | 453.78–800 |
| [C8C1Pip]2[LS] | 160.70 | 160.70–235.04 | 235.04–333.30 | 375.20–800 |
| [C12C1Pip]2[LS] | 204.87 | 204.87–254.67 | 254.67–375.20 | 333.30–800 |
| [C16C1Pip]2[LS] | 208.83 | 208.83–273.19 | 273.19–453.78 | 305.49–800 |
| Ionic Liquid | Initial Concentration (mg/L) | Linear Pseudo-First-Order | Linear Pseudo-Second Order | ||
|---|---|---|---|---|---|
| K1 | R2 | K2 | R2 | ||
| [C16C1Pip]2[LS] | 100 | 0.030 | 0.8652 | 0.035 | 0.9577 |
| Molecules | EH (eV) | EL (eV) | Orbital Energy Gap, EG (eV) |
|---|---|---|---|
| [C16C1Pip]2[LS] | 1.295 | 1.550 | 0.255 |
| Asphaltene | −7.410 | −6.131 | 1.279 |
| [C16C1Pip]2[LS] + asphaltene | −2.200 | −2.111 | 0.089 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahtar, A.; Sulaimon, A.A.; Wilfred, C.D. Lignosulfonate-Based Ionic Liquids as Asphaltene Dispersants. Molecules 2023, 28, 3390. https://doi.org/10.3390/molecules28083390
Mahtar A, Sulaimon AA, Wilfred CD. Lignosulfonate-Based Ionic Liquids as Asphaltene Dispersants. Molecules. 2023; 28(8):3390. https://doi.org/10.3390/molecules28083390
Chicago/Turabian StyleMahtar, Ariff, Aliyu Adebayo Sulaimon, and Cecilia Devi Wilfred. 2023. "Lignosulfonate-Based Ionic Liquids as Asphaltene Dispersants" Molecules 28, no. 8: 3390. https://doi.org/10.3390/molecules28083390
APA StyleMahtar, A., Sulaimon, A. A., & Wilfred, C. D. (2023). Lignosulfonate-Based Ionic Liquids as Asphaltene Dispersants. Molecules, 28(8), 3390. https://doi.org/10.3390/molecules28083390





